Abstract
Ticagrelor is an oral, third-generation reversible P2Y12 receptor antagonist used in the treatment of patients with atherosclerotic cardiovascular disease. Dyspnea is a well-recognized side effect of ticagrelor, typically occurring within hours to days after initiation. In most cases, the dyspnea is mild and resolves spontaneously without intervention. However, dyspnea can be significant and intolerable in some patients necessitating discontinuation of ticagrelor. Less commonly recognized is the association of ticagrelor with central sleep apnea. Here, we present a case of a patient who developed dyspnea shortly after starting ticagrelor but also exhibited evidence of central sleep apnea, both of which markedly improved upon discontinuation of the drug. The exact pathophysiologic mechanism underlying ticagrelor-associated dyspnea and central sleep apnea remains unclear. However, one hypothesis suggests that stimulation of pulmonary vagal C-fibers leads to increased levels of extracellular adenosine, which may heighten chemosensitivity to hypercapnia and thus contribute to these respiratory effects.
Keywords
Dyspnea, Central sleep apnea, Hypopnea, Cheyne-stokes respiration, Ticagrelor
Abbreviations
CSA: Central Sleep Apnea; OSA: Obstructive Sleep Apnea; PCI: Percutaneous Coronary Intervention; DAPT: Dual Antiplatelet Therapy; PLATO: Platelet Inhibition and Patient Outcomes; EKG: Electrocardiogram; ACS: Acute Coronary Syndrome; CHF: Congestive Heart Failure; LVEF: Left Ventricular Ejection Fraction; CVA: Cerebrovascular Accident; PTCA: Percutaneous Transluminal Coronary Angioplasty; ASV: Adaptive Servo-Ventilation; CPAP: Continuous Positive Airway Pressure; BIPAP: Bilevel Positive Airway Pressure; SDB: Sleep-Disordered Breathing; TIA: Transient Ischemic Attach
Introduction
Ticagrelor is an oral, third-generation reversible noncompetitive P2Y12 receptors antagonist used in combination with aspirin as a component of dual antiplatelet therapy (DAPT) following percutaneous coronary intervention (PCI) in patients with coronary artery disease (CAD). The primary role of this drug is to reduce the risk of adverse thrombotic events.
Several studies have demonstrated the clinical superiority of ticagrelor over clopidogrel, particularly in the first three months post-PCI, in reducing ischemic cardiovascular events and the composite outcome of cardiovascular, stroke, and myocardial infarction, with no significant increase in major bleeding [1].
Dyspnea is one of the most notable side effects associated with ticagrelor [2]. The incidence of dyspnea was reported at 13.8% in the PLATelet Inhibition and Patient Outcomes (PLATO) trial [3] and as high as 38.6% in the ONSET/OFFSET trial [4]. Importantly, dyspnea exhibited by these patients is unrelated to underlying cardiac or pulmonary dysfunction. It typically develops early in the course of treatment, often within the first 24 hours of initiation of the drug and is dose-dependent [5]. In most cases, dyspnea is mild and resolves spontaneously within days to weeks without intervention. However, some patients with moderate to severe ticagrelor-induced dyspnea may discontinue medication without first notifying their physicians. This can result in a significant increase in the risk of serious thrombotic events. Rates of drug discontinuation due to dyspnea vary between 0.9% and 10% [6]. While the precise mechanism underlying ticagrelor-induced dyspnea remains unclear [7], one theory suggests a role for increased extracellular adenosine levels [8,9], while another implicates stimulation of pulmonary vagal C-fiber receptors [10]. Interestingly, the same pathophysiologic mechanisms have also been suggested to explain ticagrelor-associated central sleep apnea (CSA) [11].
The current approach to managing ticagrelor-related dyspnea involves first ruling out other potential causes of respiratory symptoms. In most cases, patients are reassured that symptoms are typically self-limited and resolve over time. However, in moderate to severe cases in which dyspnea is intolerable, it may be necessary to discontinue ticagrelor and switch to an alternative antiplatelet agent.
Although dyspnea is a well-recognized side effect, the potential association of ticagrelor with CSA is less well-known. However, numerous reports in the literature suggest a possible link between ticagrelor use and CSA [12]. Here, we present a case of a patient who developed evidence of CSA shortly after initiating ticagrelor, with marked improvement following its discontinuation.
Case Report
A 65-year-old male with multiple cardiovascular risk factors including hypertension, nonobstructive CAD found on cardiac catheterization 3 years ago, uncontrolled hyperlipidemia with high LDL (low-density lipoprotein) not on lipid lowering medication due to statin intolerance, TIA, and family history of premature coronary artery disease, presented to the emergency room with typical chest pain radiating to his arm, accompanied by nausea and diaphoresis. His symptoms improved with sublingual nitroglycerin. An electrocardiogram showed sinus rhythm with no significant ST segment elevation or depression. His physical examination was unremarkable, and initial laboratory tests revealed a mildly elevated troponin level. Findings on chest X-ray were also unremarkable. He was diagnosed with non-ST elevation myocardial infarction (NSTEMI) and admitted for further management. His home medications include Aspirin 81 mg once a day, magnesium oxide, Vit C, Vit K, Vit E, Vit B complex, Zinc and Coenzyme Q10.
