Abstract
Pleomorphic adenoma is a benign tumor typically found in the salivary glands. Its occurrence in the trachea is rare and often overlooked due to low clinical suspicion, despite respiratory symptoms. We report the case of a 47-year-old Caucasian woman, active smoker with a 34 pack-year history, and a diagnosis of non-allergic asthma for 15 years, with progressive worsening control despite medical therapy. The main clinical symptoms were dyspnea and recurrent respiratory infections. Imaging revealed a tracheal mass causing extensive luminal obstruction. The lesion was successfully treated with bronchoscopic excision and laser therapy. Histopathological and genetic analyses confirmed the diagnosis of pleomorphic adenoma. This case highlights the importance of considering neoplastic etiologies in patients with persistent respiratory symptoms unresponsive to standard asthma treatment.
Keywords
Bronchoscopic excision, Pleomorphic adenoma, Refractory asthma, Tracheal tumor
Abbreviations
CK8/18: Cytokeratin 8/18; CT: Computed Tomography; EMA: Epithelial Membrane Antigen; FISH: Fluorescence In Situ Hybridization; GFAP: Glial Fibrillary Acidic Protein; H&E: Hematoxylin and Eosin; HMGA2: High Mobility Group AT-Hook 2; PLAG1: Pleomorphic Adenoma Gene 1; SMA: Smooth Muscle Actin (α-SMA: Alpha-Smooth Muscle Actin); TPA: Tracheal Pleomorphic Adenoma
Introduction
Pleomorphic adenoma (TPA) is the most common benign neoplasm of the salivary glands. In extremely rare cases, it may originate in the tracheobronchial tree [1]. Due to its location and slow growth, it often remains asymptomatic until it causes significant airflow obstruction, leading to persistent cough, exertional dyspnea, wheezing, or inspiratory stridor. This frequently results in an initial misdiagnosis of asthma, especially in young and middle-aged women [2,3]. When a pre-existing diagnosis of obstructive airway disease is present, patients may receive prolonged treatment with bronchodilators or corticosteroids, delaying the identification of the underlying condition by several months or years [4].
On computed tomography (CT), tracheal pleomorphic adenoma typically appears as a well circumscribed, pedunculated or polypoid lesion arising from the tracheal wall. It usually measures around 2 cm, demonstrates homogeneous soft-tissue attenuation, and shows gradual, delayed contrast enhancement. Lymphadenopathy, pleural effusion, and distant metastases are exceedingly rare. A combination of CT and endoscopic evaluation is essential for accurate diagnosis [5,6].
Histologically, TPA closely resemble pleomorphic adenomas of the salivary glands. These tumors feature a prominent ductal epithelial component mixed with spindle to plasmacytoid myoepithelial cells within a chondromyxoid stroma, sometimes with focal squamous metaplasia. While no specific immunohistochemical panel exists, markers such as cytokeratins, S100, GFAP, p40, p63, calponin, and α-SMA highlight the tumor’s biphasic nature. Molecular studies remain limited, with no confirmed PLAG1 or HMGA2 gene fusions, commonly seen in salivary pleomorphic adenomas. Rarely, malignant transformation into carcinoma ex-pleomorphic adenoma may occur, with epithelial, myoepithelial, or mixed differentiation. Diagnosis depends on features such as necrosis, marked atypia, vascular or perineural invasion, and ≥5 mitoses per 2 mm² [7–9].
Definitive treatment of TPA involves bronchoscopic or surgical resection. Rigid bronchoscopy, often using a combination of mechanical debulking, electrocautery, laser therapy, or argon plasma coagulation, has proven effective for tumors with minimal submucosal involvement and no evidence of extraluminal extension. However, surgical resection is preferred for broad-based tumors, submucosal infiltration, incomplete endoscopic excision, suspected extraluminal extension or recurrence [5].
Case Presentation
The patient was a 47-year-old Caucasian woman, active smoker with a 34 pack-year history, who presented with progressively worsening exertional dyspnea and recurrent respiratory infections. Her past medical history included a 15-year diagnosis of non-allergic asthma, vasomotor rhinitis, hypertension, and a stable thyroid nodule. She was treated with fluticasone furoate/vilanterol for asthma and bisoprolol 5 mg daily for hypertension. Family history was notable for colorectal cancer in her mother, pancreatic cancer in a maternal uncle, and breast cancer in two maternal aunts.
