Abstract
Background: Brain-computer interfaces (BCIs) are a rapidly advancing field which utilizes brain activity to control external devices for a myriad of functions, including the restoration of motor function. Clinically, BCIs have been especially impactful in patients who suffer from stroke-mediated damage. However, due to the rapid advancement in the field, there is a lack of accepted standards of practice. Therefore, the aim of this systematic review is to summarize the current literature published regarding the efficacy of BCI-based rehabilitation of motor dysfunction in stroke patients.
Methodology: This systematic review was performed in accordance with the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 statement. PubMed, Embase, and Cochrane Library were queried for relevant articles and screened for inclusion criteria by two authors. All discrepancies were resolved by discussion among both reviewers and subsequent consensus.
Results: 11/12 (91.6%) of studies focused on upper extremity outcomes and reported larger initial improvements for participants in the treatment arm (using BCI) as compared to those in the control arm (no BCI). 2/2 studies focused on lower extremity outcomes reported improvements for the treatment arm compared to the control arm.
Discussion/Conclusion: This systematic review illustrates the utility BCI has for the restoration of upper extremity and lower extremity motor function in stroke patients and supports further investigation of BCI for other clinical indications.
Keywords
Brain Computer Interfaces, Clinical Neurology, Stroke
Introduction
Brain-computer interfaces (BCIs) utilize brain activity to control external devices. BCI technology has been advancing for over five decades, and it has become a steadily more popular and reliable method of communication and motor function in patients with a variety of neurological disorders [1]. In BCIs, signals from the brain are decoded, and these signals are translated to device output, such as to control movements of a robot, prosthesis, or screen cursor, with recent research showing improvements in accuracy and convenience [2,3].
Both invasive and noninvasive techniques exist to obtain brain activity. The invasive techniques involve placing electrodes on the brain to measure neural activity [4]. Noninvasive techniques include Electroencephalography (EEG), i.e., placing sensors on the scalp to measure electrical activity (EEG); functional magnetic resonance imaging (fMRI), or magnetic energy (MEG) [4]. Among these, EEG is most often utilized because of the method’s non-invasive nature and practicality [4,5]. In addition to its level of invasiveness, BCI technology can be classified according to its function as assistive or rehabilitative. Notably, assistive BCI replaces motor functions that have been lost or assists patients to control robots which aid them with the performance of daily tasks [3]. Further, rehabilitative BCIs help restore brain function by influencing the neurophysiology of the brain [3].
Clinically, BCIs have been an important tool for patients’ motor dysfunction due to CNS damage, especially in stroke-mediated damage [3,6]. Devices leveraging BCIs, such as robotic prosthesis and speech generators, have successfully supplemented or substituted CNS functions to improve patients’ quality of life by facilitating the performance of manual tasks [6]. Moreover, with ever-growing number of patients needing rehabilitation services following strokes, and an increasingly aging population, and not enough physical therapists to meet the need, BCIs provide a way to answer this demand with effective and lasting treatment [2]. Currently, there are wide ranging options of BCI assisted therapy, each with different efficacy. Being that the application of BCIs is so diverse and novel, it is essential for the clinician to be aware of the technology’s effectiveness when using it to restore motor function in the setting of post-stroke rehabilitation. Due to the lack of a universally accepted standard of practice in the emerging field, the onus is upon the clinician to review the published literature and apply it to their practice. Consequently, the aim of this review is to highlight the efficacy of BCI-based rehabilitation of motor dysfunction in stroke patients.
Methods
Literature search
We performed this systematic review in accordance with the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2020 statement [7]. A comprehensive search was performed for all human clinical trial articles which met our pre-defined search terms and were published in English in electronic databases including PubMed, Embase, and Cochrane Library between 01.01.2018 and 11.20.2022. The following search terms were used: “Brain-Computer Interfaces” AND (“Amyotrophic Lateral Sclerosis” OR “Spinal Cord Injury” Or “Stroke”). Where possible, these terms were expanded using the Medical Subject Headings (“[MeSH]”) and Explosion (“/exp”) controlled vocabulary functions in PubMed and Embase, respectively.
