Abstract
Lung cancer represents the second malignancy in both men and women and the first cause of cancer-related mortality. Because of worldwide diffusion of lung cancer screening programs, major lung resections for initial stage diseases have been increasingly performed in past decades. Video-assisted thoracic surgery (VATS) emerged as a new and feasible approach representing a viable alternative to classic open thoracotomy. Between January 2017 and September 2018, we performed 130 VATS major lung resections with uniportal technique. We compared this sample to 48 previous or simultaneous lobectomies performed with a lateral thoracotomy (NO-VATS group). Total morbidity resulted significantly lower in the VATS group (p=0.0046). VATS technique resulted in a significant mean decrease in time of surgery of 20 minutes (p=0.013). We did not observe a significant difference in the number of nodes sampled. VATS technique resulted in an average of 3.57 days reduction in hospital stay (5.45 vs 9.02, 95% CI: 1.27-5.85; significant with t-test: p=0.0028). We compared post-operative pain among the two groups: we observed a small advantage for pain level in the VATS group, but without statistical significance. Surgical costs were significantly greater for VATS (p<0.05) while recovery costs were higher for NO-VATS (p<0.05). Overall costs resulted to be lower in the VATS group (significant with t-test, p=0.026). VATS showed same results in terms of oncological outcome compared to lateral thoracotomy. We observed an advantage in VATS group patients according to morbidity, pain level, hospital stay and overall costs.
Keywords
Uniportal-VATS, Early stage lung cancer, Surgical morbidity, Costs analysis
Abbreviations
VATS: Video-Assisted Thoracic Surgery; RATS: Robotic-Assisted Thoracic Surgery; OR: Operating Room; ICU: Intensive Care Unit; IQRs: Interquartile Ranges (IQRs); ANOVA: Analysis of Variance
Background
In last decades, video-assisted thoracic surgery (VATS) together with robotic-assisted thoracic surgery (RATS) can be considered the biggest innovation in thoracic surgery. This approach drastically changed the way of performing surgical operations, improving patient’s outcome undergoing thoracic surgery. Initially performed for minor pulmonary resections [1], VATS has been subsequently applied for major operations such as lobectomies with many authors pointing out possible risks in terms of oncological radicality, safety (complications and mortality), advantages for patients in terms of pain level and better quality of life. Initial worries have been completely wiped out with the improvement of surgical equipment and the development of new technologies, with more and more sophisticated and demolitive surgical operations being performed, with indications ranging from pulmonary or mediastinal biopsies to lung cancer major resections [2].
The evidence about the advantages of VATS in terms of reduction in patient’s morbidity and better surgical outcomes has increased [3-13] showing that, when compared to open thoracotomy, VATS lobectomy allows a faster post-operatory recovery, a decreased incidence of prolonged air leak, arrhythmias, pneumonia and pain, a reduction of inflammatory parameters and a better esthetic outcome [14-20]. As such, VATS progressively replaced traditional approach for minor pulmonary resections [21].
In today’s cost-conscious health care environment, it is necessary to assess the real burden of VATS lobectomy in terms of costs and to prove whether above-mentioned advantages justify the use of this procedure: indeed, some studies focused on the economic analysis of VATS procedure [22-29]. The present study reports the experience in our institution facing VATS in lung cancer major resections, analyzing data in terms of oncological results, morbidity, mortality and hospitalization costs.
Methods
Patients recruitment
We retrospectively reviewed clinical and surgical outcomes of pulmonary lobectomies performed between January 2017 and September 2018 at our institution. Patients were stratified in two groups - namely “VATS” and “NO-VATS group” - according to the undertaken procedure; patients undergoing conversion thoracothomy during VATS procedure were allocated in the VATS group. Most of these procedures were performed for initial stage Non-small-cell lung carcinoma (NSCLC). Demographics, clinical and surgical data were gathered, retrospectively analyzed and stratified based on the chosen surgical approach. 30 days morbidity and mortality rates were evaluated. The eligibility criteria for VATS lobectomy included: disease confined to the lung parenchyma, lesion’s diameter smaller than 5 cm and absent N1 or N2 lymph node involvement from the pre-operatory radiological investigations (CT and PET scan).