The patient underwent cardiac stress testing in conjunction with a myocardial perfusion imaging study, which yielded abnormal results. An echocardiogram revealed normal left ventricular systolic function with an ejection fraction of 60-65%, grade 1 diastolic dysfunction, and no significant valvular abnormalities. Coronary angiography revealed high-grade stenosis of the proximal right coronary artery (RCA), for which he underwent successful PCI. He began on DAPT with ticagrelor in addition to low-dose aspirin. His cardiac medical regimen was optimized starting him on metoprolol, lisinopril with the plan to initiate PCSK9 inhibitor therapy in the outpatient setting for management of uncontrolled hyperlipidemia.
The patient developed dyspnea within 24 hours of starting ticagrelor with no other plausible etiology for his symptoms. It was explained to the patient that his dyspnea was probably a side effect of ticagrelor that was likely to resolve with time but that the current antiplatelet regimen would be changed if symptoms persisted. He was discharged home in stable condition.
At 3 months follow-up, the patient continued to report significant dyspnea accompanied by episodes of apnea when lying down. He described these episodes as he cannot catch his breath when he relaxed and resting as well as having daytime fatigue and excessive sleepiness. His wife noted that he had occasionally snored in the past but that his apneic episodes had become more pronounced since starting on ticagrelor. She also reported witnessing episodes of breathing cessation while he was asleep.
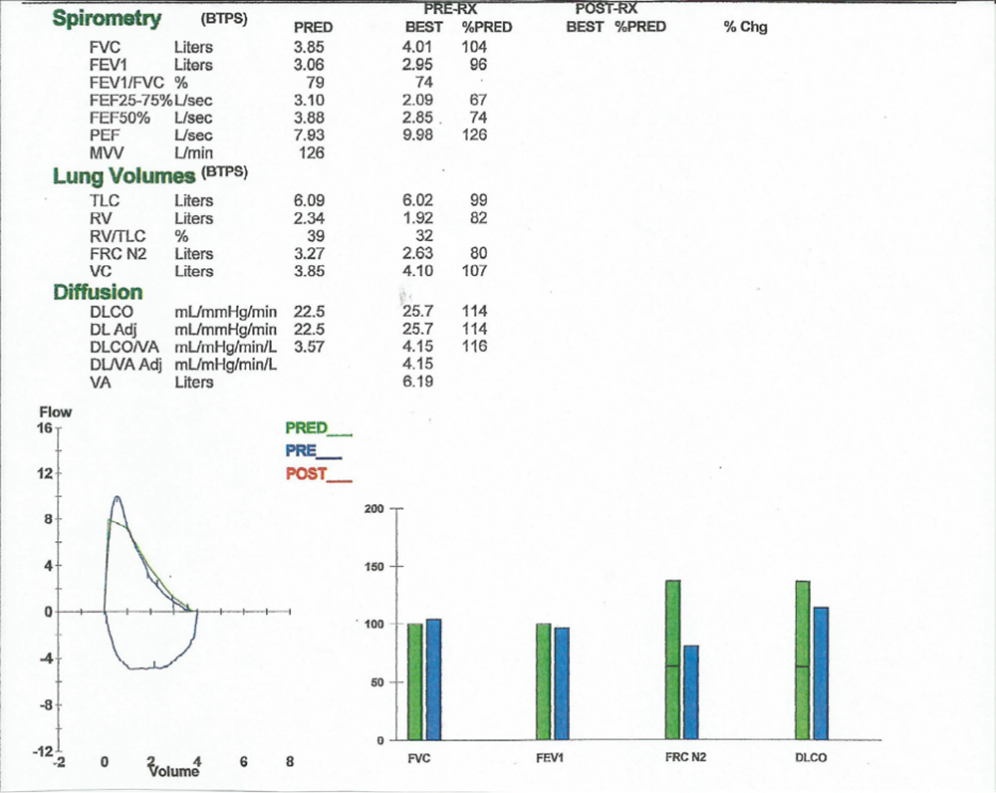
To further evaluate his symptoms, the patient underwent pulmonary function testing. The results of this study were unremarkable (Figure 1).