Screening & eligibility criteria
Two reviewers (KR and YP) screened the included studies independently at every stage. All discrepancies were resolved by discussion among both reviewers and subsequent consensus. Inter-rater reliability (IRR) and Cohen’s kappa (k) were quantified using Covidence systematic review software.
Specifically, we were interested in articles reporting on human clinical trials for patients recovering from stroke using BCI technology during the rehabilitative process and comparing their outcomes to a non-BCI subject group using standardized clinical outcome measures of motor and communication functionality. The inclusion criteria for the articles were: (1) publications that reported on clinical trials of a patient population recovering from stroke, SCI, or ALS; (2) interventional clinical trials which reported on the use and effectiveness of a BCI; (3) trials which reported results in terms of a validated clinical measure of communication or motor function. The exclusion criteria for the articles were: (1) clinical trials without randomization; (2) clinical trials without a non-BCI control group; (3) trials in fewer than 3 patients (Figure 1).
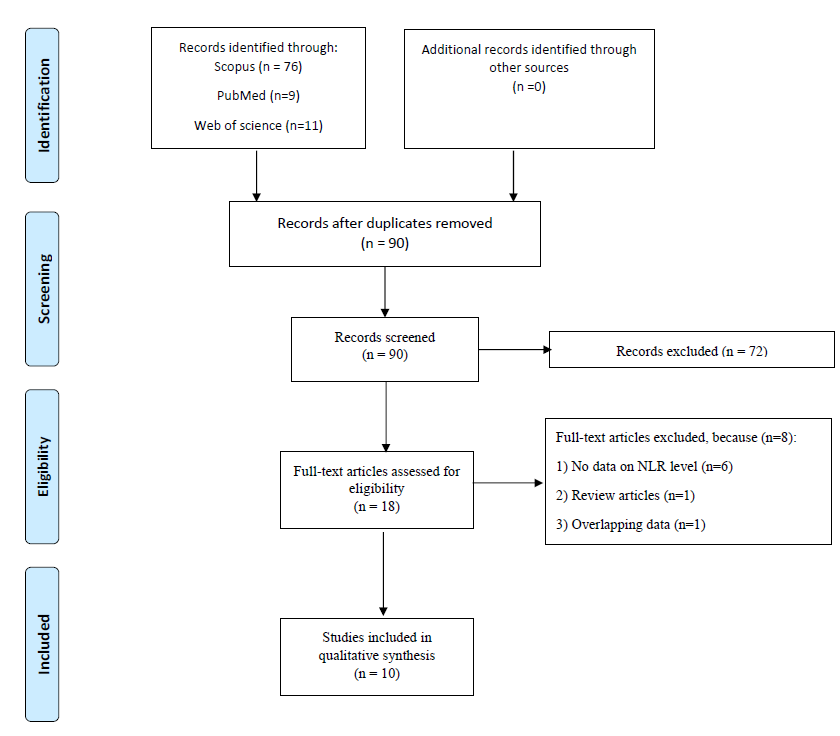
Figure 1. PRISMA flow diagram for the selection of studies in the systematic review.
Results
A total of 55 records were identified using the search terms defined above across PubMed, Embase, and Cochrane Libraries. Following removal of duplicate records, 46 research articles underwent initial screening of title and abstract. Among the 30 articles selected for full text review (IRR = 0.76, k = 0.45), a further 9 were excluded due to wrong study design (e.g., no randomization), 5 due to measuring results using a unique/ non-standard clinical outcome measure, and 2 for using the wrong comparator (e.g., no control group), where IRR = 0.87 and k = 0.72. The details of the entire study selection process with the respective number of articles included/ excluded at every stage are described in Figure 1 above.
Among the studies included in this review, 12 focused on clinical applications of BCI systems for improvements in upper extremity (UE) function in a total of 266 participants (147 treatment and 119 controls, with 10 crossover participants). A further 2 studies focused on BCI applications for lower extremity (LE) function with a total of 58 participants (30 treatment and 28 controls). Two studies excluded during the final screening process reported outcomes only on whole body motor activity or brain activity, respectively, and an additional 3 reported outcomes using non-standardized clinical outcome measures. None of the selected studies reported clinical outcomes on communication functions.
