Abstract
Managing vertebrobasilar (VB) stroke, particularly basilar artery occlusion (BAO), presents challenges due to diverse clinical presentations and intricate diagnostics, risking delays in acute-phase reperfusion therapies. The diagnostic complexities of VB stroke prompt questions about whether presentation tempo influences outcomes. For non-urgent cases of basilar artery stenosis (BAS) lacking guidelines, clinical management becomes case-dependent. Current options include aggressive medical therapy (AMT) and percutaneous transluminal angioplasty and/or stenting (PTAS). However, the choice of PTAS remains debated, with recent trials observing higher-than-expected recurrence rates, particularly in intracranial stenosis. Understanding hemodynamic status is crucial in predicting stroke risk, especially in atherosclerotic VB disease. Recent studies highlighted the role of distal flow status in predicting stroke risk, emphasizing the importance of hemodynamic assessment beyond anatomic measures. Neurosonology, especially transcranial color-coded duplex sonography (TCCS), emerges as a valuable tool for assessing hemodynamics. Despite TCCS being operator-dependent and technically challenging for BAS evaluation, it effectively detects significant hemodynamic changes, providing real-time information on collateral flow. This review explores the potential role of TCCS in managing BA hemodynamic failure in VB stroke, with particular regard to the selection of BAS patients who may benefit from PTAS.
Keywords
Basilar artery stenosis, Basilar artery stenosis, Vertebrobasilar stroke, Hemodynamics, Neurosonology, Transcranial color doppler, Percutaneous transluminal angioplasty and/or stenting
Introduction
In the field of cerebrovascular pathology, vertebrobasilar (VB) stroke represents one of the most difficult scenarios to manage. The variable clinical presentation of VB stroke, together with the complexity of instrumental investigations, sometimes makes it difficult to obtain a timely diagnosis. Diagnostic errors or delays risk compromising acute phase reperfusion therapies, when indicated [1]. Recent pivotal randomized clinical trials, namely ATTENTION and BAOCHE, published in October 2022, decisively demonstrated the superiority of endovascular treatment (EVT) over medical management within 24 hours of basilar artery occlusion (BAO) onset [2]. Subsequent meta-analysis corroborated this, revealing a two-fold increase in favorable outcomes and reduced mortality at 3 months with EVT.
The heterogeneity in symptoms of VB stroke poses a diagnostic challenge, raising the question of whether the tempo of presentation influences patient outcomes. A recent study by Zhu et al., drawing data from the BASILAR study, highlighted that earlier treatment correlated with better outcomes in particular in patients suffering from VB stroke with sudden onset to maximal deficit [3].
In VB strokes characterized by a subtle, less severe or fluctuating clinical course (e.g. repeated transient ischaemic attacks), which do not undergo urgent endovascular treatment, clinical management is not supported by specific guidelines and is therefore assessed on a case-by-case basis. In such instances, beyond the clinical evaluation and morphological diagnosis of basilar artery (BA) pathology, neurosonology, thanks to the haemodynamic evaluation of the intracranial circulation, could be a useful support in the choice of the most useful therapy in the individual patient. In this paper, we aim to present a case series of patients suffering from BA hemodynamic failure and, consequently, discussing a systematic review of the literature regarding the potential role of neurosonology in the management of these subtype of VB stroke. Specifically, this systematic review is focused on the potential role of transcranial color-coded duplex sonography (TCCS) in managing BA hemodynamic failure in VB stroke, with particular regard to the selection of BA stenosis (BAS) patients who may benefit from percutaneous transluminal angioplasty and/or stenting (PTAS).
Methods
Literature search
We performed a systematic review of available evidence on the role of TCCS in selecting BAS patients for stenting, following the PRISMA Guidelines [4].
A literature search that included English language publications from December 1986 to January 2024 was performed, using PubMed (National Center for Biotechnology Information, US National Library of Medicine). The key search parameters included: “transcranial ultrasonography AND basilar artery stenosis” (230 results) “transcranial color-coded sonography AND basilar artery stenosis” (23 papers), “transcranial sonography AND basilar artery stroke” (189 results), “transcranial ultrasonography AND basilar artery stenosis stenting” (20 papers) and “transcranial ultrasonography AND basilar artery stenting” (13 papers). Furthermore, we searched the reference lists of all relevant articles to identify additional published studies for possible inclusion in the review (3 papers).
Study selection
Pre-defined inclusion and exclusion criteria were set. Two reviewers (SC and MF) independently screened the titles and abstracts to exclude articles that conflicted with one of the inclusion criteria or fulfilled one of the exclusion criteria. Remaining articles were reviewed in full and retained only if they met all the inclusion criteria, and no single exclusion criteria had been identified. Any disagreements were resolved by consensus discussion between the two reviewers. The article types included were clinical study, clinical trial, comparative study, controlled clinical trial, journal article, multicentre study, observational study, randomized controlled trial, systematic and narrative review. Studies were excluded if they (1) were letters, editorials, or conference abstracts; (2) involved patients with BAS from different etiologies (e.g. vasospasm after subarachnoid hemorrhage); (3) involved patients with basilar artery occlusion; (4) did not differentiate patients with BAS from patients with vertebral artery stenosis (VAS) or with anterior circulation stenosis (5) included patients aged less than 19 years (6) were studies on animal models. A total of 478 articles were identified from PubMed. After evaluating the titles and abstracts of these articles, 71 remained eligible for assessment. After removing duplicates and assessing the full texts of these papers, 36 articles were considered.
Data extraction
Data were extracted by MF and then confirmed by SC. Extracted data included authors, year, cohort size, age, gender, risk factors, clinical presentation, peak systolic velocity (PSV), end diastolic velocity (EDV), stenotic-to-prestenotic ratio (SPR), stenosis location, intervention protocol (i.e., access, angioplasty and/or stenting, angioplasty-assisted stenting protocol, and type of stent), degree of BAS at baseline and post-intervention, and survival status.
Data synthesis and quality assessment
The aim of the present systematic review was to analyze the literature data about the role of TCCS on selecting patients for BAS. For each article, level of evidence was independently assessed by two reviewers upon the 2011 Oxford Centre For Evidence-Based Medicine guidelines [5], and risk of bias was evaluated using the JBI checklists [6]. This review’s overall risk of bias was estimated by considering the risk of bias of all included studies in aggregate.
Vertebrobasilar Stroke: Pathophysiology, Clinical Course and Outcome
Stroke in the VB territory accounts for around 20% of all strokes, and in particular, stroke within the BA territory represents approximately 10% of all strokes [7]. BAO can lead to clinical manifestation ranging from mild and transient symptoms to life-threatening strokes. BA originates from the conjunction of the two vertebral arteries (VAs) at the intracranial level. Anatomically, BA can be divided into three segments: proximal, middle, and distal [1]. The proximal segment is comprised from the VAs junction to the anterior inferior cerebellar artery; the middle segment from the anterior inferior cerebellar artery to the superior cerebellar artery; and the area above the orifices of the superior cerebellar artery is known as the distal segment [7].
Occlusions of the proximal or middle segments of the BA generally lead to large pontine strokes with either hemiplegia or quadriplegia; other clinical presentations include reduced consciousness, bilateral extensor plantar sign, dysarthria, dysphagia, horizontal gaze paresis, and other cranial nerve palsies. Occlusions in the distal segment of the BA cause strokes bilaterally in the mesencephalon and thalamus with decreased consciousness, quadriparesis, and nuclear or supranuclear oculomotor and pupillomotor dysfunctions [8].
The main causes of BAO are atherosclerotic occlusions following local thrombosis due to severe stenosis and embolization from cardiac and large artery sources. According to anatomopathological studies, atherosclerosis is the most common cause of BAO [9]. In imaging-based studies, atherosclerosis accounted for 26–36% of BAO, emboli for 30–35%, other (for example dissection of the VAs) for 6–8%, and causes were undetermined in 22–35% of patients [10,11]. Atherosclerosis often affects both VAs and provokes mostly occlusion of the proximal and middle segments of the BA.
The size of the infarct resulting from BAO is mainly determined by the status of collateral pathways. Progressively occluding atherosclerotic lesions allow the formation of collateral circles, thus BAO leads to little ischemic lesions and transient symptoms. Conversely, sudden embolic or thrombotic occlusions can cause extensive ischemic damage and severe clinical deficits [8].
Management of Basilar Artery Stroke: Current Pitfalls and Perspectives
Strategies for treatment of patients with acute stroke due to BAO and with underlying intracranial atherosclerotic stenosis (ICAS) should be different from those without it. VB stenosis is associated with high risk of stroke recurrence, despite medical therapy; in particular, 90-days risk of stroke from the first event is 24.6% in patients with VB stenosis versus 7.2% in those without. Recurrence is more likely (33%) with intracranial than extracranial stenosis. Risk from the presenting event is 9.6% in patients with stenosis versus 2.8% in those without, and it is higher with ICAS [12].
Current treatment options for BAS range from medical therapy, including aggressive medical therapy (AMT), combining dual antiplatelet therapy (DAPT) with risk factors control, and PTAS. The choice of PTAS may be based on high-grade stenosis, poor collateralization, insufficient control of vascular risk factors or transient ischemic attack (TIA)/ischemic stroke under pre-existing antiplatelet and lipid-lowering therapy [13,14].