Surgical approaches
We perform video-assisted lobectomy procedures with a mini-thoracotomy on the 4h-5th intercostal space at the anterior axillary line level, following Gonzales- Riva’s method [30]. The same access is then used to insert pleural drainage at the end of the operation; in case of conversion, thoracotomies are mostly performed laterally. Both the surgeon and the assistant stand in front of the patient in order to have the same thoracoscopic view during all the steps of the procedure and to allow more coordinated movements. The scrub nurse should be positioned behind the patient.
Although vision is only obtained through the front access site, the combined movements of the thoracoscope along the incision will create different viewing angles (the 10 mm 30 degrees thoracoscope is strongly recommended for a broad vision). Coordinating the thoracoscope with the other instruments, we get a targeted vision which allows us to place surgical instruments in order to focus the target tissue from a sagittal perspective. The instrument must be long and curved to allow their simultaneous insertion. Proper lung exposure is essential to facilitate the dissection of structures and to prevent interference between the different instruments.
The patient is placed in a lateral decubitus position in the same manner as in the conventional VATS, under general anesthesia and ventilated with a double lumen tube. The incision, about 4-5 cm long, is preferably performed at the fourth or fifth intercostal space in the anterior axillary line. From this position, according to the author, we are able to acquire the best angles to perform the dissection of the hilum and the insertion of the staplers.
In the minimally invasive approach the major vascular structures are generally managed with the use of staplers, as well as the lobar bronchus: in our Thoracic Surgery Unit there are two types of mechanical staplers and relative cartridges: the ECHELON FLEX™ Powered Vascular StaplerFlex 35 mm (Ethicon) and the ECHELON FLEX™ ENDOPATH® Staplers (45 mm). For most surgical operations the thoracoscope is placed backward to the thoracotomy site, while the anterior part is thought for the rest of surgical equipment. For the lower lobectomies the normal dissection sequence is: pulmonary ligament, inferior pulmonary vein, pulmonary artery, bronchus and finally completion of the fissure. In case of superior lobectomies, the pulmonary artery is normally dissected first, followed by the dissection of the vein, the bronchus and ultimately the fissure.
When the lobectomy is completed, the lobe is removed via an Endo-bag and then a routine lymph node dissection is performed. A drainage tube is inserted at the back of the thoracotomy. Besides the canonical contraindications of VATS Lobectomy, Gonzalez-Rivas adds the discomfort for the surgeon and the presence of voluminous neoplasms that require the enlargement of the thoracotomy [30]. Regardless of the surgical approach, hylar dissection and lymphadenectomy were performed following the same steps.
Postoperative management
Thoracic operations were performed together with a dedicated anesthesiological team. Most patients belonging to the NO-VATS group received a perioperative analgesic therapy administrated with different approaches (such as opiods and/or NSAID i.v. or epidural analgesia with naropine) and after 24 hours they were shifted to a standard anthalgic therapy based on paracetamol (1g every 8 hours i.v.) with NSAID (30 mg i.v.) for breakthrough pain.
Patients managed with a VATS approach underwent a peri-operative eco-guided serratus plane analgesic block generally performed 1 hour before surgery in the operating room by the anesthesiologist.
Chest drainage (digital Thopaz – Medela©) were kept to suction until perioperative chest X-ray execution and were then switched to valve in case of good lung expansion. Criteria for chest drainage removal were an output lower than 200 cc/day and absence of air leakage.
Regardless of the type of surgical approach they underwent, in our Unit patients were considered to be dischargeable if in a good general condition, with good pain control and after pleural drain removal - that is when the imaging yields a good radiological picture.
Cost Analysis
Hospitalization costs were calculated considering together disposable devices, operating room (OR) occupation time, staff, instrumental investigations (e.g. chest radiographies) and the daily cost of conventional ward and/or intensive care unit (ICU) stay; all data were provided by the financial department of our institution. Operation time and in-hospital stays were collected retrospectively from the company databases; several disposable devices consumption has been deduced from the operative notes. Items common to both approaches were not taken into consideration (e.g. the cost of double-lumen orotracheal tube, gowns and gloves, etc.). In the same way, the cost of the multi-use material (e.g. surgical instruments, monitors, ventilators, etc.), either purchased or rented, was not taken into consideration in the cost analysis since it is part of the usual supply of an Operating Unit of Thoracic Surgery. All the costs were determined and stratified according to the type of surgical approach.