Eleven out of the twelve studies focused on UE outcomes reported larger initial improvements for participants in the treatment arm (using the BCI) as compared to those in the control arm (no BCI). Four studies reporting follow-up data collection at 3 or 6 months (including the study which did not report higher initial improvements using BCI) reported higher longstanding benefits of BCI as compared to control. Both studies focused on LE outcomes reported larger improvements for participants in the treatment arm as compared to those in the control arm (Table 1).
|
Authors and Years |
Study Design N (Treatment, Control) |
Baseline: Treatment Mean (SD), Control Mean (SD), P = X* |
Outcome: Treatment Mean = X (SD), Control Mean = X (SD), P = X* |
Findings |
|---|---|---|---|---|
| Biasiucci et al., 2018 [9] | Nonrandomized Controlled Trial (14, 13) | 1' FMA-UE: 21.6 (10.8), 19.9 (11.2) | 1' FMA-UE: T = 28.3 (14.5), C = 22.0 (12.2). p = 0.03 Follow up (6-12 mo post): 1' FMA-UE: T = 28.6 (12.2), C = 22.7 (13.1) |
When paired with functional electric therapy, BCI is associated with significant functional recovery and plasticity. |
| Cantillo-Negrete et al., 2021 [21] | Crossover Feasibility Study (5, 5 + crossover) | 1' FMA-UE: 17.5 (15) | 1' FMA-UE: 23.1 (16), 8.4 (10) | In patients who have had severe strokes, BCI intervention with a robotic hand orthosis results in neuroplasticity-related mechanisms comparable to conventional therapy. |
| Caria et al., 2020 [22] | Randomized Controlled Trial (16, 14) | 1' cFMA (FMA hand/finger + FMA arm): 11.16(1.73), 13.29 (2.86) | 1' cFMA: 14.56 (1.95), 13.64 (2.91) | BCI reinforces ipsilesional brain activity and enhances proprioceptive function of affected hands in stroke patients. |
| Cheng et al., 2020 [8] | Randomized Controlled Feasibility Study (5, 5) | 1' FMA-UE: 24.2 (7.01), 30 (7.84) | 1' FMA-UE: 28 (5.36), 35.60(2.61) Follow up 24 weeks 1' FMA-UE: 28.8 (4.77), 32.00 (4.64) |
BCI based soft robotic glove may lead to sustained functional improvements in stroke patients. |
| Chen et al., 2020 [23] | Randomized Controlled Trial (7, 7) | 1' FMA-UE: 31.3 (11.5), 32.3 (11.8) | 1' FMA-UE: 39.71 (6.85), 37 (3.91) | BCI interface training with exoskeleton feedback has use in subacute stroke patients. |
| Foong et. al., 2020 [24] | Single-arm Study with Historical Control (11, 7) | 1' FMA-UE: 35.5 (12.6), 23.4 (14.0) | 1' FMA-UE: 39.9 (3.6), 28.3 (4.1) Week 24 FMA-UE: 41.3 (5.6), 27 (5.9) |
The results of the BCI-FES training show potential advantages on walking abilities in individuals with chronic hemiparetic stroke. |
| Gou et al., 2022 [25] | Randomized controlled trial (T = 10, CC = 10, RC = 10) | 1' FMA-UL: T = 14.9 (7.98), CC 14.4 (8.69), RC 14.9 (5.23) | 1' FMA: T = 28.6 (14.0), CC 15.2 (9.48), RC 21.9 (7.62) 3 month follow up FMA: T = 30.80 (14.31), CC 16.3 (9.24), RC 23.55 (7.76) |
Steady-state visually evoked potentials-BCI controlled soft robotic glove rehabilitation system is feasible for post-stroke hand function rehabilitation. |
| Hu et al., 2021 [26] | Randomized Controlled trial (7, 5) | 1' FMA-UE: 12.7 (8.88), 13.8 (6.65) | 1' FMA: 15.4 (10.11), 20.6 (12.67) | Motor imagery-based BCI combined with sensory and visual feedback might improve severe upper limb and hand impairment in chronic stroke patients, showing the potential for application in rehabilitation medicine. |
| Lee et al., 2022 [27] | Randomized Controlled trial (13, 13) | 1' FMA-UE: 30.53 (5.05), 29.84 (3.64) | 1' FMA: 33.84 (7.45), 29.84 (3.53) | Action observation training plus BCI-functional electronic stimulation can enhance motor function of upper extremity and cortical activation in patients with stroke. |