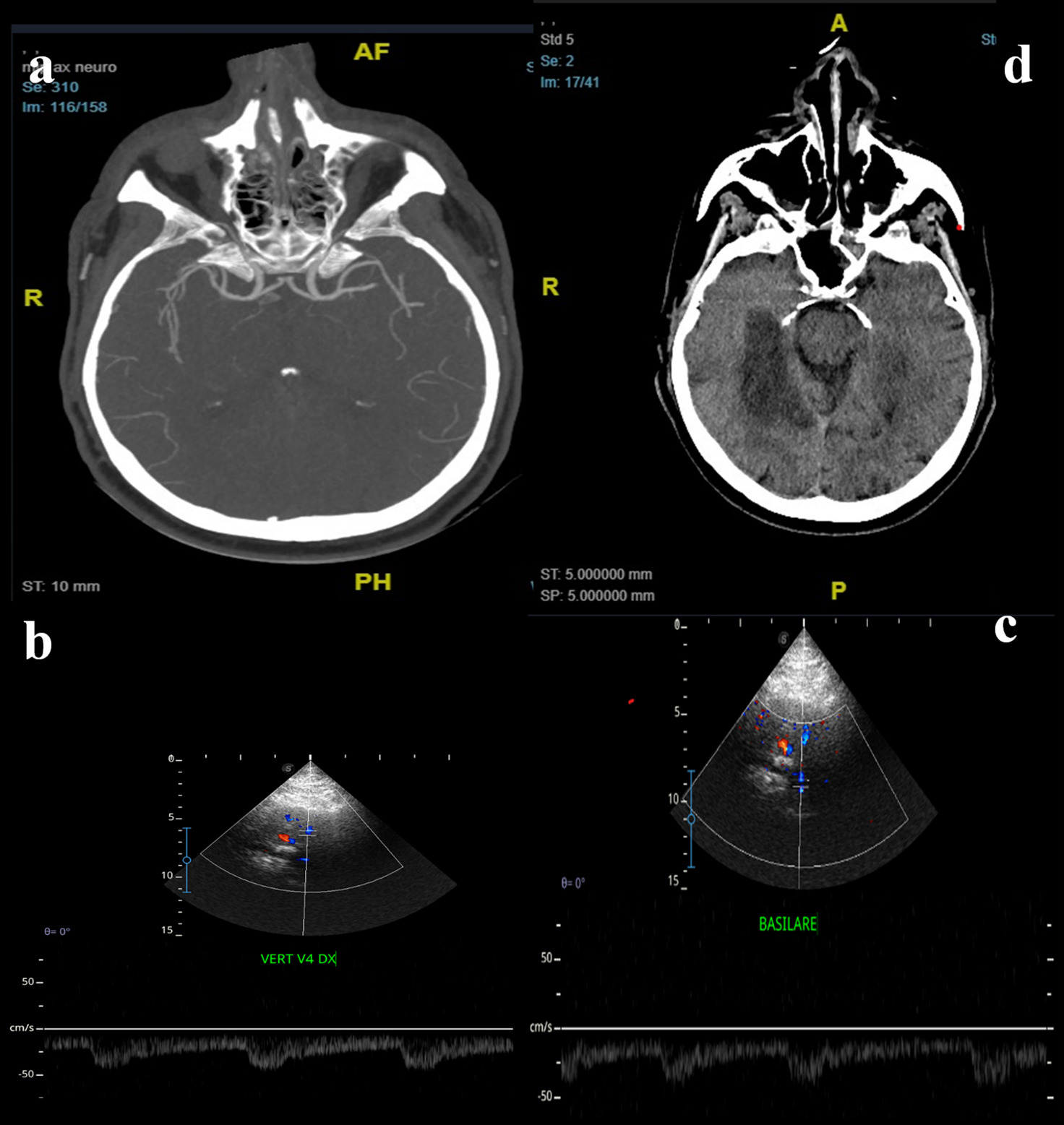
The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) group compared the safety and efficacy of the two antithrombotic agents in a multicentric post hoc analysis of 68 patients with a stroke secondary to a 50–99% BAS [15]. They found that 23% of patients taking aspirin had a subsequent stroke in the same vascular territory as the stenotic artery compared with 10% for warfarin. The WASID group concluded that the high rate of subsequent strokes in the stenotic territories suggests that endovascular therapy may be needed for symptomatic severe stenosis of the VB circulation. The WEAVE trial [16] demonstrated that PTAS was safe with a low periprocedural complication rate. Conversely, the SAMMPRIS [17] and VISSIT [18] trials observed higher than-expected rates of recurrent 30-day strokes after PTAS in patients with intracranial stenosis compared to AMT. Lutsep et al. in a post-hoc analysis on subpopulations of patients of the SAMMPRIS trial, including those with qualifying event hypoperfusion symptoms, did not found benefit from stenting procedure even in those populations [19]. However, the trials mentioned above were not focused on BAS. As regards BAS, in the acute setting, the combined approach of emergency thrombectomy and stent placement may be the most proper. In this context, one of the most complex aspects is the therapeutic management of BAS after EVT, especially in cases characterized by a higher risk of hemorrhagic infarction (e.g. in patients also treated with intravenous thrombolysis, in patients treated late, or in cases where perfusion studies have shown a larger ischaemic core) (Figure 1).
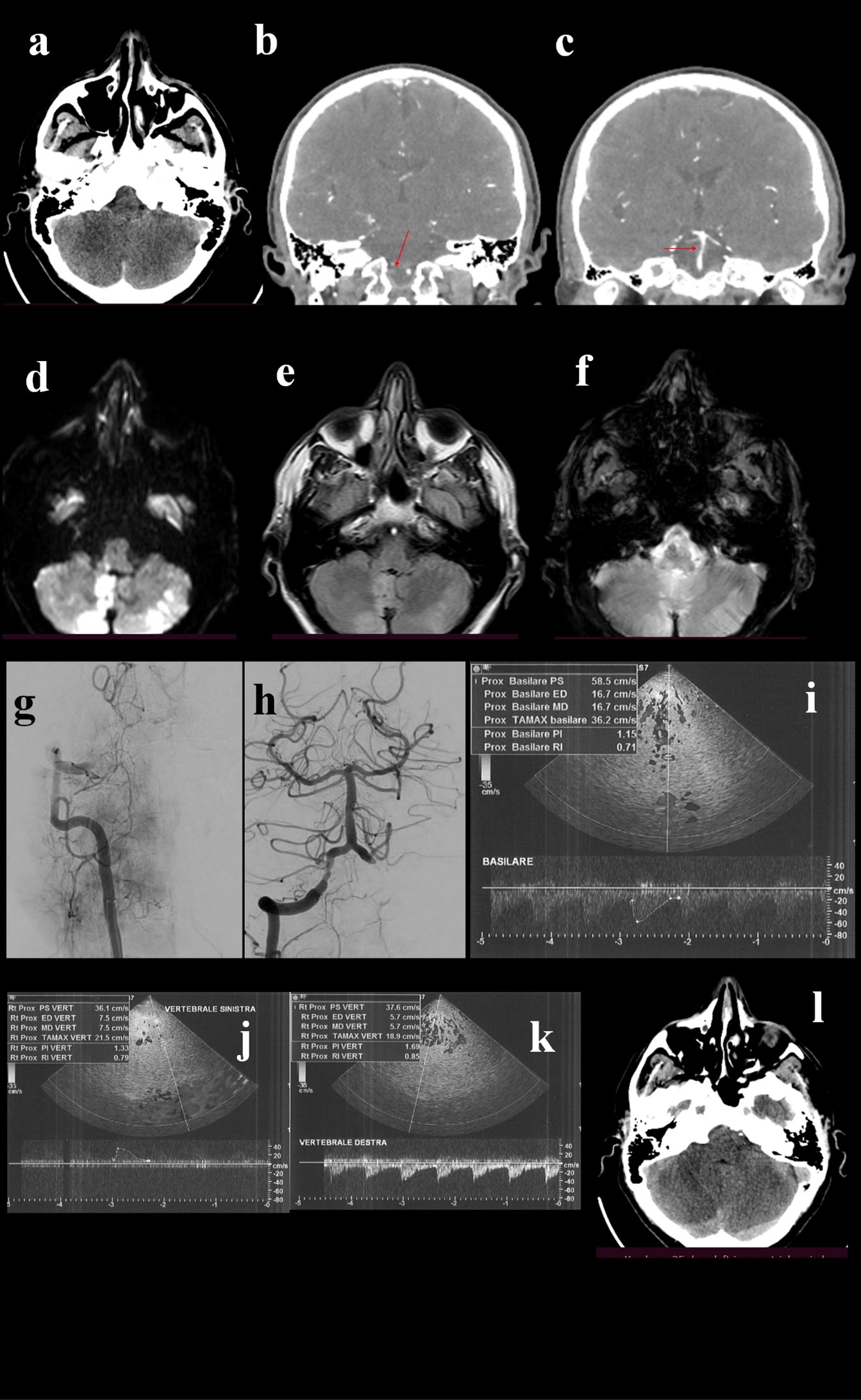
Figure 1. A 58 year old male patient, with history of smoking and hypertension, was urgently admitted for wake-up stroke, characterized by vertigo, vomit, diplopia, ataxia and progressive alteration of mental status. Head CT (a) scan did not detected acute lesions, CTA showed occlusion of V4 tract of right vertebral artery (b, arrow) with stenotic extension to the basilar artery (c, arrow). Urgent MRI showed bilateral cerebellar and bulbar DWI hyperintensities (d), less evident in FLAIR sequences (e) and with no hypointensities in GE sequences (f). DSA confirmed V4 occlusion of right vertebral artery (g), that was treated with mechanical thrombectomy. The final angiogram showed post-procedural right V4 and basilar stenosis (h). Given the risks of the complex stenting of both arteries, VB hemodynamics were analyzed by TCCS, that showed diffuse angiosclerotic flow without significant focal alterations (i, j, k). Based on these findings stenting was not performed. The patients slightly improved and 24-hours control brain CT scan (l) did not point out a limited right cerebellar hypointensity.