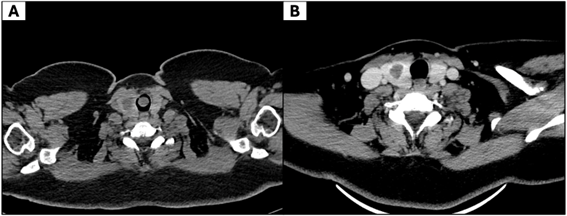
Since 2018, her asthma symptoms had progressively worsened despite adherence to treatment. At that time, pulmonary function tests revealed no significant abnormalities. Due to persistent symptoms, computed tomography (CT) of the neck and chest was performed, which revealed a pedunculated, hypodense mass measuring 14 × 13 × 3 mm, arising from the anterior tracheal wall at the first tracheal ring, causing significant luminal obstruction (Figure 1A), while post-excision CT showed no residual disease (Figure 1B).
Figure 1. Computed tomography (CT) of the trachea. (A) Axial CT demonstrates a pedunculated, solid mass in the proximal trachea, arising from the anterior wall and partially obstructing the airway lumen. (B) Post-excision CT shows no residual disease, with no pathological wall thickening or abnormal enhancement.
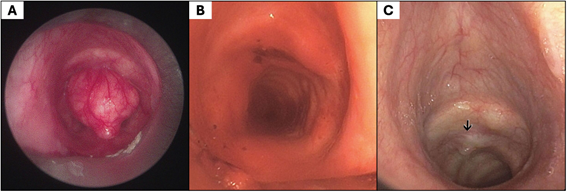
Bronchoscopy demonstrated a well-circumscribed, pedunculated mass originating from the anterior tracheal wall and occupying approximately 90% of the tracheal lumen, causing critical airway narrowing, as shown in Figure 2A. Rigid bronchoscopy with mechanical debulking achieved complete macroscopic excision, after which laser therapy was applied to the tumor implantation site (Figure 2B). Airway patency was fully re-established immediately after the procedure, with resolution of dyspnea.
Figure 2. Bronchoscopic views of the trachea. (A) A pedunculated lesion arising from the anterior tracheal wall caused near-complete airwayobstruction. (B) Mechanical debulking achieved complete macroscopic excision, and laser therapy was applied to the anterior wall at the tumor implantation site. (C) Follow-up bronchoscopy revealed a mucosal protrusion (black arrow), which was biopsied and confirmed absence of residual neoplasm.
Histopathological examination revealed a well-defined, lobulated neoplasm composed of ductal epithelial cells (positive for CK8/18 and EMA), arranged in tubular structures and surrounded by a myoepithelial layer (positive for S100, p40, and p63). Focal squamous metaplasia with keratin cysts and adipose tissue infiltration was present, without evidence of mitosis, necrosis, or per neural/lymphovascular invasion. Figure 3 illustrates these findings, including H&E and Alcian Blue stains, as well as CK8/18 and p63 immunostaining. Fluorescence in situ hybridization (FISH) detected a PLAG1 gene rearrangement in 84% of nuclei, confirming the diagnosis of tracheal pleomorphic adenoma (TPA).
Figure 3. Histopathological features of tracheal pleomorphic adenoma. (A) Hematoxylin and eosin staining shows a well-circumscribed benign mixed tumor composed of ductal and myoepithelial cells. (B) Alcian Blue highlights the chondromyxoid stromal component. (C) CK8/18 immunostaining marks the ductal epithelial component. (D) p63 immunostaining highlights the myoepithelial cells.
Following bronchoscopic intervention, the patient’s respiratory symptoms resolved completely. At six-month follow-up, CT imaging (Figure 1B) and bronchoscopy were performed. The bronchoscopy revealed a mucosal protrusion, as shown in Figure 2C, which was biopsied and showed respiratory epithelium without evidence of residual neoplasm. Clinically, the patient was symptom-free, with unrestricted daily activities and no radiological or endoscopic signs of recurrence.
Discussion
Tracheal pleomorphic adenoma (TPA) is a rare cause of central airway obstruction, with fewer than 75 cases reported in the literature [2]. Its clinical presentation often mimics chronic pulmonary conditions such as asthma, contributing to delayed or missed diagnosis, as illustrated in our case [2–4]. The patient had a long-standing history of smoking and a 15-year diagnosis of non-allergic asthma, raising the possibility that her initial respiratory symptoms, previously attributed to asthma, may have been caused by this indolent tumor [4].