| Lyukmanov et al., 2019 [28] | Randomized Controlled trial (35, 20) | FMA-Proximal UE: 7.0 (6.0; 17.0), 9.0 (7.0; 10.0) FMA-Distal UE: 1.0 (0.0; 4.0), 1.0 (0.0; 1.0) | FMA-Proximal UE: T =14.0 [8.0; 19.0] P<0.005, C = 11.0 [7.0; 12.0] FMA-Distal UE: T= 3.0 [0.0; 6.0] P <0.05, C = 1.0 [1.0; 4.0] | Inclusion of the BCI-exoskeleton system into the complex rehabilitation of patients with post stroke upper limb paresis significantly improves a number of measures of grasping and movement functions in the proximal segments of the upper limb. |
| Miano et al., 2020 [29] | Randomized Controlled Trial (8, 8) | FMA-UL:19.5 (9.9), 20.6(9.7) | FMA-UE: 23.0(11.4), 21.5 (10.0) | BCI rehabilitation provides a larger functional improvement in upper extremity motor functions in stroke patients compared to control. |
| Ramos-Murguialday et al., 2019 [10] | Randomized Controlled Trial (16, 12) | FMA-UE: 11.16 (1.73), 13.29 (2.86) | FMA-UE Treatment 13.44 (1.96) P=0.015, Control 14.75(2.71), P=0.070 | BMI-based rehabilitation promotes lasting improvements in motor function of chronic stroke patients with severe paresis. |
| Yuan et al., 2021 [11] | Randomized Controlled Trial (16, 14) | FMA-LE: 10.4 (6.8), 10.1 (6.8) | FMA-LE: Treatment mean = 14.9, Control mean = 12.2, P=value 0.022 | The use of BCI-PT significantly improved the patient’s lower limb motor function by increasing the patient’s participation. |
| Zhao et al., 2022 [12] | Randomized Controlled Trial (14, 14) | FMA-LE: 10.4 (5.7), 10.1 (6.8) | 1' FMA-LE [18.21 (7.59), 16.36(7.63) P=0.540 | The use of a BCI-controlled robot combined with traditional physiotherapy promotes cognitive function recovery and enhances motor functions of the lower extremity in patients with subacute stroke. |
| *P-value and variance was provided when given | ||||
| BCI: Brain Computer Interface; FMA: Fugl-Meyer Assessment; UE: Upper Extremity; UL: Upper Limb; LE: Lower Extremity; cFMA: Combined Fugl-Meyer Assessment; T: Treatment; CC: Conventional Control; RC: Robotic Control | ||||
Discussion
This systematic review demonstrates that BCIs have many current and emerging applications with high efficacy for the restoration of motor function in stroke patients. An overwhelming 91.7% majority of studies comparing BCIs vs. control therapy for post-stroke upper extremity deficits using standardized clinical outcome measures favored the application of BCIs. Similarly, both studies reporting on lower extremity improvements which were included in this review favored the application of BCIs as compared to placebo or sham treatments.
Several clinical trials identified in our review document are not just significant, but lasting improvement following BCI-mediated intervention. Notably, these include the only study in our systematic review which initially found BCI results to be no better than conventional treatment. Namely, in 2020 Cheng et al. [8] reported that a soft robotic glove therapy alone was more beneficial than BCI-based soft robotic glove immediately following a 6-week intervention; however, BCI effects proved more significant at both 12- and 24-week follow up intervals, speaking to the technology’s long-lasting clinical effectiveness. Similarly, in the 2018 nonrandomized controlled trial, Biasiucci et al. [9] found significant FMA-UE measured improvement compared to the control group when a BCI was paired with functional electric therapy [Treatment mean = 28.3 (SD = 14.5), Control mean = 22.0 (SD = 12.2), p = 0.03]. Importantly, these results were found to persist in a six to twelve months follow up, providing yet another testament to the longevity of rehabilitative results achieved via incorporating BCIs as compared to the standard of care only.