In a retrospective analysis on endovascular treatment of acute BAO, 38 patients with associated VB steno-occlusive disease were presented. In 52.6% of patients, additional stent angioplasty was performed and successful reperfusion (TICI 2b–3) was achieved in 86.8% of cases. The in-hospital mortality rate was similar with in stent and non-stent angioplasty groups [20]. Behme et al., in a series of 7 patients who underwent stenting for high-grade BAS, 6 of whom also received mechanical thrombectomy, reported a successful recanalization (TICI 2b–3) in all patients and one in-hospital death [21]. Another small study reported high rates of successful revascularization (100%) and favorable outcome (60%), without any symptomatic hemorrhage, and a low mortality rate (13.3%) in patients with acute BAO and underlying ICAS who were treated with intracranial angioplasty with or without stent placement after first-line mechanical thrombectomy [22].
Beyond cases treated with ENT, the appropriateness of elective PTAS for BAS in secondary prophylaxis is certainly one of the most complex and debated issues to be addressed in routine clinical practice. A recent systematic review and meta-analysis pointed out that, in experienced centers, elective PTAS appears to be safe and effective in selected patients with medically refractory, severe, symptomatic, and non-acute BAS [23] However, periprocedural risks are high with VB stenting, ranging up to 20%, particularly for BA disease [24,25]. The potential complications associated with this treatment option include periprocedural risks of ischemia, hemorrhage, intimal dissection, postoperative neurological deficits, and the possibility of in-stent restenosis [13,17,26,27]. Moreover, there is the risk of BA branch occlusion due to the “snow ploughing” effect (displacing plaque into branch/perforating arteries during the process of stent deployment) in branches such as the anterior inferior cerebellar artery or the pontine arteries [28]. In a recent study investigating angiographic and clinical differences in BAO subtypes undergoing mechanical thrombectomy, despite comparable rates of successful recanalization, outcome of the treatment seemed to depend on pathogenesis with less clinical benefit for underlying BAS, raising the question of early identification of this subgroup [29]. Therefore, there is an urgent need for a tool which could allow to select patients with BAS and a potential favorable outcome after PTAS. To improve the advantage of PTAS treatment, detecting the risk factors of long-term recurrent VB stroke is crucial.
Literature data have shown the relevance of the hemodynamic status in predicting the risk of stroke recurrence in patients with atherosclerotic VB disease [30-32]. The failure of prior stenting trials may be partly explained by the fact that they missed to select the highest-risk hemodynamically compromised subgroup, perhaps weakening a possible treatment effect [33].
Role of Hemodynamics in Posterior Circulation Stroke Risk and Recurrence in the Setting of Vertebrobasilar Atherosclerotic Disease
Along with thromboembolism, local hypoperfusion due to hemodynamic VB disease is believed to be a main cause of posterior circulation stroke [34]. In fact, chronic cerebrovascular hypoperfusion may injure the vascular endothelium, leading to local neurovascular unit dysfunction and disruption [35,36]; moreover, hypoperfusion decreases the capacity of bloodstream to wash out emboli and limits the available blood flow to areas made ischemic by emboli in distal arteries [37].
Hemodynamic compromise has already been identified as a risk factor for stroke associated with cerebrovascular occlusive diseases affecting the anterior circulation [38-40].
In the Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (VERiTAS) study quantitative magnetic resonance angiography (QMRA) was employed to assess the hemodynamic compromise and its role in prediction of stroke risk in symptomatic VBD [41]. The flows in affected vessels correlated with residual diameter and stenosis; the association was more robust with diameter, and only evident with stenosis in the BA but not VAs. However, according to the authors, the distal flow status provides a hemodynamic assessment which, by including collateral capacity, is different from anatomic measures such as stenosis and diameter and, therefore, can be independent of the local hemodynamic impact of the disease [42,43].
In the VERiTAS cohort, those patients with VB disease and impaired distal flow were found to be at significantly higher risk of subsequent vertebrobasilar stroke, with a 22% one-year stroke risk compared to 4% in patients with normal distal flow; also the risk of stroke recurrence was higher, with 12- and 24-month event-free survival rates of 78% and 70%, respectively, in the low-flow group vs 96% and 87%, respectively, in the normal-flow group [30].
Also another study on patients from the VERiTAS cohort demonstrated that those with symptomatic atherosclerotic VB disease and low distal flow are at higher risk of hemodynamic infarction [33].
The relevance of distal flow status in predicting stroke risk had been underlined also in a previous work. In a retrospective single center cohort, patients with >50% VB stenosis or occlusion with low distal flow status presented a significantly worse stroke free survival compared to patients with normal distal flow status. On multivariate analysis, distal flow status was an independent predictor of recurrent stroke after adjusting for stenosis severity and location of the disease [44]. These results were confirmed by further post-hoc analyses performed in the same cohort of patients, indicating that flow represents a strong predictor of stroke across different predictive models, and suggesting that prediction of future stroke risk can be optimized by use of vascular anatomy and age-specific normalized flows [45] (Figure 2).
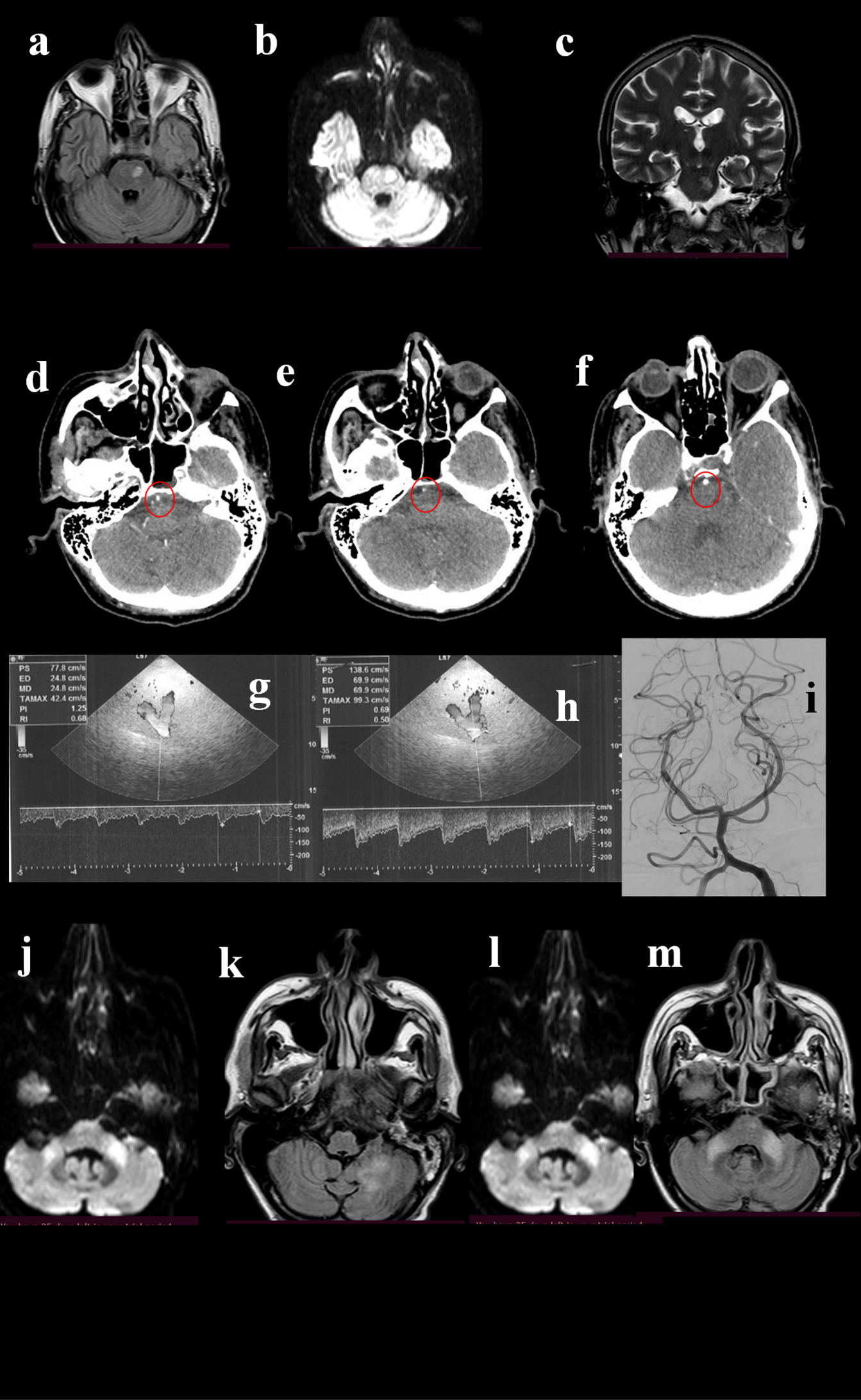
Figure 2. A 49-year-old man was admitted to the hospital for repetitive episodes of acute reduced strength of the right limbs and subsequent fall in last weeks. His past medical history revealed smoking, past episodes of syncope and diplopia started a few months before. The neurological examination showed deficit of left lateral rectus muscle, right VII cranial nerve palsy, mild hyposthenia of right limbs; normal deep tendon reflexes; ataxia. The brain MRI showed FLAIR-(a), DWI-(b), and T2-(c) positive lesion in the left pons. The patient underwent CTA, that showed a normal basilar origin (d, circle) followed by a narrowing (e, circle), and then a normalization of caliber (f, circle). TCCS detected normal flow at the basilar origin (g), while a 1 cm after the origin of the vessel an increase of the flow velocity was observed (h), indicating moderate stenosis (>50%). DSA pointed out the stenosis (i), even if it was not considered suitable for stenting. Thus, the best medical treatment was suggested. One month later the patient was re-admitted for novel clinical worsening, and MRI showed hyperintensities in DWI and FLAIR sequences at bilateral middle cerebellar peduncles (j, k) and at left cerebellum (l, m).