Pulmonary function tests were unremarkable, likely reflecting the initially limited degree of airway obstruction. As the tumor progressed, it may have caused fixed central airway obstruction, typically seen as flattening of both the inspiratory and expiratory limbs of the flow–volume loop [3]. Recognizing this pattern is key to suspecting an intraluminal lesion. This case underscores that normal spirometry does not exclude early-stage central airway tumors.
TPA typically consists of epithelial and myoepithelial cells embedded within a chondromyxoid stroma. In our case, the diagnosis was further supported by the detection of a PLAG1 gene rearrangement, a molecular feature that helps distinguish pleomorphic adenoma from malignant salivary-type tumors such as adenoid cystic carcinoma or mucoepidermoid carcinoma [7–9]. Although TPA is classified as a benign tumor, rare cases of malignant transformation into carcinoma ex pleomorphic adenoma have been reported in the tracheobronchial tree, underscoring the need for complete excision and appropriate follow-up [9].
Bronchoscopic excision combined with laser ablation was successfully performed in this case, given the lesion’s pedunculated morphology, minimal submucosal involvement, and absence of extraluminal extension. This outcome supports the use of endoscopic approaches as a less invasive and effective treatment option for selected patients with TPA [5].
Follow-up CT and bronchoscopy at six months were unremarkable, and the patient remains asymptomatic. However, delayed recurrence of TPA has been reported up to ten years post-resection, particularly in cases with positive or uncertain margins, and long-term surveillance with periodic imaging and bronchoscopy is recommended for follow-up [5].
This case illustrates how rare conditions such as tracheal tumors can remain undiagnosed for years when symptoms mimic common diseases like asthma. Delayed recognition may result in prolonged ineffective treatment and worsening obstruction. In patients with respiratory symptoms unresponsive to therapy, thorough diagnostic evaluation, including imaging and bronchoscopy, should be considered to rule out structural causes.
Conclusion
This case demonstrates a rare but critical cause of severe tracheal obstruction due to pleomorphic adenoma, which posed a significant risk of life-threatening respiratory compromise. The tumor’s favorable morphology enabled complete endoscopic excision via bronchoscopic debulking and laser ablation, with subsequent histological confirmation of its benign nature, thus avoiding the need for more invasive surgical intervention.
This report is limited by being a single case with short follow-up. Nevertheless, it underscores the importance of considering tracheal tumors in patients with refractory asthma-like symptoms and highlights the role of imaging and bronchoscopy in early diagnosis. Timely recognition and treatment can restore airway patency, relieve symptoms, and prevent life-threatening complications.
Conflicts of Interest
The authors declare no conflicts of interest.
References
2. Schultz K, Al-Tarbsheh AH, Railwah CN, Bejarano P, Woytanowski JR, Romero-Legro I. Pleomorphic adenoma: a rare cause of central airway obstruction. Chest. 2024 Oct 1;166(4):A4870–1.
3. Kushima N, Himeji D, Yanagihara T, Maekawa K, Marutsuka K. Tracheal Pleomorphic Adenoma With Severe Airway Obstruction. Cureus. 2024 Dec 24; 16(12):e76341.
4. Aribas OK, Kanat F, Avunduk MC. Pleomorphic adenoma of the trachea mimicking bronchial asthma: report of a case. Surgery today. 2007 Jun; 37(6):493–5.
5. Liao QN, Fang ZK, Chen SB, Fan HZ, Chen LC, Wu XP, et al. Pleomorphic adenoma of the trachea: a case report and review of the literature. World Journal of Clinical Cases. 2020 Dec 6;8(23):6026–35.
6. Inomata M, Kuroki S, Oguri N, Sato Y, Kawano F, Maeda R. Pleomorphic adenoma of the trachea: a case report. International Journal of Surgery Case Reports. 2023 Aug 1;109:108499.
7. Falk N, Weissferdt A, Kalhor N, Moran CA. Primary pulmonary salivary gland-type tumors: a review and update. Advances in Anatomic Pathology. 2016 Jan 1; 23(1):13–23.
8. Katabi N, Xu B, Jungbluth AA, Zhang L, Shao SY, Lane J, et al. PLAG1 immunohistochemistry is a sensitive marker for pleomorphic adenoma: a comparative study with PLAG1 genetic abnormalities. Histopathology. 2018 Jan;72(2):285–93.
9. Weissferdt A, Moran CA. Pulmonary Salivary Gland–Type Tumors With Features of Malignant Mixed Tumor (Carcinoma Ex Pleomorphic Adenoma) A Clinicopathologic Study of Five Cases. American journal of clinical pathology. 2011 Nov 1; 136(5):793–8.