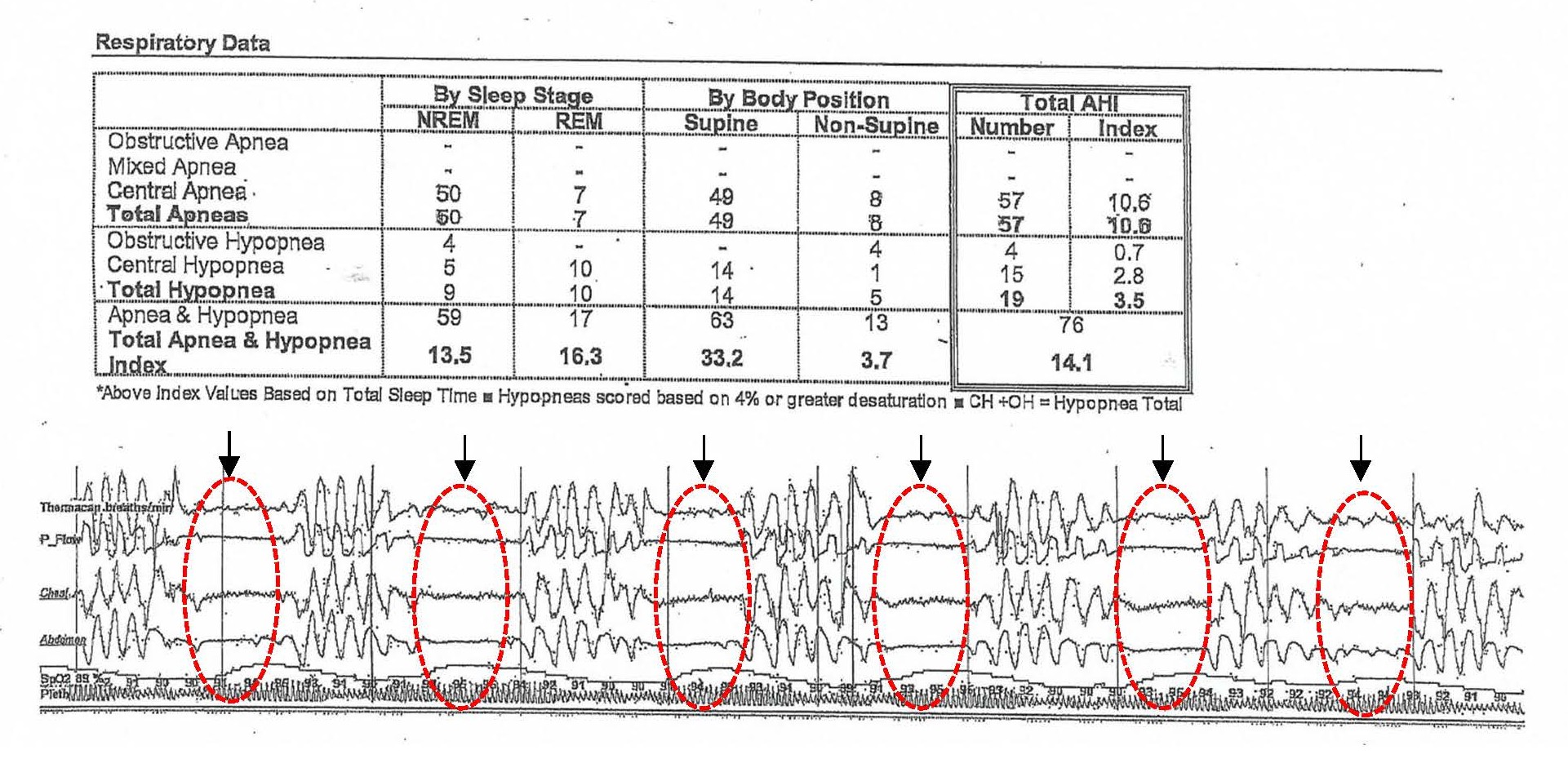
The patient subsequently underwent in-laboratory polysomnography, which revealed 57 episodes of central sleep apneas, 15 episodes of central hypopnea, and 4 obstructive hypopnea events. Collectively, these findings resulted in an apnea-hypopnea index (AHI) of 14.1 events per hour of sleep (Figure 2).
Given these findings, ticagrelor was discontinued, and the patient was transitioned to clopidogrel.
Within two days of ticagrelor discontinuation, the patient reported complete resolution of his dyspnea.