Early neurologic deterioration (END) is correlated with poor clinical outcome in patients with medically treated acute symptomatic BAS. Several factors have been associated with END in these patients, including proximal FLAIR-hyperintense vessel (FHV), elevated C-reactive protein level, high NIHSS score, and poor DW imaging–based pc-ASPECTS. FHV was found to be also an independent predictor of unfavorable functional outcome [46]. FHV reflects altered hemodynamics, representing the slow flow of blood proximal and distal to large arterial occlusion [47].
Huang et al. performed a longitudinal study to assess the prognostic value of hemodynamic features in patients with posterior ICAS treated with PTAS, using the quantitative DSA (q-DSA) technique. They measured hemodynamic parameters such as relative time to peak (rTTP) and trans-stenotic TTP difference before and after PTAS. Prolonged TTP reflects low blood flow velocity implying a low local blood pressure gradient and therefore decreased perfusion. The stenosis increases focal flow velocity, which is manifested as a shortening of trans-stenotic TTP difference. They found that prolongation of preprocedural rTTP of the target vessel and shortened trans-stenotic TTP at the preprocedural DSA imaging were associated with recurrent events. Furthermore, despite PTAS significantly improved perfusion status, patients with more restricted baseline blood flow still showed higher risks of stroke relapse even after endovascular treatment [48] (Figures 3 and 4).
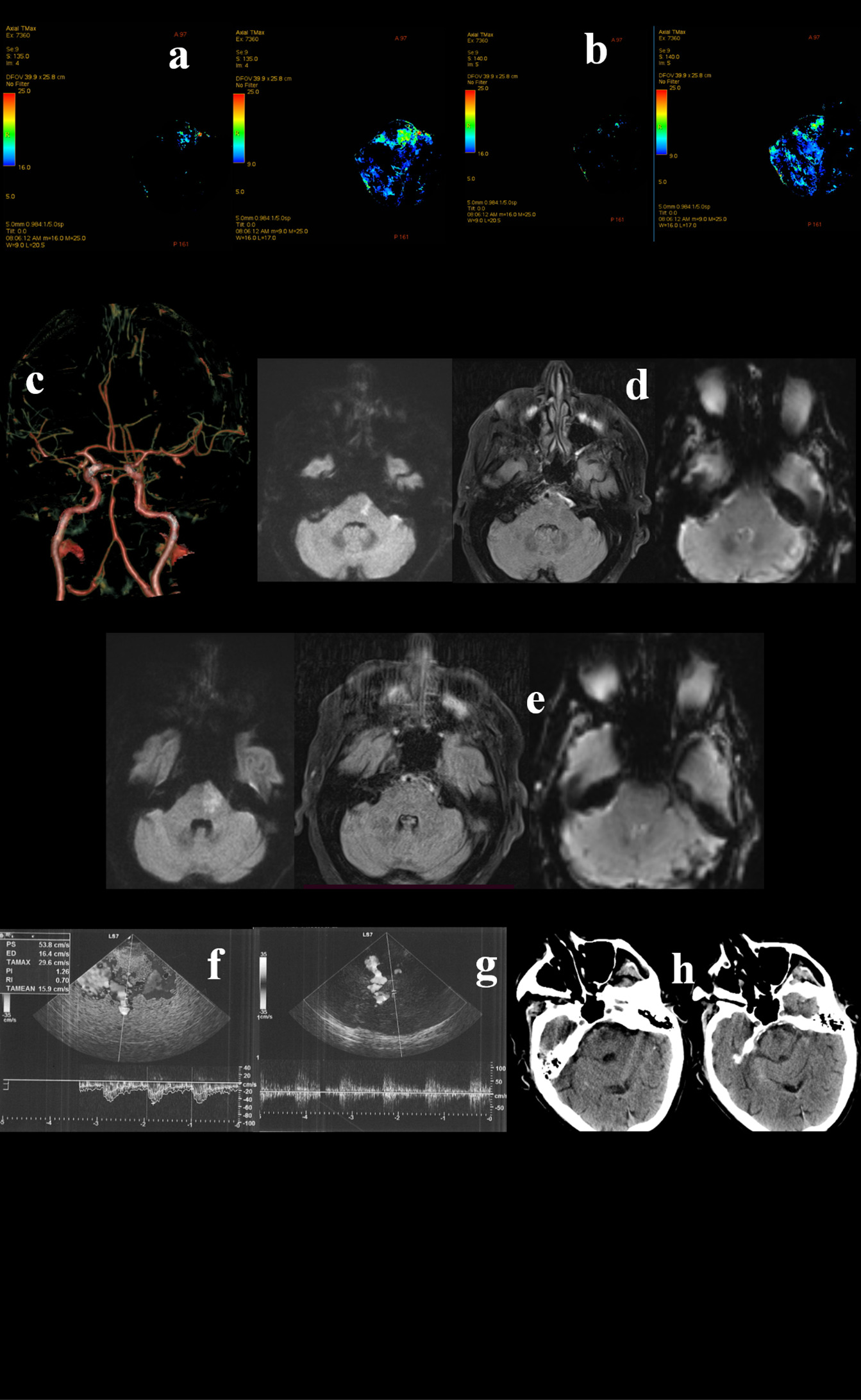
Figure 3. A 79-year-old Caucasian man awoke with right deviation of the head and right hemiparesis. His last known normal was 10 p.m. the previous night. His past medical history revealed arterial hypertension, diabetes mellitus, and dislypidemia. The patient was presented to the emergency department at 09:00 a.m. The neurological examination showed coniugate left gaze palsy, dysarthria, and right hemiparesis, with a fluctuating course. Urgent Head CT did not show acute lesions and the CT perfusion documented an area of ischemic core in the left pons (a) and penumbra in pons, medulla and bilateral superior cerebellar hemispheres (a,b). CTA documented distal basilar artery stenosis. The patient underwent urgent MRI which confirmed a right paramedian pontine DWI hyperintensity, in the context of which a moderate hyperintensity on FLAIR sequences was observed (c,d). Although basilar artery was associated with brainstem hypoperfusion, the FLAIR-positive lesion determined a significant risk for hemorrhagic transformation in case of revascularization. Hence, TCCS was performed and did not reveal hemodynamic demodulation in the proximal tract (f) and in the top (g) of basilar artery. Thus, DSA and PTAS were avoided. Dual antiplatet therapy was administered. In the following days the patient showed a gradual progressive improvement, with the evidence on a small lesion on control head CT (h).