Statistics and data handling
Mean and standard deviation or median and interquartile ranges (IQRs) were used to describe, respectively, parametric and non-parametric data. Unpaired T tests was used to investigate differences between unpaired groups of parametric data; analysis of variance (ANOVA) was used for the same purpose when comparing more than 2 groups. Mann Whitney test was used to compare the difference between non-parametric distributions. Chi squared test was used to investigate differences in frequencies of categorical data. All data were imported and handled with R and R studio (v 3.5.1). R package ggplot2 was used to make plots.
Results
Patients characteristics
A total of 178 pulmonary lobectomies were performed: 130 patients in the VATS group and 48 patients in the NO-VATS group. Patients demographics (age, sex) and cancer histotypes and stages are shown in (table 1). Moderate imbalances can be observed for sex, cancer histotypes and stages.
| NO-VATS (n=48) | VATS (n=130) | p value | |
|---|---|---|---|
| Age | 68.5 ± 16.7 | 69.0 ± 18.0 | 0.7054 |
| Sex | 0.8081 | ||
| Female | 15 (31.0%) | 45 (35.0%) | |
| Male | 33 (69.0%) | 85 (65.0%) | |
| Histotype | 0.3675 | ||
| adenocarcinoma | 23 (48.0%) | 90 (69.0%) | |
| adenosquamous | 0 (0.0%) | 2 (1.5%) | |
| squamous cells | 10 (21.0%) | 15 (11.5%) | |
| large cells neuroendocrine | 4 (8.0%) | 9 (7.0%) | |
| small cells neuroendocrine | 1 (2.0%) | 1 (1.0%) | |
| other tumors | 5 (11.0%) | 2 (1.5%) | |
| metastases | 1 (2.0%) | 3 (2.5%) | |
| not cancer | 4 (8.0%) | 8 (6.0%) | |
| Stage | 0.0742 | ||
| 0 | 0 (0.0%) | 0 (0.0%) | |
| I | 14 (29.1%) | 81 (62.3%) | |
| II | 8 (16.6%) | 19 (14.7%) | |
| III | 16 (33.3%) | 17 (13.0%) | |
| not primary lung cancer | 10 (21.0%) | 13 (10.0%) | |
| Lobe | 0.4679 | ||
| Bilobectomy | 3 (6.0%) | 2 (2.0%) | |
| RLL | 6 (12.0%) | 27 (21.0%) | |
| LLL | 6 (12.0%) | 20 (15.0%) | |
| ML | 3 (6.0%) | 6 (5.0%) | |
| RUL | 17 (35.0%) | 42 (32.0%) | |
| LUL | 13 (27.0%) | 33 (25.0%) |
As illustrated, in 153 patients there was a pathologic diagnosis of primary NSCLC lung cancer, in 2 case we found a small cell lung cancer (SCLC), 4 patients were diagnosed with metastasis from other primitive cancers, 7 patients with other rare tumors and 12 patients did not receive a diagnosis of cancer.
Lobe; ML: Middle Lobe; RUL: Right Upper Lobe; LUL: Left Upper Lobe.