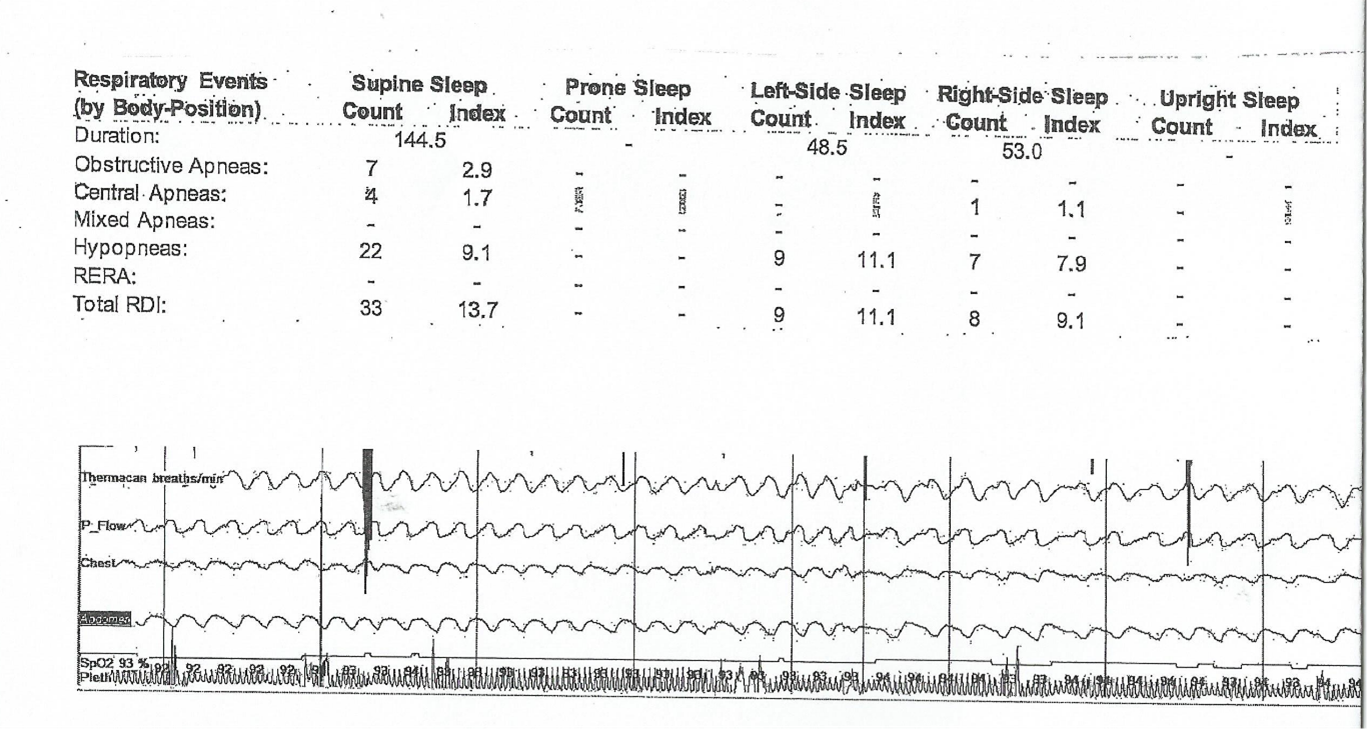
Three weeks later, without any additional intervention, the patient underwent another in-laboratory polysomnography study. This follow-up study demonstrated a significant reduction in central apnea, with a total of 12 apneic events, five central apneas (central apnea index of 1.7), and seven obstructive apneas (obstructive apnea index of 2.9). Additionally, 38 hypopneic events were recorded, resulting in an AHI of 12.2 events per hour of sleep, findings consistent with mild obstructive sleep apnea (OSA) primarily due to hypopneas. No periodic leg movements were observed during the study (Figure 3). These findings indicate a clear improvement in CSA in this patient following the discontinuation of ticagrelor. Collectively, these results suggest a potential association between ticagrelor use and the development of CSA.
Discussion
Ticagrelor is a cyclopentyl-triazolo-pyrimidine currently administered as an oral, third-generation reversible, non-competitive P2Y12 receptor antagonist that inhibits adenosine diphosphate (ADP)-mediated platelet signaling and aggregation [13]. Unlike clopidogrel, ticagrelor binds to a distinct site on the P2Y12 receptor, separate from the ADP binding site. When bound to this site, ticagrelor inhibits receptor conformational change and G-protein activation induced by ADP binding, leading to decreased intracellular cyclic AMP (cAMP) level within platelets, thereby preventing aggregation [14,15]. Ticagrelor does not alter the configuration of P2Y12 receptors, and its dissociation from these receptors is relatively rapid [16].
Ticagrelor is used in combination with aspirin as a component of DAPT for patients with coronary artery disease, most notably those undergoing PCI with stent placement to prevent thrombotic events [17]. Several controlled trials, including PLATO, DISPERSE-2, RESPONSE and ONSET/OFFSET, have demonstrated that ticagrelor, when combined with aspirin, is superior to clopidogrel plus aspirin in reducing ischemic vascular events, cardiovascular death, recurrent myocardial infarction, and stroke, particularly in the first three months following PCI, without significantly increasing major bleeding risk [18-21].
Ticagrelor and its active metabolite are rapidly absorbed in the gastrointestinal tract and exhibit rapid onset and termination of action. Unlike clopidogrel, ticagrelor does not require hepatic biotransformation for activation and is primarily metabolized by cytochrome P450 (CYP)3A4 with a half-life of 7-12 hours [22,23]. No renal dose adjustment is necessary. The antiplatelet effect of ticagrelor is dependent on plasma drug concentration, which determines receptor occupancy.