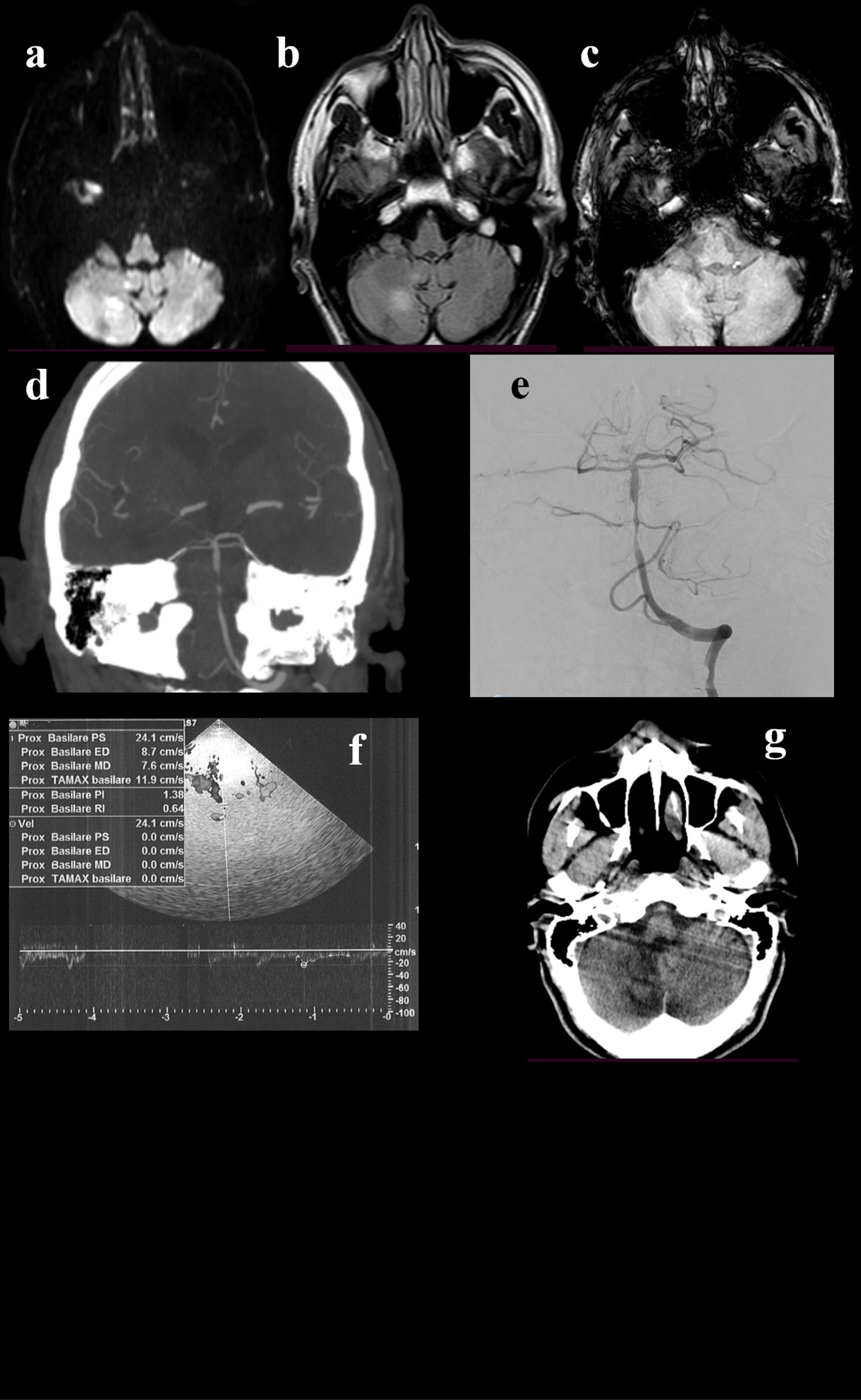
Figure 4. A 68-year-old man presented to the emergency department because of a 4-days-history of brief recurrent episodes of dizziness, nausea, vomiting, and sweating. He suffered from arterial hypertension, diabetes mellitus, and he had a spontaneous subarachnoid hemorrhage 4 years before. He was taking clopidogrel. During the episodes described before, the blood glucose was normal. The initial neurological examination did not show any abnormal findings. The brain CT scan and ORL examination were unremarkable. The brain MRI showed DWI-(a) and FLAIR-(b) positive lesion in the right cerebellar hemisphere, without hypointensities in SWI (c). CTA revealed stenosis at the middle portion of basilar artery (d), that was confirmed by DSA (e). TCCS was performed and pointed out basilar artery hemodynamics within normal range (f). In light of this, stenting was not performed. The 24-hours control head CT confirmed the right cerebellar region, without signs of further expansion of the ischaemic area or hemorrhage (g). The best medical treatment was adopted. The patient showed a progressive clinical improvement.
Transcranial Color-Coded Duplex Sonography in the Evaluation and Management of Basilar Artery Stenosis
DSA is the gold standard to detect intracranial artery stenosis [49-51], but it has some limitations: it is expensive, invasive, not ubiquitously available, and it does not allow to study hemodynamic changes [50,52].
TCCS has been validated as a noninvasive screening tool to identify intracranial large artery stenosis [53,54]. Compared with transcranial Doppler (TCD), a closer approximation of true flow velocities is obtained by measuring the insonation angle [55,56]. TCCS far exceeds sonological evaluation of the extracranial VA in detecting significant haemodynamic changes in the VB vasculature [57-61].
Transcranial ultrasound has a very high negative predictive value in excluding a 50–99% stenosis and occlusion, whereas it has only a moderate positive predictive value [62-64].
As regards the anterior circulation, TCCS can detect stenosis of internal carotid artery (ICA) with high sensitivity (87.1%), specificity (89.8%), accuracy (88.8%) with an excellent agreement level compared to DSA [49,65].
Focusing on the evaluation of BA, the main concern about the use of TCCS is that it is technically challenging, especially regarding the distal segments, so that the sensitivity is lower compared to the anterior circulation [66-68]. Being an operator-dependent technology, it requires the availability of experienced operators [69]. Furthermore, there are some diagnostic limitations of the imaging technique, including difficulties obtaining a proper sonographic window, the effect of overweight, variations in vessel morphology [69,70].
Indeed, transforaminal scanning may be impaired by excessive fat in the neck region or in patients with a limited range of motion of the cervical spine. Visualization is markedly improved after administration of an echo-contrast agent [55,69]; nevertheless, its application may be limited for reasons of cost or time [67].
Pade et al. demonstrated that a complete visualization of BA was feasible by using combined transforaminal and transtemporal approach [71].
Some studies found that the validity of TCD in the assessment of the degree of BA stenosis or in-stent restenosis was poor, whereas TCCS exhibited excellent sensitivity and specificity in detecting in-stent BAS [50,72,73].
Nasr et al. showed that TCCS allowed to detect symptomatic steno-occlusive lesions of the large intracranial arteries with perfect agreement with MR 3D TOF angiography. Demonstration of such a lesion by TCCS was strongly predictive of early recurrent TIA/stroke independent of the clinical ABCD2 score. On the other hand, the sensitivity of TCCS was relatively low, implying that a negative TCCS cannot rule out subsequent TIA/stroke recurrence [52].
Diagnostic criteria have been developed to identify hemodynamically significant or not significant stenosis in various intracranial arteries [74,75]. Furthermore, in a more recent study TCCS criteria for BAS degree of 50% and 70% showed a satisfactory predictive value and good agreement with DSA. In particular, MFV >90 cm/s and stenotic-to-prestenotic ratio (SPR) ≥ 2 achieved an overall accuracy of 92.4 for >50% BAS, while MFV >120 cm/s and stenotic-to-prestenotic ratio (SPR) ≥ 3 demonstrated an overall accuracy of 92.4 for >50% BAS [76]. These results were consistent with other observations obtained in different studies [77-79].
Once the anatomical diagnosis of intracranial stenosis is made, it is crucial to understand the functional significance and the hemodynamic effects of stenosis.
TCCS can provide real-time information on collateral flow [80] and in case of vessel obstruction, activation of collateral pathways is very important for the clinical outcome of the patient [81]. A complete circle of Willis and the possibility to activate primary collaterals (ACoA, PCoA) or secondary collaterals ([ophthalmic artery, leptomeningeal arteries) decrease the risk of hemodynamic ischemic stroke; in patients with intracranial artery obstruction, the most frequent collaterals are the leptomeningeal arteries [82].
When interpreting hemodynamic changes, the complexity of cerebral circulation should be kept in mind. Elevated flow velocities in the intracranial arteries should not be read in isolation for diagnosing an intracranial stenosis, and the patency of major cervicocranial arteries should be considered. In a study conducted by Zhong et al., the authors reported that one-third of increased velocity in BA was due to BA stenosis, one-third was due to ICA severe stenosis or occlusion, and a quarter of that was due to VA severe stenosis or occlusion. These findings can be explained by the fact that, in patients with symptomatic carotid artery occlusion, alterations of blood flow through contralateral ICA, BA, and middle cerebral artery (MCA) and the development of intracranial collateral circulation may work for compensate the reduced cerebral perfusion caused by anterior artery disease [51,83] (Figures 5 and 6).