Moreover, significant findings of BCI-mediated rehabilitation were found to be clinically meaningful in the rehabilitation of upper extremity motor function in a variety of stroke severities. In addition to the results reported by the 2020 Cheng et al. [8] study referenced above, in 2019, Ramos-Murguialday et al. [10] identified parallel findings when using BCI-based rehabilitation in chronic stroke patients with severe paresis [FMA-UE Treatment mean = 13.44 (SD = 1.96) P=0.015; Control mean = 14.75 (SD = 2.71), P = 0.070]. Collectively, these highlight the applicability and clinical utility of the technology in mild as well as severe disease rehabilitation.
Our search additionally identified two clinical studies which demonstrated the positive impact of BCI-based therapy in the rehabilitation of lower extremity motor function. Of note, in a 2021 randomized controlled trial, Yaun et al. [11] found the use of BCI-integrated physical therapy significantly improved lower limb function in subacute stroke patients [FMA-LE: Treatment mean = 14.9, Control mean = 12.2, P=value 0.022]. Considering that the results of both this study and the one by Zhao et al. [12] in 2022 favor BCI treatment, this provides a strong signal for further research into the applications of BCI for post-stroke lower extremity therapy as well as other motor pathologies.
More clinical investigations should be done to assess the use of BCI-based rehabilitation in other clinical settings such as in the rehabilitation of communication in stroke patients or patients with other diseases. As one example, among the studies excluded from analysis in our systematic review due to a non-stroke clinical indication, one study investigated the use of BCIs in patients with degeneration of motor pathways necessary for speech such as in amyotrophic lateral sclerosis (ALS) [13]. Many of these individuals rely on methods of augmented and alternative communication (AAC), such as eye gaze tracking or computer peripherals (joystick, stylus, mouse), to communicate [14]. These methods require a certain level of volitional control which limits their target populations. Similarly, for patients with locked in syndrome (LIS) where there is total paralysis except for eye movements, many methods of AAC are not feasible. BCIs have been investigated for use in conjunction with AAC to allow for use of AAC devices without volitional control [15]. The majority of research in this area involves electrocorticography (ECoG), the invasive placement of electrodes into the subdural space to measure cortical EEG activity [13]. Early studies in this area worked on decoding the phenomes of speech [16,17]. Sokumbi et al. [18] created a real-time neural speech recognition (rtNSR) package that classified speech at both the phonetic and sentence level. Herff et al. [19] created a Brain-to-Text model that could reconstruct a limited set of spoken phrases from neural data obtained from ECoG. More recent studies have focused on using a vocoder, which produces naturally sounding speech from either text or acoustic features of decoded speech [20]. Non-invasive methods such as steady state evoke potentials (SSVEP) have also been studied in patients with LIS and ALS, but their lower signal quality restricts their potential for decoding speech [14]. However, despite these promising results, the paucity of clinical trials related to the application of BCI in communication, ALS, or spinal injury patients demonstrates the need for further investigation.
Our systematic review had several limitations. The relatively small number of eligible studies with varying demographic characteristics of patients may affect the generalizability of the findings, especially for studies assessing the effects of BCIs on LE function recovery where only two studies made it to the final analysis stage. The filtering was further narrowed by the exclusion of non-English literature and studies without standardized clinical outcomes and/ or non-BCI control groups. Among the included studies, there was heterogeneity in the type of BCI technology utilized, baseline clinical characteristics of patients, and the outcomes evaluated. The heterogeneity in the evaluated outcomes emphasizes the importance of establishing a more standardized protocol and reporting method to assess the clinical and technical outcomes of BCI applications. Despite the limitations listed here, the authors believe this systematic review provides important guidance and validation on the use of BCI based motor rehabilitation for stroke patients.