Ticagrelor-associated dyspnea
Dyspnea is a well-recognized side effect of ticagrelor. The PLATO trial reported an incidence of dyspnea of 13.8% in the ticagrelor group versus 7.8% in the clopidogrel group. By contrast, the ONSET/OFFSET study reported a 38.6% incidence of dyspnea in the ticagrelor group compared to 9.3% in the clopidogrel group and 8.3% in the placebo group [24]. Ticagrelor-induced dyspnea typically occurs within hours to days after drug initiation and is usually mild to moderate, transient, and self-limiting, resolving spontaneously in most patients within a few weeks [25]. Patients often describe dyspnea as a sensation of air hunger or unsatisfied inspiration at rest, unrelated to exercise, activity, or body position [26,27]. The dyspnea is often episodic, lasting up to two minutes, and is sometimes associated with anxiety [28]. Between episodes, patients are usually asymptomatic. However, in some cases, dyspnea can be severe and persistent, leading to non-adherence and self-discontinuation of the drug, which significantly increases the risk for cardiovascular thrombotic events [29]. Discontinuation rates due to dyspnea vary between 0.9 % and 10%, with self-discontinuation reported in up to 6% of patients [30,31].
Interestingly, all three reversible P2Y12 receptor inhibitors (ticagrelor, cangrelor, and elinogrel) are associated with an increased incidence of dyspnea compared to irreversible P2Y12 inhibitors such as clopidogrel.
Ticagrelor and CSA
CSA syndrome is a sleep disorder characterized by recurrent episodes of reduced or absent ventilatory effort that leads to disrupted sleep and intermittent hypoxia. Several studies have suggested an association between ticagrelor and CSA, with symptoms improving upon discontinuation and returning when ticagrelor is reintroduced [32-34].
Although some cases of ticagrelor-associated CSA may be mild and asymptomatic, moderate to severe CSA can lead to fatigue, poor sleep quality, insomnia, frequent nocturnal awakenings, morning headache, poor concentration, and excessive daytime sleepiness, similar to OSA [35]. One study reported persistent CSA in 30% of patients after 40 days of ticagrelor use, raising concerns about the potential impact of this drug on cardiovascular health; CSA is associated with increased sympathetic activity, ventricular remodeling, and negative cardiac outcomes [36,37].
A recent meta-analysis synthesizing data from multiple clinical trials demonstrated a statistically significant association between ticagrelor use and the incidence of CSA-related events [38]. By contrast, a post-hoc analysis of randomized, double-blinded clinical trials revealed no significant relationship between ticagrelor use and sleep apnea [39]. However, post-hoc analyses are inherently limited in detecting asymptomatic CSA, as these were not included among the pre-specified adverse events in these trials [40].
Proposed mechanisms of ticagrelor-related dyspnea and CSA
The exact mechanism underlying ticagrelor-associated dyspnea and CSA remains unclear. However, several hypotheses have been proposed:
Adenosine-mediated mechanism: Ticagrelor inhibits adenosine reuptake by blocking the activity of sodium-independent equilibrative nucleoside transporter 1 (ENT1), leading to increased extracellular adenosine levels. Elevated adenosine levels stimulate hyperventilation, hypocapnia, and central apnea, contributing to Cheyne-Stokes respiration [41,42].
P2Y12 receptor antagonism in the nervous system: P2Y12 receptors are expressed not only on platelets but also on pulmonary vagal sensory neurons. Ticagrelor may directly stimulate these neurons, enhancing neuronal signaling and increasing vagal fiber conductivity, thereby triggering dyspnea and CSA [43]. Ticagrelor may also increase carbon dioxide chemosensitivity, leading to a heightened hypercapnic ventilatory response while maintaining normal hypoxic chemosensitivity [44]. Inhibition of P2Y12 receptors on microglial cells in the central nervous system has been suggested as another possible mechanism [45].
Clinical implications and management
There is currently no data about whether patients with preexisting cardiopulmonary conditions (e.g., chronic obstructive pulmonary disease, asthma, congestive heart failure [CHF]) are at high risk for ticagrelor-induced dyspnea. However, misattribution of dyspnea to these cardiopulmonary disorders may lead to unnecessary treatments (e.g., steroids, diuretics, and inhalers), inappropriate medication adjustments, and poor adherence to ticagrelor.
Management strategies
Mild cases: Provide reassurance; dyspnea is often transient and self-limiting
Moderate to severe cases: Consider switching to an alternative antiplatelet agent (e.g., clopidogrel or prasugrel) if symptoms are persistent and intolerable.