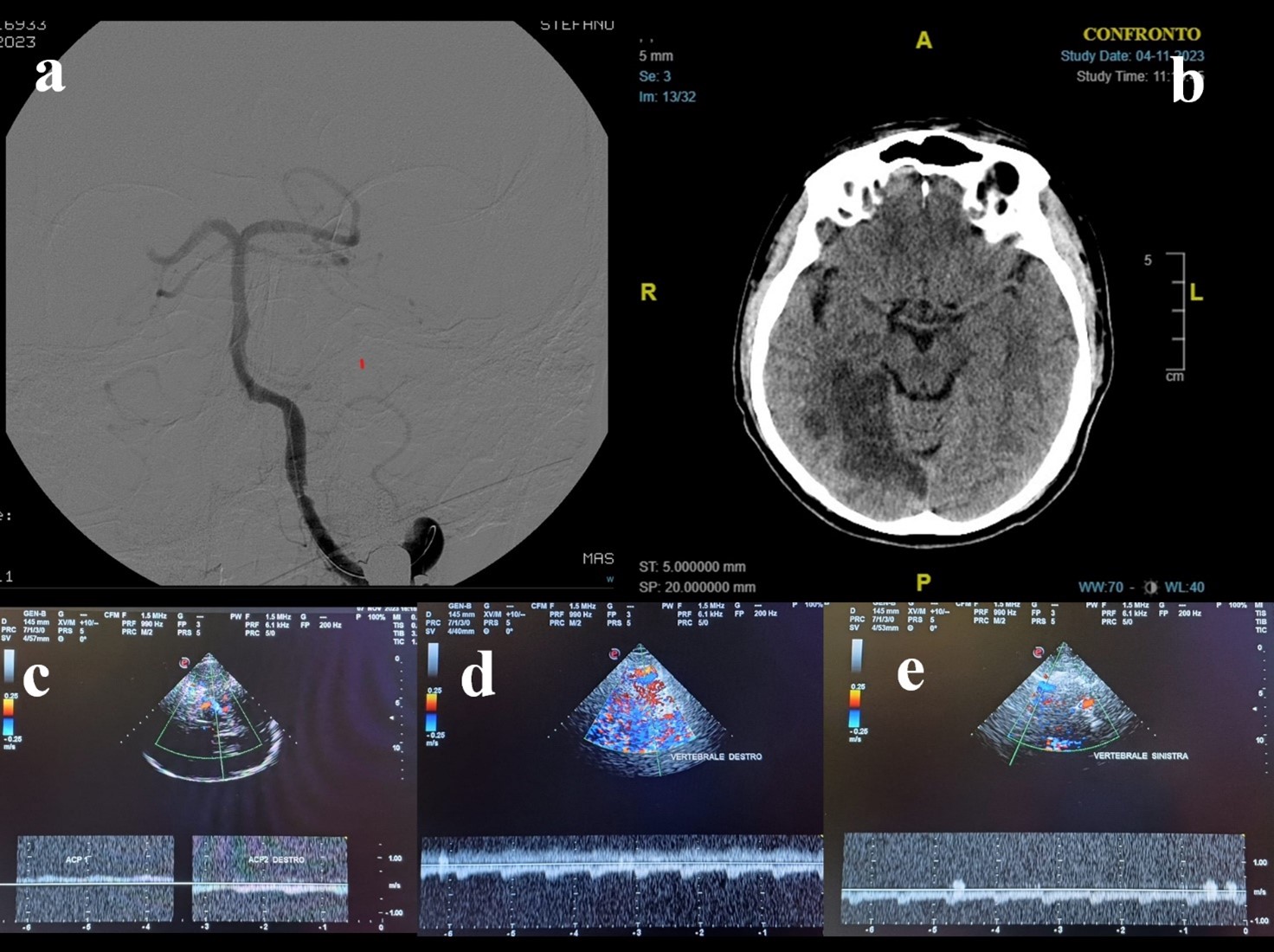
Figure 5. A 65-years-old man came to the hospital for acute loss of consciousness. His medical history revealed paroxysmal atrial fibrillation, hypertension, smoking, dyslipidemia, and COPD. In ER department he underwent CTA that showed thrombotic occlusion of the top of basilar artery (a), with normal flow of the vertebral-basilar axis near the occlusion. The patient also had an aortic dissection, so thrombolysis was contraindicated. The patient was treated by mechanical thrombectomy: DSA showed occlusion of the distal third of the basilar artery and of P3-segment of right cerebral posterior artery. After the successful procedure, TCCS was employed to monitor the posterior hemodynamic pattern. TCCS detected normal flow at the origin of the basilar (b) and at the vertebral arteries (c). The patient was admitted to the Intensive Care department, and the 24-hour control brain CT showed a right cerebellar infarct and bilateral thalamic infarcts (d). Neurological examination showed right facio-brachio-crural hemiparesis. Anticoagulant therapy was administrated. The patient showed an important improvement of his conditions and at the time of discharge, associated with regular flow in basilar artery.
Figure 6. A 57-years-old man was admitted to the ER department for acute dysarthria, dysphagia, and dysmetria; the neurological examination showed left facio-brachio-crural hemiparesis, ocular ptosis (left eye), left facial hypoesthesia, dysmetria and dysarthria. He underwent CTA that showed left vertebral artery occlusion and left cerebellar and brainsteam infarction. The patient was treated by thrombolysis and mechanical thrombectomy: DSA showed occlusion of the vertebro-basilar junction and of P1-segment of right cerebral posterior artery (a), so a stent was placed at the vertebro-basilar junction. Control brain CT (b) showed multiple infarcts in bilateral cerebellar territory and right temporo-occipital hemisphere. TCCS (c) showed normal hemodynamics at right ACP, so medical treatment was continued. A TCCS (d,e) performed after one week showed normal flow velocity in the basilar and vertebral arteries. At discharge, neurological examination showed improvement in symptomatology.
A Potential, Pivotal, Role for Neurosonology in Patients Selection for Elective Stenting of Basilar Artery Stenosis
Whether in VB strokes undergoing emergency EVT, PTAS on BAS can be considered a reasonable therapeutic approach in order to stabilize cerebral perfusion and reduce the risk of early ischemic recurrence on a hemodynamic basis, in patients not eligible for acute phase therapy, the management of BAS lacks obvious and widely agreed solutions. Although thanks to the widely available neuroradiological examinations an accurate anatomical diagnosis is easily and readily obtained, the clinical decision on the best treatment for each individual patient must be based on the assessment of cerebral hemodynamics. TCCS represents a non-invasive, bedside, widely available, real-time technology that, under experienced hands, can offer a diagnostic tool with appropriate predictive value. As found in the cases presented (Figures 1-6), TCCS can add a crucial piece of information in the interpretation of BAS pathophysiological and prognostic aspects, contributing decisively to the choice of the most suitable treatment in the individual patient.
Among neurosonological parameters, peak systolic velocity (PSV), end-diastolic velocity (EDV), mean flow velocity (MFV) are usually considered criteria for the haemodynamic assessment of stenosis of extracranial and intracranial vessels. In particular, ≥ 50% BAS can be defined by PSV ≥ 140 cm/s, while for ≥ 70% BAS PSV ≥ 140 cm/s associated with post-stenotic flow pattern in posterior cerebral arteries [84]. These criteria are characterized by good reliability, especially for the higher degree of stenosis [85]. Moreover, it has been observed that the best diagnostic accuracy of BAS using TCCS is obtained combining PSV ≥110 cm/s, MFV ≥ 70 cm/s, and (BA PSV)/(VA PSV) ≥ 1.5 for ≥ 50% stenosis, and PSV ≥ 150 cm/s, MFV ≥ 90 cm/s, and (BA PSV)/(VA PSV) ≥ 2 for ≥ 70% stenosis [86].
Despite the usefulness of defining the degree of stenosis, other haemodynamic parameters can help in order to assess the potential instability of the VB circulation. Specifically, pulsatility index (PI) is a measure of the variability of blood velocity in a vessel, sensitively reflecting changes in the cerebral arteriolar tone. In fact, PI decreases during vasodilation and increases after vasoconstriction of the cerebral arterioles. In addition, Pourcelot/resistance index (RI) is a sensitive measure of the distal vascular resistance [80]. The accuracy of PI and RI in reflecting cerebral hemodynamics has been recently highlighted comparing TCD parameters and angiographic pressure wire measurements [87].
As observed in previous studies, pooling velocity and hemodynamic parameters may be possible to define the risk of haemodynamic instability of an intracranial stenosis, particularly in the VB circulation [88,89]. Hence, in case of BAS, if PI<1 and RI<0.5 AMT should be considered; on the other hand, if PI>1.5 and RI>1 acute PTAS should be taken into account.
Beyond all this, TCCS operator dependency remains an important limit, which must be overcome in order to achieve the appropriate degree of sensitivity, specificity and reproducibility. For these reasons, a clinical trial on the ability of TCCS in selecting BAS patients to undergo PTAS would be highly needed.
Technical and Procedural Limitations
Beyond the discussed important benefits provided, due to limited anatomical information, TCCS provides few structural details about distal BA compared to other imaging techniques, like MRI or computed tomography angiography. This can be a drawback when detailed anatomical information is crucial for treatment planning. The quality of TCCS examinations can be highly dependent on the operator's skill and experience. Variability in operator expertise may affect the accuracy and reliability of the results. TCCS has limitations in visualizing vessels deep within the brain, particularly in obese patients or those with challenging cranial anatomy. This limitation may compromise the ability to assess the full extent of BAS. TCCS primarily evaluates blood flow and velocity but does not directly visualize the vessel wall. This limitation may make it challenging to differentiate between various pathologies affecting the BA, such as atherosclerosis or vasculitis. Hence even though TCCS offers several advantages in terms of non-invasiveness, real-time monitoring, and cost-effectiveness, it also has limitations related to anatomical detail, operator dependence, and penetration depth. The choice of imaging modality for the management of BAS should be based on a thorough consideration of the clinical context and the specific information required for treatment decisions.
Conclusion
The complexities of diagnosing and treating BAS call for a nuanced and evolving strategy. Although progress in imaging technologies and a deeper comprehension of the disease bring optimism, ongoing research and collaboration remain crucial. Improving diagnostic precision and refining therapeutic approaches are key to enhancing outcomes for BAS. The continuous interaction between clinical expertise, technological advancements, and research initiatives will play a pivotal role in shaping the future of diagnosis and treatment for this intricate cerebrovascular condition.
Within this context, TCCS can represent a safe, reproducible technique providing a real-time dynamic assessment of BAS, which may help clinicians in the difficult therapeutic management of this type of patient, both in the acute and elective phases. Notwithstanding, technical and operator limitations imply a necessary caution in basing the therapeutic decision solely on the TCCS findings. For this reason, there is a strong need for further research in order to validate the role of TCCS in guiding BAS treatment decisions.
Conflicts of Interest
The authors do not declare any conflict of interest.
Funding
None.
Acknowledgments
The authors would like to thank Neurosonology Nurse Stefania Borelli, for her indispensable help.
References
2. Nguyen TN, Fisher M, Schonewille WJ. Evolution of endovascular therapy trials for basilar artery occlusion. Journal of Cerebral Blood Flow & Metabolism. 2023 Nov;43(11):2005-7.