Conclusion
This study addresses the lack of current standards of practice in BCI-mediated motor rehabilitation in stroke patients though a systematic review of the literature. Our findings demonstrate the utility of BCI in this context, with 91.6% of identified studies focused on UE and 100% of LE studies reporting larger initial improvements for participants in the treatment arm. These results support the wider adoption of BCIs for upper as well as lower motor improvements at stroke rehabilitative programs and facilities. Further research is needed to explore the technology’s application in communication improvements and its clinical utility for other medical indications such as ALS, LIS, and others. To reach the technology’s full clinical potential, researchers and healthcare workers should unite around a common set of practice protocols and clinical outcome measures in their future efforts in the field.
Source of Funding
No sources of funding.
Acknowledgements
None.
Conflicts of Interest
None.
Author Contributions
Conceptualization, K.R., Y.P. and B.L.-W.; methodology, M.J.D., A.B., K.R., Y.P.; investigation, K.R. Y.P.; writing—original draft preparation, K.R., Y.P.P., A.B., E.M., S.K., M.R., M.J.D., J.L.R.; writing—review and editing, K.R., Y.P.P., A.B., E.M., S.K., M.R., M.J.D., J.L.R.; supervision, B.L.-W.
References
2. Baniqued PDE, Stanyer EC, Awais M, Alazmani A, Jackson AE, Mon-Williams MA, et al. Brain-computer interface robotics for hand rehabilitation after stroke: a systematic review. J Neuroeng Rehabil. 2021;18(1):15.
3. Chaudhary U, Birbaumer N, Ramos-Murguialday A. Brain-computer interfaces for communication and rehabilitation. Nat Rev Neurol. 2016;12(9):513-525.
4. Belwafi K, Gannouni S, Aboalsamh H. Embedded Brain Computer Interface: State-of-the-Art in Research. Sensors (Basel). 2021;21(13):4293.
5. Sadagurski M, Leshan RL, Patterson C, Rozzo A, Kuznetsova A, Skorupski J, et al. IRS2 signaling in LepR-b neurons suppresses FoxO1 to control energy balance independently of leptin action. Cell Metab. 2012;15(5):703-712.
6. Mridha MF, Das SC, Kabir MM, Lima AA, Islam MR, Watanobe Y. Brain-Computer Interface: Advancement and Challenges. Sensors (Basel). 2021;21(17):5746.
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
8. Cheng N, Phua KS, Lai HS, Tam PK, Tang KY, Cheng KK, et al. Brain-Computer Interface-Based Soft Robotic Glove Rehabilitation for Stroke. IEEE Trans Biomed Eng. 2020;67(12):3339-3351.
9. Biasiucci A, Leeb R, Iturrate I, Perdikis S, Al-Khodairy A, Corbet T, et al. Brain-actuated functional electrical stimulation elicits lasting arm motor recovery after stroke. Nat Commun. 2018;9(1):2421.
10. Ramos-Murguialday A, Curado MR, Broetz D, Yilmaz Ö, Brasil FL, Liberati G, et al. Brain-Machine Interface in Chronic Stroke: Randomized Trial Long-Term Follow-up. Neurorehabil Neural Repair. 2019;33(3):188-198.
11. Yuan Z, Peng Y, Wang L, Song S, Chen S, Yang L, et al. Effect of BCI-Controlled Pedaling Training System With Multiple Modalities of Feedback on Motor and Cognitive Function Rehabilitation of Early Subacute Stroke Patients. IEEE Trans Neural Syst Rehabil Eng. 2021;29:2569-2577.
12. Zhao CG, Ju F, Sun W, Jiang S, Xi X, Wang H, et al. Effects of Training with a Brain-Computer Interface-Controlled Robot on Rehabilitation Outcome in Patients with Subacute Stroke: A Randomized Controlled Trial. Neurol Ther. 2022;11(2):679-695.
13. Luo S, Rabbani Q, Crone NE. Brain-Computer Interface: Applications to Speech Decoding and Synthesis to Augment Communication. Neurotherapeutics. 2022;19(1):263-273.
14. Brumberg JS, Pitt KM, Mantie-Kozlowski A, Burnison JD. Brain-Computer Interfaces for Augmentative and Alternative Communication: A Tutorial. Am J Speech Lang Pathol. 2018;27(1):1-12.