CSA monitoring: Patients with persistent CSA symptoms should undergo polysomnography and in-laboratory positive airway pressure (PAP) titration study to determine the optimal setting needed to overcome CSA such as continuous positive airway pressure (CPAP), bilevel positive airway pressure (BIPAP), or adaptive servo- ventilation (ASV), with or without low flow oxygen, and be closely monitored for potential cardiovascular consequences. However, the use of ASV mode in patients with CSA who have congestive heart failure and a left ventricular ejection fraction ≤ 45 percent is contraindicated due to an increase in cardiovascular mortality seen with ASV use among this patient population [46].
Discontinuation of ticagrelor typically results in the resolution of symptoms within 24-48 hours, although some cases may persist for up to one month. Persistent dyspnea can have a significant impact on quality of life, including an increase in emergency visits, additional testing (e.g., pulmonary computed tomography angiography, echocardiography), and more frequent physician consultations.
Potential role of caffeine and theophylline
Caffeine, an adenosine antagonist, has been investigated as a potential treatment for ticagrelor-associated dyspnea, but data are limited. Regular caffeine consumption was not associated with a lower incidence of dyspnea in ticagrelor-treated patients [47]. Theophylline, a non-selective phosphodiesterase inhibitor, has been used in acute care settings for ticagrelor-induced dyspnea and Cheyne-Stokes respiration but is not routinely recommended [48].
Risk factors and cardiac implications of CSA
CSA is associated with several risk factors, including advanced age, renal failure, substance abuse, male sex, CHF, cerebrovascular accident (CVA), primary mitochondrial disease, acromegaly, and hypothyroidism. Additionally, certain medications (e.g., chronic opioid therapy, baclofen, gabapentin, benzodiazepines, methadone maintenance therapy, and antidepressants) have been linked to an increased risk of CSA.
Sleep-disordered breathing (SDB) including both OSA and CSA, is particularly common following a stroke, with approximately 70% of patients with CVA developing sleep apnea within 72 hours after CVA. In most cases, SDB after a CVA is self-limiting and resolves over time [49]. An increased prevalence of CSA has also been reported after acute coronary syndrome; however, many studies assessing sleep disorders in this context do not differentiate between CSA and OSA [50].
Conclusion
While ticagrelor is an essential component of DAPT, clinicians should be mindful of its association with dyspnea and CSA. Awareness of these potential adverse effect is essential to prevent medication non-adherence or premature discontinuation, which can increase morbidity and mortality [51]. Patient monitoring, appropriate symptom management, and timely medication adjustment can help mitigate these adverse effects while ensuring optimal cardiovascular protection.
Ticagrelor-induced dyspnea is well-documented and is mild and self-limiting in most patients. However, the factors leading to persistence and the long-term implications of ticagrelor-related CSA remain unclear.
Patients with OSA receiving CPAP therapy who started on ticagrelor should be monitored for increased residual AHI, which may indicate ticagrelor-associated CSA [52]. In such cases, treatment adjustments, including switching to an alternative antiplatelet agent or using a more advanced positive airway pressure devices such as ASV or BIPAP with a back-up respiratory rate may be necessary to manage both obstructive and central apneas.
Further studies are needed to confirm the association, elucidate underlying mechanisms, and assess the incidence, clinical consequences, and long-term outcomes of ticagrelor-associated CSA.
Conflicts of Interest
No conflicts of interest.
Funding Statement
None.
Acknowledgments
None.
References
2. Serebruany VL, Sibbing D, DiNicolantonio JJ. Dyspnea and reversibility of antiplatelet agents: ticagrelor, elinogrel, cangrelor, and beyond. Cardiology. 2014;127(1):20-4.
3. Storey RF, Becker RC, Harrington RA, Husted S, James SK, Cools F, et al. Pulmonary function in patients with acute coronary syndrome treated with ticagrelor or clopidogrel (from the Platelet Inhibition and Patient Outcomes [PLATO] pulmonary function substudy). Am J Cardiol. 2011 Dec 1;108(11):1542-6.
4. Storey RF, Bliden KP, Patil SB, Karunakaran A, Ecob R, Butler K, et al. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol. 2010 Jul 13;56(3):185-93.
5. Caldeira D, Pinto FJ, Ferreira JJ. Dyspnea and reversibility profile of P2Y₁₂ antagonists: systematic review of new antiplatelet drugs. Am J Cardiovasc Drugs. 2014 Aug;14(4):303-11.
6. Fiocca L, Rossini R, Carioli G, Carobbio A, Piazza I, Collaku E, et al. Adherence of ticagrelOr in real world patients with aCute coronary syndrome: The AD-HOC study. Int J Cardiol Heart Vasc. 2022 Jul 18;42:101092.
7. Burki NK, Lee LY. Mechanisms of dyspnea. Chest. 2010 Nov;138(5):1196-201.
8. Ortega-Paz L, Brugaletta S, Ariotti S, Akkerhuis KM, Karagiannis A, Windecker S, et al. Adenosine and Ticagrelor Plasma Levels in Patients With and Without Ticagrelor-Related Dyspnea. Circulation. 2018 Aug 7;138(6):646-8.