3. Zhu L, Liu W, Hu Z, Li Z, Duan Z, Guo Z, et al. Endovascular therapy for basilar artery occlusion in sudden onset to maximal deficit ischemic events. Journal of the American Heart Association. 2024 Jan 16; 13(2):e030713.
4. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29:372:n71.
5. Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). In: Oxford Cent. Evidence-Based Med. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence
6. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Systematic reviews of etiology and risk. In: Aromataris E, Munn Z (Editors). JBI Manual for Evidence Synthesis. JBI, 2020.
7. Chung JW, Park SH, Kim N, Kim WJ, Park JH, Ko Y, et al. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion‐weighted imaging. Journal of the American Heart Association. 2014 Aug 11;3(4):e001119.
8. Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke. 1977 May;8(3):383-90.
9. Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G. Basilar artery occlusion. The Lancet Neurology. 2011 Nov 1; 10(11):1002-14.
10. Castaigne P, Lhermitte F, Gautier JC, Escourolle R, Derouesne C, AGOPIAN PD, et al. Arterial occlusions in the vertebro-basilar system: a study of 44 patients with post-mortem data. Brain. 1973 Mar 1; 96(1):133-54.
11. Jung S, Mono ML, Fischer U, Galimanis A, Findling O, De Marchis GM, et al. Three-month and long-term outcomes and their predictors in acute basilar artery occlusion treated with intra-arterial thrombolysis. Stroke. 2011 Jul; 42(7):1946-51.
12. Schonewille WJ, Wijman CA, Michel P, Rueckert CM, Weimar C, Mattle HP, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. The Lancet Neurology. 2009 Aug 1; 8(8):724-30.
13. Gulli G, Marquardt L, Rothwell PM, Markus HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke. 2013 Mar; 44(3):598-604.
14. Maier IL, Karch A, Lipke C, Behme D, Mpotsaris A, Kabbasch C, et al. Transluminal angioplasty and stenting versus conservative treatment in patients with symptomatic basilar artery stenosis: perspective for future clinical trials. Clinical Neuroradiology. 2018 Mar; 28:33-8.
15. Machado M, Borges de Almeida G, Sequeira M, Pedro F, Fior A, Carvalho R, et al. Percutaneous transluminal angioplasty and stenting in acute stroke caused by basilar artery steno-occlusive disease: the experience of a single stroke Centre. Interventional Neuroradiology. 2022 Oct; 28(5):547-55.
16. Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Prognosis of patients with symptomatic vertebral or basilar artery stenosis. Stroke. 1998;29:1389-92.
17. Alexander MJ, Zauner A, Chaloupka JC, Baxter B, Callison RC, Gupta R, et al. WEAVE Trial Investigators. WEAVE trial: final results in 152 on-label patients. Stroke. 2019 Apr;50(4):889-94.
18. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. New England Journal of Medicine. 2011 Sep 15;365(11):993-1003.
19. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. Jama. 2015 Mar 24;313(12):1240-8.
20. Lutsep HL, Lynn MJ, Cotsonis GA, Derdeyn CP, Turan TN, Fiorella D, et al. SAMMPRIS Investigators. Does the stenting versus aggressive medical therapy trial support stenting for subgroups with intracranial stenosis?. Stroke. 2015 Nov;46(11):3282-4.
21. Siebert E, Bohner G, Zweynert S, Maus V, Mpotsaris A, Liebig T, et al. Revascularization techniques for acute basilar artery occlusion: technical considerations and outcome in the setting of severe posterior circulation steno-occlusive disease. Clinical Neuroradiology. 2019 Sep 1;29:435-43.
22. Behme D, Weber W, Mpotsaris A. Acute basilar artery occlusion with underlying high-grade basilar artery stenosis: multimodal endovascular therapy in a series of seven patients. Clinical Neuroradiology. 2015 Sep; 25:267-74.
23. Lee YY, Yoon W, Kim SK, Baek BH, Kim GS, Kim JT, et al. Acute basilar artery occlusion: differences in characteristics and outcomes after endovascular therapy between patients with and without underlying severe atherosclerotic stenosis. American Journal of Neuroradiology. 2017 Aug 1; 38(8):1600-4.
24. Palmisciano P, Hoz SS, Algburi HA, Ventre G, Street S, Agyeman N, et al. Percutaneous transluminal angioplasty and/or stenting for the treatment of basilar artery stenosis: a systematic review and meta-analysis. Neuroradiology. 2023 Jun;65(6):985-1000.
25. Fiorella D, Derdeyn CP, Lynn MJ, Barnwell SL, Hoh BL, Levy EI, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS). Stroke. 2012 Oct;43(10):2682-8.
26. Nahab F, Lynn MJ, Kasner SE, Alexander MJ, Klucznik R, Zaidat OO, et al. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009 Jun 9;72(23):2014-9.
27. Wolfe TJ, Fitzsimmons BF, Hussain SI, Lynch JR, Zaidat OO. Long term clinical and angiographic outcomes with the Wingspan stent for treatment of symptomatic 50–99% intracranial atherosclerosis: single center experience in 51 cases. Journal of NeuroInterventional Surgery. 2009 Jul 1; 1(1):40-3.
28. Daga K, Taneja M, Ahmad MT, Venketasubramanian N. Isolated Focal Basilar Artery Stenosis with Acute Stroke Treated with Emergency Thrombectomy and Stenting. Case Reports in Neurology. 2020 Dec 14; 12(Suppl. 1):27-33.
29. Berndt MT, Pree D, Kaesmacher J, Maegerlein C, Friedrich B, Zimmer C, et al. From perviousness to plaque imaging in acute basilar occlusions: the impact of underlying stenosis and how to detect it. Stroke. 2020 Mar;51(3):766-74.
30. Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurology. 2016 Feb 1;73(2):178-85.
31. Leng X, Lan L, Ip HL, Abrigo J, Scalzo F, Liu H, et al. Hemodynamics and stroke risk in intracranial atherosclerotic disease. Annals of Neurology. 2019 May;85(5):752-64.
32. Tian JW, Sun LT, Zhao ZW, Gao J. Transcranial color Doppler flow imaging in detecting severe stenosis of the intracranial vertebral artery: a prospective study. Clinical Imaging. 2006 Jan 1;30(1):1-5.
33. Schaafsma JD, Silver FL, Kasner SE, Caplan LR, Rose-Finnell L, Charbel FT, et al. VERiTAS Study Group. Infarct patterns in patients with atherosclerotic vertebrobasilar disease in relation to hemodynamics. Cerebrovascular Diseases Extra. 2020 Apr 6;9(3):123-8.
34. Caplan LR, Chung CS, Wityk RJ, Glass TA, Tapia J, Pazdera L, et al. New England medical center posterior circulation stroke registry: I. Methods, data base, distribution of brain lesions, stroke mechanisms, and outcomes. Journal of Clinical Neurology. 2005 Apr;1(1):14-30.
35. Dudvarski Stankovic N, Teodorczyk M, Ploen R, Zipp F, Schmidt MH. Microglia–blood vessel interactions: a double-edged sword in brain pathologies. Acta Neuropathologica. 2016 Mar;131(3):347-63.
36. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. Journal of Translational Medicine. 2009 Nov 17:7:97.
37. Caplan LR, Hennerici M. Impaired clearance of emboli [washout] is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998; 55(11):1475-82.
38. Grubb RL, Derdeyn CP, Fritsch SM, Carpenter DA, Yundt KD, Videen TO, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA. 23 1998;280(12):1055-60.
39. Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke. 2012;43(11):2884-91.
40. Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial [NASCET] Group. Stroke. 2000;31(1):128-32.
41. Amin-Hanjani S, Du X, Rose-Finnell L, Pandey DK, Richardson D, Thulborn KR, et al. Hemodynamic Features of Symptomatic Vertebrobasilar Disease. Stroke. 2015;46(7):1850-6.
42. Zhou Y, Hua Y, Jia L, Wang L, Liu B, Duan C, et al. Evaluation of Interventional Therapy for Patients with Intracranial Vertebral Artery Stenosis by Transcranial Color-Coded Sonography. Ultrasound Med Biol. 2016;42:44-50.
43. Shao JX, Ling YA, Du HP, Zhai GJ, Xu Y, Cao YJ. Comparison of hemodynamic changes and prognosis between stenting and standardized medical treatment in patients with symptomatic moderate to severe vertebral artery origin stenosis. Medicine (Baltimore). 2019;98:e14899.
44. Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36(6):1140-5.
45. See AP, Pandey DK, Du X, Rose-Finnell L, Charbel FT, Derdeyn CP, et al. Optimized Hemodynamic Assessment to Predict Stroke Risk in Vertebrobasilar Disease: Analysis From the VERiTAS Study. J Am Heart Assoc. 2020;9(12):e016406.
46. Lee WJ, Jung KH, Ryu YJ, Lee KJ, Lee ST, Chu K, et al. Acute Symptomatic Basilar Artery Stenosis: MR Imaging Predictors of Early Neurologic Deterioration and Long-term Outcomes. Radiology. 2016;280(1):193-201.
47. Sanossian N, Saver JL, Alger JR, Kim D, Duckwiler GR, Jahan R, et al. Angiography reveals that fluid-attenuated inversion recovery vascular hyperintensities are due to slow flow, not thrombus. AJNR Am J Neuroradiol. 2009;30(3):564-8.