Clinical outcomes
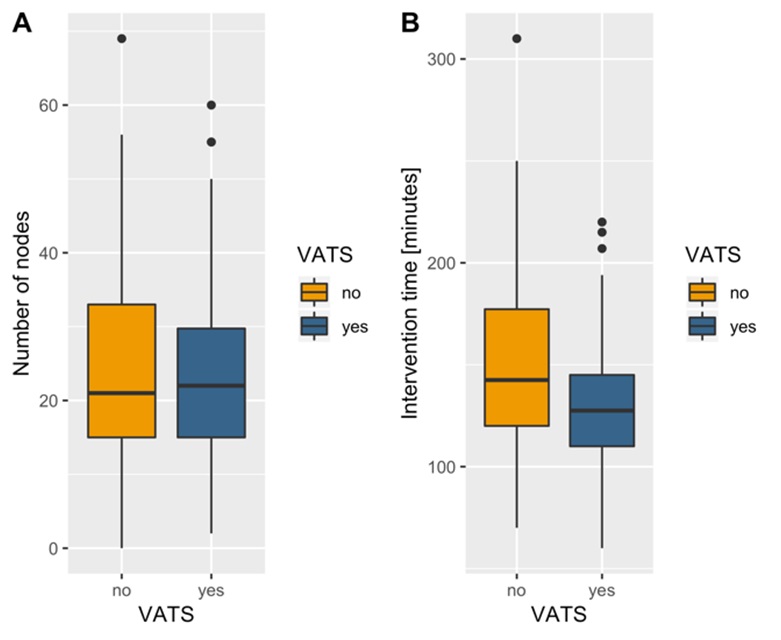
VATS techniques resulted in a mean decrease in operating time of about 20 minutes (128.9 ± 28.04 minutes for VATS, 148.8 ± 49.16 for NO-VATS, p=0.01; (Figure 1). We did not observe a significant difference in the number of nodes sampled between VATS and NOVATS groups: the mean number of nodes sampled was 23.3 in the VATS cohort and 24.1 in the NO-VATS one (p=0.75), (Figure 1).
Figure 1. Surgical outcomes.
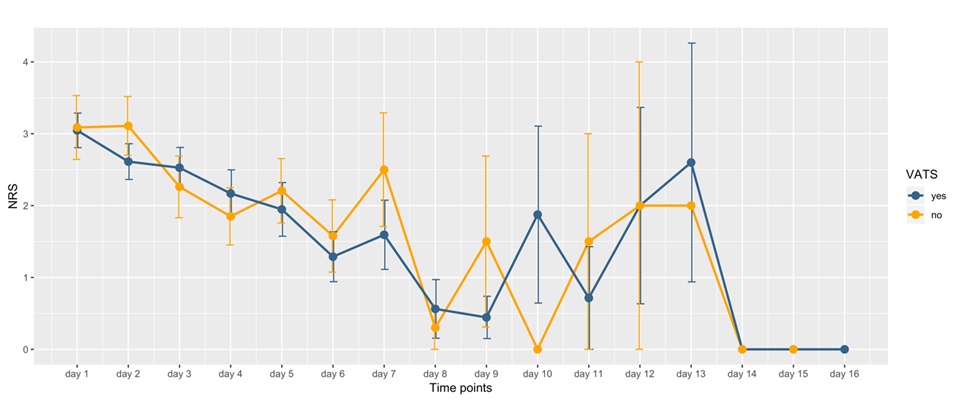
We next compared day-by-day collected self-reported post-operative pain among the two groups. In the first week - except for days 3 and 4 - the mean pain level was higher in the NO-VATS group; this difference, though, didn’t reach statistical significance (Figure 2). A comparison of the pain levels after the eight day is harder due to the great amount of discharged patients and the subsequent decrease in the numerosity of the population.
Figure 2. Pain level.
Finally, the median of doses of as needed pain medications was lower in the VATS group; nevertheless, this didn’t reach statistical significance (p=0.54).
Incidence of 30 days postoperative complications are shown in (Table 2). We considered broncho-pleural, respiratory failure, acute kidney injury and acute coronary syndrome as major complications. Prolonged air leak was the most frequent event in both groups but its incidence was higher in NO-VATS group (not significant, p=0.5536).