9. Cattaneo M, Schulz R, Nylander S. Adenosine-mediated effects of ticagrelor: evidence and potential clinical relevance. J Am Coll Cardiol. 2014 Jun 17;63(23):2503-9.
10. Cattaneo M, Faioni EM. Why does ticagrelor induce dyspnea? Thromb Haemost. 2012 Dec;108(6):1031-6.
11. Davtyan C, Cheng K. Central Sleep Apnea Caused by ticagrelor. Proceeding of UCLA health. 2022;Volume 26.
12. Kado J, Farrehi P, Shelgikar AV. Central Sleep Apnea with Cheyne-Stokes Breathing induced by Use of Ticagrelor. Sleep.2018; volume 41, Abstract Supplement. DOI:10.1093/sleep/zsy063.1139
13. Husted S, van Giezen JJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009 Winter;27(4):259-74.
14. van Giezen JJ, Sidaway J, Glaves P, Kirk I, Björkman JA. Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. J Cardiovasc Pharmacol Ther. 2012 Jun;17(2):164-72.
15. Gurbel PA, Kuliopulos A, Tantry US. G-protein-coupled receptors signaling pathways in new antiplatelet drug development. Arterioscler Thromb Vasc Biol. 2015 Mar;35(3):500-12.
16. Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther. 2014 Mar;19(2):209-19.
17. Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009 Dec 22;120(25):2577-85.
18. Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010 Mar 16;121(10):1188-99.
19. Husted SE, Storey RF, Bliden K, Tantry US, Høimark L, Butler K, et al. Pharmacokinetics and pharmacodynamics of ticagrelor in patients with stable coronary artery disease: results from the ONSET-OFFSET and RESPOND studies. Clin Pharmacokinet. 2012 Jun 1;51(6):397-409.
20. Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol. 2007 Nov 6;50(19):1844-51.
21. Chen W, Zhang C, Zhao J, Xu X, Dang H, Xiao Q, et al. Effects of clopidogrel, prasugrel and ticagrelor on prevention of stent thrombosis in patients underwent percutaneous coronary intervention: A network meta-analysis. Clin Cardiol. 2021 Apr;44(4):488-94.
22. Cattaneo M. New P2Y(12) inhibitors. Circulation. 2010 Jan 5;121(1):171-9.
23. Dobesh PP, Oestreich JH. Ticagrelor: Pharmacokinetics, pharmacodynamics, Clinical efficacy, and Safety. Pharmacotherapy. 2014 Aug 28;34(10):1077-90.
24. Giannoni A, Emdin M, Passino C. Cheyne-Stokes Respiration, Chemoreflex, and Ticagrelor-Related Dyspnea. N Engl J Med. 2016 Sep 8;375(10):1004-6.
25. Krakowiak A, Kuleta J, Plech I, Zarębiński M, Wojciechowska M, Wretowski D, et al. Ticagrelor-Related Severe Dyspnoea: Mechanisms, Characteristic Features, Differential Diagnosis and Treatment. Clin Med Insights Case Rep. 2020 Oct 8;13:1179547620956634.
26. Tamakauskas V, Žaliūnas R, Lesauskaitė V, Kupstytė-Krištaponė N, Šakalytė G, Jurgaitytė J, et al. Factors determining ticagrelor-induced dyspnea in patients with acute coronary syndrome. Appl Sci. 2022 Oct 6;12(19):10021.
27. Serebruany V, Pokov I, Kuliczkowski W, Vahabi J, Atar D. Incidence and causes of new-onset dyspnea in 3,719 patients treated with clopidogrel and aspirin combination after coronary stenting. Thromb Haemost. 2008 Aug;100(2):314-8.
28. Hai-Ling LI. Risk factors of ticagrelor-associated dyspnea in patients with acute coronary syndrome. Acad J Second Mil Med Univ. 2020;12:11-7.
29. Kinlay S, Quach L, Cormack J, Morgenstern N, Hou Y, Young M, et al. Premature Discontinuation of Dual Antiplatelet Therapy After Coronary Stenting in Veterans: Characteristics and Long-Term Outcomes. J Am Heart Assoc. 2021 May 4;10(9):e018481.
30. Lombardi N, Lenti MC, Matucci R, Mugelli A, Vannacci A. Ticagrelor-related dyspnea: an underestimated and poorly managed event? Int J Cardiol. 2015 Jan 20;179:238-9.
31. Giannoni A, Borrelli C, Gentile F, Mirizzi G, Coceani M, Paradossi U, et al. Central apnoeas and ticagrelor-related dyspnoea in patients with acute coronary syndrome. Eur Heart J Cardiovasc Pharmacother. 2021 May 23;7(3):180-8.