48. Huang K, Yao W, Zha M, Qin S, Li Y, Xu Y, et al. Angiography-based hemodynamic features predict recurrent ischemic events after angioplasty and stenting of intracranial vertebrobasilar atherosclerotic stenosis. Eur Radiol. 2024;34(4):2352-63.
49. Assarzadegan F, Mohammadi F, Safarpour Lima B, Mansouri B, Aghamiri SH, Sahebi Vaighan N. Evaluation of neurosonology versus digital subtraction angiography in acute stroke patients. J Clin Neurosci Off J Neurosurg Soc Australas. 2021;91:378-82.
50. Koh W, Kallenberg K, Karch A, Frank T, Knauth M, Bähr M, et al. Transcranial doppler sonography is not a valid diagnostic tool for detection of basilar artery stenosis or in-stent restenosis: a retrospective diagnostic study. BMC Neurol. 2017;17(1):89.
51. Zhong J, Chen XY, Leung TWH, Ou A, Shi X, Cai Y, et al. Significance of Raised Flow Velocity in Basilar Artery in Patients with Acute Ischemic Stroke: Focal Stenosis, Coexistent Stenosis, and Collateral Flow. J Neuroimaging Off J Am Soc Neuroimaging. 2015;25(6):922-6.
52. Nasr N, Ssi-Yan-Kai G, Guidolin B, Bonneville F, Larrue V. Transcranial color-coded sonography to predict recurrent transient ischaemic attack/stroke. Eur J Neurol. 2013;20(8):1212-7.
53. Alexandrov AV, Sloan MA, Tegeler CH, Newell DN, Lumsden A, Garami Z, et al. Practice standards for transcranial Doppler (TCD) ultrasound. Part II. Clinical indications and expected outcomes. J Neuroimaging Off J Am Soc Neuroimaging. 2012;22(3):215-24.
54. Wong KS, Li H, Chan YL, Ahuja A, Lam WW, Wong A, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. 2000;31(11):2641-7.
55. Tateishi Y, Iguchi Y, Kimura K, Inoue T, Shibazaki K, Eguchi K. Contrast-enhanced transcranial color-coded duplex sonography criteria for basilar artery stenosis. J Neuroimaging Off J Am Soc Neuroimaging. 2008;18(4):407-10.
56. Postert T, Federlein J, Przuntek H, Büttner T. Power-based versus conventional transcranial color-coded duplex sonography in the assessment of the vertebrobasilar-posterior system. J Stroke Cerebrovasc Dis. 1997;6:398-404.
57. Lee SJ, Lee TK, Moon JE. Vertebral artery foraminal segment doppler sonography to detect vertebral and basilar artery stenosis or occlusion. J Neuroimaging Off J Am Soc Neuroimaging. 2023;33(5):852-9.
58. Becker G, Lindner A, Bogdahn U. Imaging of the vertebrobasilar system by transcranial color-coded real-time sonography. J Ultrasound Med. 1993; 12:395-401.
59. Shepard DL, Halsey JH Jr. Transcranial Doppler in acute brain stem infarction. Neurol Res. 1989;11:49-50.
60. Kaps M, Seidel G, Bauer T, Behrmann B. Imaging of the intracranial vertebrobasilar system using color-coded ultrasound. Stroke. 1992;23:1577-82.
61. Barrett KM, Ackerman RH, Gahn G, Romero JM, Candia M. Basilar and middle cerebral artery reserve: a comparative study using transcranial Doppler and breath-holding techniques. Stroke. 2001;32:2793-2796.
62. Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68(24):2099-106.
63. Felberg RA, Christou I, Demchuk AM, Malkoff M, Alexandrov AV. Screening for intracranial stenosis with transcranial Doppler: the accuracy of mean flow velocity thresholds. J Neuroimaging. 2002;12:9-14.
64. Mead GE, Wardlaw JM, Dennis MS, Lewis SC, Warlow CP. Relationship between pattern of intracranial artery abnormalities on transcranial doppler and Oxfordshire Community Stroke Project clinical classification of ischemic stroke. Stroke. 2000;31:714-749.
65. De Bray JM, Missoum A, Dubas F, Emile J, Lhoste P. Detection of vertebrobasilar intracranial stenoses: transcranial Doppler sonography versus angiography. J Ultrasound Med. 1997;16:213-18.
66. Brandt T, Knauth M, Wildermuth S, Winter R, von Kummer R, Sartor K, et al. CT angiography and Doppler sonography for emergency assessment in acute basilar artery ischemia. Stroke. 1999;30(3):606-12.
67. Stolz E, Nückel M, Mendes I, Gerriets T, Kaps M. Vertebrobasilar transcranial color-coded duplex ultrasonography: improvement with echo enhancement. AJNR Am J Neuroradiol. 2002;23(6):1051-4.
68. Schöning M, Walter J. Evaluation of the vertebrobasilar-posterior system by transcranial color duplex sonography in adults. Stroke. 1992;23:1280-86.
69. Koga M, Kimura K, Minematsu K, Yamaguchi T. Relationship between findings of conventional and contrast-enhanced transcranial color-coded real-time sonography and angiography in patients with basilar artery occlusion. AJNR Am J Neuroradiol. 2002;23(4):568-71.
70. Palazzo P, Ruff M, Lyerly MJ, Alexandrov AV. Basilar artery thrombus vs. fenestration: a differential diagnostic challenge in acute ischemic stroke. J Neuroimaging. 2014;24:607-09.
71. Pade O, Eggers J, Schreiber SJ, Valdueza J. Complete basilar artery assessment by transcranial color-coded duplex sonography using the combined transforaminal and transtemporal approach. Ultraschall Med Stuttg Ger 1980. 2011;32 Suppl 2:E63-8.
72. Oehm E, Els T, Spreer J, Kassubek J, Hetzel A. Transcranial color-coded sonography in basilar artery stenting. Ultrasound Med Biol. 2002;28:383-87.
73. Vicenzini E, Puccinelli F, Ricciardi MC, Guidetti G, Delfini R, Lenzi GL. Contrast-enhanced transcranial color-coded duplex sonography versus computed tomography and magnetic resonance angiography in the follow-up of basilar stenting. J Ultrasound Med. 2007;26:543-46.
74. Baumgartner RW, Mattle HP, Schroth G. Assessment of >/=50% and <50% intracranial stenoses by transcranial color-coded duplex sonography. Stroke. 1999;30(1):87-92.
75. Bartels E, Fuchs HH, Flügel KA. Color Doppler imaging of basal cerebral arteries: normal reference values and clinical applications. Angiology. 1995;46:877-84.
76. Zhao L, Barlinn K, Sharma VK, Tsivgoulis G, Cava LF, Vasdekis SN, et al. Velocity criteria for intracranial stenosis revisited: an international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke. 2011;42(12):3429-34.
77. Fujiwara Y, Kuroda H, Abe T, Ishida K, Oguri T, Noguchi S, et al. The B-Mode Image-Guided Ultrasound Attenuation Parameter Accurately Detects Hepatic Steatosis in Chronic Liver Disease. Ultrasound Med Biol. 2018;44(11):2223-32.
78. Klötzsch C, Bozzato A, Lammers G, Mull M, Noth J. Contrast-enhanced three-dimensional transcranial color-coded sonography of intracranial stenoses. AJNR Am J Neuroradiol. 2002;23:208-212.
79. Rorick MB, Nichols FT, Adams RJ. Transcranial Doppler correlation with angiography in detection of intracranial stenosis. Stroke. 1994;25:1931-34.
80. Csiba L, Baracchini C. Manual of Neurosonology. Cambridge University Press Ed; 2016.
81. Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69(6):963-74.
82. Baumgartner RW, Baumgartner I, Mattle HP, Schroth G. Transcranial color-coded duplex sonography in the evaluation of collateral flow through the circle of Willis. AJNR Am J Neuroradiol. 1997;18:127-33.
83. Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Rossini PM, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001;32(7):1552-8.
84. Tayeshi Y, Iguchi Y, Kimura K, Inoue T, Shibazaki K, Eguchi K. Contrast-enhanced transcranial color-coded duplex sonography criteria for basilar artery stenosis. J Neuroimaging. 2008;18:407-10.
85. Danyel LA, Hadzibegovic S, Valdueza JM, Tietze A, Fuchs S, Schreiber SJ, et al. Classification of Intracranial Stenoses: Discrepancies between Transcranial Duplex Sonography and Computed Tomography Angiography Ultrasound Med Biol 202;46:1889-95.
86. Yang J, Hua Y, Li X, Gao M, Li Q, Liu B, et al. The Assessment of Diagnostic Accuracy for Basilar Artery Stenosis by Transcranial Color-Coded Sonography Ultrasound Med Biol. 2018;44:995-1002.
87. Xu X, Raynald, Li X, Li R, Yang H, Zhao X, Miao Z, et al. New evidence for fractional pressure ratio prediction by pulsatility index from transcranial Doppler in patients with symptomatic cerebrovascular stenosis disease. Quant Imaging Med Surg. 2024;14:264-72.
88. Okamura M, Takekawa H, SJSUM, Okabe R, Suzuki K, Hirata K. Vertebral artery Doppler waveform patterns for exclusive diagnosis of basilar artery stenosis and occlusion. J Med Ultrason (2001). 2016;43:83-89.
89. Yoo IH, Kim JM, Han SH, Ryu J, Jung KH, Park KY. Increased pulsatility index of the basilar artery is a risk factor for neurological deterioration after stroke: a case control study. Clin Hypertens. 2022;28:27.