| complication | NO- VATS (n=48) | VATS (n=130) | p value |
|---|---|---|---|
| Wound infections | 4 (8%) | 0 (0%) | 0.0057 |
| Prolonged air leak | 15 (31%) | 33 (25%) | 0.5536 |
| Anemia | 4 (8%) | 4 (3%) | 0.2737 |
| Arrhythmias | 5 (1%) | 3 (2%) | 0.0561 |
| Any minor complication | 27 (56%) | 41 (32%) | 0.0045 |
| Broncho-pleural fistula |
1 (2%) | 1 (1%) | 1 |
| Respiratory failure | 4 (8%) | 2 (2%) | 0.0781 |
| Acute kidney injury | 1 (2%) | 5 (4%) | 0.9120 |
| Acute coronary syndrome | 2 (4%) | 0 (0%) | 0.1237 |
| Any major complication | 7 (15%) | 7 (5%) | 0.0873 |
| Any complication | 29 (60%) | 46 (35%) | 0.0046 |
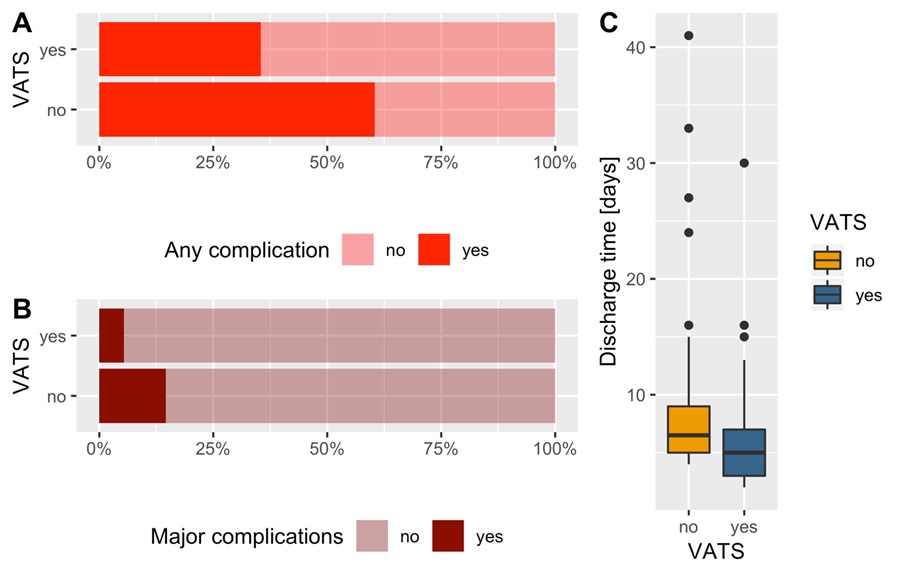
Minor complications rate was significantly lower in the VATS group (p=0.0045) and the same we found out for major complications but without reaching the statistical significance (p=0.0873).
Total morbidity (including major and minor complications) resulted to be significantly lower in the VATS group (p=0.0046).
VATS technique operations led to a significant reduction in mean hospital stay: patients undergoing VATS had an average earlier discharge of 3.57 days compared to the NO-VATS group (5.45 ± 3.46 days vs 9.02 ± 7.62 days, p=0.0028; Figure 3). We did not observe in both our cohort’s perioperative mortality cases or cases of readmission within 30 days from the operation.
Figure 3. Surgical complications.