32. Meurin P, Ben Driss A, Defrance C, Renaud N, Dumaine R, Weber H, Grosdemouge A, Mouram S, Pericart L, Bonnevie L, Tabet JY. Ticagrelor could cause central sleep apnea after acute coronary syndrome in patients without left ventricular dysfunction or heart failure. Eur Heart J. 2020 Nov;41(Supplement_2):ehaa946-2885.
33. Meurin P, Ben Driss A, Defrance C, Dumaine R, Weber H, Renaud N, et al. Central sleep apnea after acute coronary syndrome and association with ticagrelor use. Sleep Med. 2021 Apr;80:39-45.
34. Puel V, Théophile H, Godard I, Raymond N, Miremont-Salamé G, Gosse P, et al. Ticagrelor and central sleep apnoea: Impact of withdrawal and reintroduction. Br J Clin Pharmacol. 2019 Aug;85(8):1855-8.
35. Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: Pathophysiology and treatment. Chest. 2007 Feb;131(2):595-607.
36. Javaheri S, Sharma RK, Bluemke DA, Redline S. Association between central sleep apnea and left ventricular structure: the Multi-Ethnic Study of Atherosclerosis. J Sleep Res. 2017 Aug;26(4):477-80.
37. Sanchez AM, Germany R, Lozier MR, Schweitzer MD, Kosseifi S, Anand R. Central sleep apnea and atrial fibrillation: A review on pathophysiological mechanisms and therapeutic implications. Int J Cardiol Heart Vasc. 2020 May 22;30:100527.
38. Shrestha A, Mainali N, Rajak K, Goyal A, Acharya B, Yadav R. Abstract 4144926: Central Sleep Apnea with Ticagrelor in Patients with Coronary Syndrome; A Meta-Analysis. Circulation. 2024;150(Suppl_1). doi:10.1161/circ.150.suppl_1.4144926.
39. Sabatine MS, Hiatt WR, Goto S, Johnston SC, Bonaca MP, Steg PG, et al. No Significant Relationship Between Ticagrelor and Sleep Apnea in Large, Randomized, Blinded Trials. JACC Cardiovasc Interv. 2020 Apr 27;13(8):1012-4.
40. Borrelli C, Gentile F, Mirizzi G, Passino C, Emdin M, Giannoni A. Hide and seek. Ticagrelor and central apneas after acute coronary syndrome. Sleep Med. 2021 Oct;86:125.
41. Belchikov YG, Koenig SJ, Dipasquale EM. Potential role of endogenous adenosine in ticagrelor-induced dyspnea. Pharmacotherapy. 2013 Aug;33(8):882-7.
42. Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014 Mar 11;63(9):872-7.
43. Van Giezen JJ. Optimizing Platelet Inhibition. Eur Heart J Suppl. 2008;10(Suppl_D):D23-9.
44. Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, van Giezen JJ, Jonasson J, Nylander S, et al. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol. 2013 Feb 19;61(7):723-7.
45. Parodi G, Storey RF. Dyspnoea management in acute coronary syndrome patients treated with ticagrelor. Eur Heart J Acute Cardiovasc Care. 2015 Dec;4(6):555-60.
46. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med. 2015 Sep 17;373(12):1095-105.
47. Furtado RHM, Venkateswaran RV, Nicolau JC, Gurmu Y, Bhatt DL, Storey RF, et al. Caffeinated Beverage Intake, Dyspnea With Ticagrelor, and Cardiovascular Outcomes: Insights From the PEGASUS-TIMI 54 Trial. J Am Heart Assoc. 2020 May 18;9(10):e015785.
48. Sanmartin-Fernandez M, Zamorano JL. Theophylline for Attenuating Ticagrelor-Related Dyspnea. Arq Bras Cardiol. 2021 Jul;117(1):146-8.
49. Blissitt PA. Sleep-Disordered Breathing After Stroke: Nursing Implications. Stroke. 2017 Mar;48(3):e81-4.
50. Ludka O, Stepanova R, Vyskocilova M, Galkova L, Mikolaskova M, Belehrad M, et al. Sleep apnea prevalence in acute myocardial infarction--the Sleep Apnea in Post-acute Myocardial Infarction Patients (SAPAMI) Study. Int J Cardiol. 2014 Sep;176(1):13-9.
51. Arora S, Shemisa K, Vaduganathan M, Qamar A, Gupta A, Garg SK, et al. Premature Ticagrelor Discontinuation in Secondary Prevention of Atherosclerotic CVD: JACC Review Topic of the Week. J Am Coll Cardiol. 2019 May 21;73(19):2454-64.
52. Paboeuf C, Priou P, Meslier N, Roulaud F, Trzepizur W, Gagnadoux F. Ticagrelor-Associated Shift From Obstructive to Central Sleep Apnea: A Case Report. J Clin Sleep Med. 2019 Aug 15;15(8):1179-82.