15. Peters B, Eddy B, Galvin-McLaughlin D, Betz G, Oken B, Fried-Oken M. A systematic review of research on augmentative and alternative communication brain-computer interface systems for individuals with disabilities. Front Hum Neurosci. 2022;16:952380.
16. Pei X, Barbour DL, Leuthardt EC, Schalk G. Decoding vowels and consonants in spoken and imagined words using electrocorticographic signals in humans. J Neural Eng. 2011;8(4):046028.
17. Ikeda S, Shibata T, Nakano N, Okada R, Tsuyuguchi N, Ikeda K, et al. Neural decoding of single vowels during covert articulation using electrocorticography. Front Hum Neurosci. 2014;8:125.
18. Sokunbi MO, Linden DEJ, Habes I, Johnston S, Ihssen N. Real-time fMRI brain-computer interface: development of a “motivational feedback” subsystem for the regulation of visual cue reactivity. Front Behav Neurosci. 2014;8:392.
19. Herff C, Heger D, de Pesters A, Telaar D, Brunner P, Schalk G, et al. Brain-to-text: decoding spoken phrases from phone representations in the brain. Front Neurosci. 2015;9:217.
20. Akbari H, Khalighinejad B, Herrero JL, Mehta AD, Mesgarani N. Towards reconstructing intelligible speech from the human auditory cortex. Sci Rep. 2019;9(1):874.
21. Cantillo-Negrete J, Carino-Escobar RI, Carrillo-Mora P, Rodriguez-Barragan MA, Hernandez-Arenas C, Quinzaños-Fresnedo J, et al. Brain-Computer Interface Coupled to a Robotic Hand Orthosis for Stroke Patients’ Neurorehabilitation: A Crossover Feasibility Study. Front Hum Neurosci. 2021;15:656975.
22. Caria A, da Rocha JLD, Gallitto G, Birbaumer N, Sitaram R, Murguialday AR. Brain-Machine Interface Induced Morpho-Functional Remodeling of the Neural Motor System in Severe Chronic Stroke. Neurotherapeutics. 2020;17(2):635-650.
23. Chen S, Cao L, Shu X, Wang H, Ding L, Wang SH, et al. Longitudinal Electroencephalography Analysis in Subacute Stroke Patients During Intervention of Brain-Computer Interface With Exoskeleton Feedback. Front Neurosci. 2020;14:809.
24. Foong R, Ang KK, Quek C, Guan C, Phua KS, Kuah CWK, et al. Assessment of the Efficacy of EEG-Based MI-BCI With Visual Feedback and EEG Correlates of Mental Fatigue for Upper-Limb Stroke Rehabilitation. IEEE Trans Biomed Eng. 2020;67(3):786-795.
25. Guo N, Wang X, Duanmu D, Huang X, Li X, Fan Y, et al. SSVEP-Based Brain Computer Interface Controlled Soft Robotic Glove for Post-Stroke Hand Function Rehabilitation. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2022;30:1737-1744.
26. Hu YQ, Gao TH, Li J, Tao JC, Bai YL, Lu RR. Motor Imagery-Based Brain-Computer Interface Combined with Multimodal Feedback to Promote Upper Limb Motor Function after Stroke: A Preliminary Study. Evid Based Complement Alternat Med. 2021;2021:1116126.
27. Lee SH, Kim SS, Lee BH. Action observation training and brain-computer interface controlled functional electrical stimulation enhance upper extremity performance and cortical activation in patients with stroke: a randomized controlled trial. Physiother Theory Pract. 2022;38(9):1126-1134.
28. Lyukmanov RKh, Aziatskaya GA, Mokienko OA, Varako NA, Kovyazina MS, Suponeva NA, et al. Brain-Computer Interfaces in Poststroke Rehabilitation: a Clinical Neuropsychological Study. Neurosci Behav Physi. 2019;49(8):1038-1046.
29. Miao Y, Chen S, Zhang X, Jin J, Xu R, Daly I, et al. BCI-Based Rehabilitation on the Stroke in Sequela Stage. Neural Plast. 2020;2020:8882764.