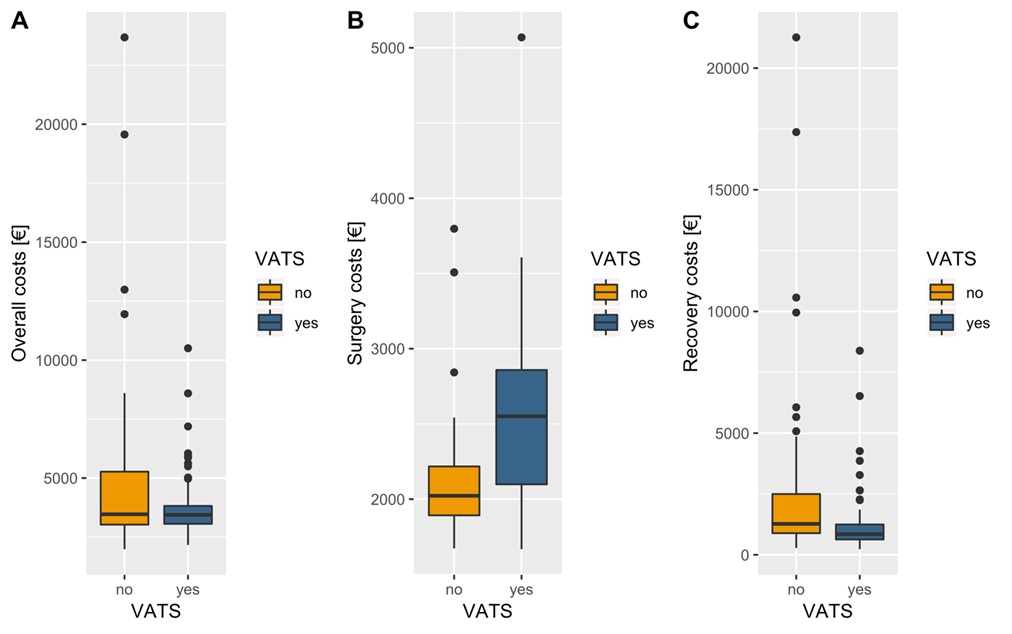
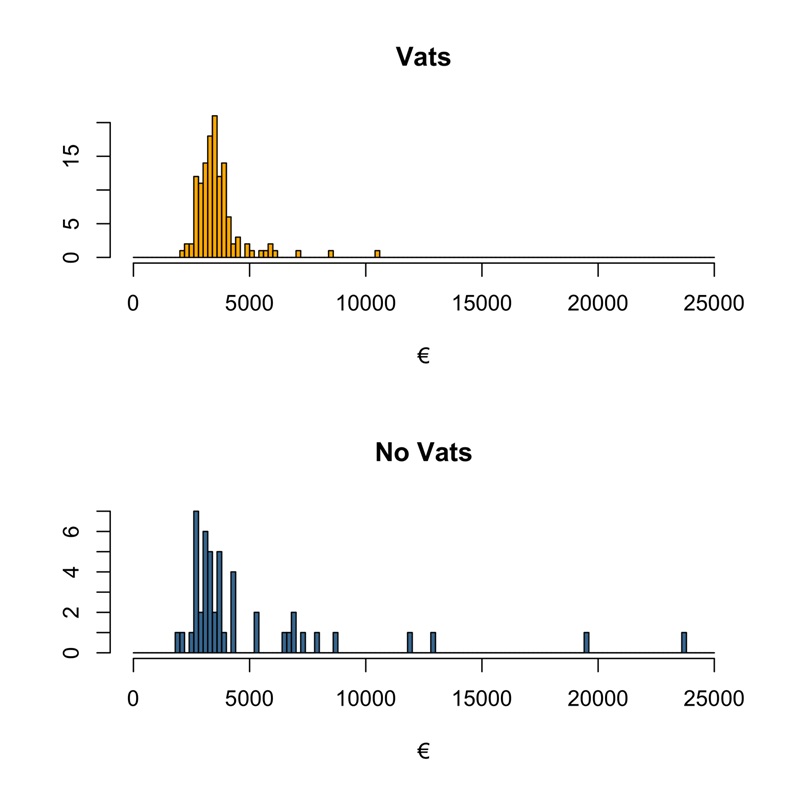
As expected, the surgical costs were sensibly higher for VATS procedures (€ 2546.26 ± 489.12) than NO-VATS ones (€ 2138.58 ± 451.99) mainly because of a larger use of staplers and the disposable instruments needed (p<0.05); (Figure 4). Conversely, the hospital stay costs were significantly higher for the NO-VATS group (€ 2899.57 ± 4144.37) than the VATS group (€ 1084.71 ± 1047.11, p<0.05; (Figure 4). Finally, despite the greater surgical cost, mean overall costs turn up to be lower for the VATS group (€ 3625.11 ± 1079.13) than the NO-VATS group (€ 5038.16 ± 4211.80) with statistical significance (p= 0.026), (Figure 4).
Figure 4. A. Overall costs; B. Surgery costs; C. Recovery costs.
(Figure 5) shows the impact of outliers in the mean costs: indeed, in the VATS group there is a significant lower number of patients exceeding the cost of 5000 € (27.1% for NO-VATS and 7.0% in VATS, p=0.0007). This is probably due to a longer stay in ICU that we observed in these outliers’ patients which is due to the higher complications incidence that we found in the NO-VATS group.
Figure 5. Procedural costs.
Discussion
Lung cancer represents the second malignancy in both men and women and the first cause of cancerrelated mortality. Because of the worldwide diffusion of lung cancer screening programs [31,32], major lung resections for initial stage diseases (stage I and II) have been increasingly performed in past decades even thanks to advances in surgical techniques and equipment and the development of new technologies [33,34]. In this scenario, VATS emerged as a new and feasible approach representing a viable alternative to classic open thoracotomy.
With the widespread application of VATS for major lung resections, many authors reported different experiences, trying to investigate the efficacy and feasibility of this technique mainly in oncological terms. Despite VATS advantages have been already largely evaluated and approved, its costs have been considered as an obstacle to the diffusion of the thoracoscopic technique [35,36].
Gossot et al. reported that VATS surgical costs resulted to be higher than classical thoracotomy with a small but significant difference (€ 2861 ± 458 vs € 2260 ± 398, p<0.001) [36]. Other authors, such as Casali and Walker outlined a greater difference in costs among the two techniques (€ 2533 ± 230 vs € 1280 ± 54, p<0.001) [23].
As illustrated, we found similar results with VATS lobectomy having significantly higher surgery-related costs compared to NO-VATS. This data came out from the addition of operating room costs, the staplers’ cartridge and fixed costs of both approaches. The operating room costs are mainly related to the surgery’s duration and the equipment costs (e.g. staplers’ cartridge and the endobags). The staplers’ cartridge cost is obviously determined by their usage, sensibly higher in thoracoscopic procedures. The employment of the endobags is necessary to remove the anatomical pieces in order to reduce the risk of malignant cells’ implants in the surgical accesses [37,38].
About the OR time, in our experience the surgical time in the NO-VATS group was longer of an average of 20 minutes (p= 0.013); conversely Gossot reports that his thoracoscopic technique was more time consuming than a posterolateral thoracotomy (time difference of about 77 minutes), with much greater averages compared to the other authors [36].
Focusing on the different kind of lobectomies, in the VATS group we observed an higher surgical cost for upper-left lobectomies (€2895.84 ± 446,96) compared to the others (€2255.91 ± 267.74 for lower-right lobectomy, €2397.04 ± 340.14 for middle lobectomies, €2519.60 ± 356.68 for upper-right lobectomy and €2283.05 ± 277.33 for lower-left lobectomy): this is probably due to the longer duration and the use of an higher number of staplers’ cartridge that are related to this procedure. This data is in contrasts with other authors, who found the upper-right lobectomy to be the most expensive procedure to be performed with VATS.
Regarding the hospital stay, we highlighted significant differences between the two groups: patients who underwent VATS had a reduction in hospital stay of 3.57 days. This evidence, together with the lower length of ICU stay and number of chest radiographies performed in the VATS group, can explain the significant difference observed in the overall cost.
The decrease in the hospital stay that we reported for VATS is also related to our improvement in postoperative management. Indeed, we have replaced the most traditional draining systems with the latest ones, currently used in our Unit (e.g. Thopaz – Medela©), equipped with a digital display: this could provide real time objective data on air leakage and liquid output which allowed us to easily monitor the clinical progression. Even though the difference was not significant, the advantage in pain control for the VATS group allowed us to perform an immediate post-operative respiratory kinesis physiotherapy with these patients.
In our economic analysis, we did not included outpatient clinical related costs and this element could enhance the advantage of performing VATS rather than open surgery.
Indeed, Bendixen et al. has recently reported a costeffectiveness analysis based on their randomized controlled trial where they compared VATS against lateral thoracotomy for stage I lung cancer [28]. Nevertheless, VATS showed a brief hospital stay, recovery costs resulted to be similar for both the approaches, but assessing the overall costs - where they also included outpatient clinical charge (e.g. number of medicine’s prescription, number of general practitioner visits, etc.) - VATS resulted to be cheaper than lateral thoracotomy.
Conclusions
VATS yielded similar surgical outcomes to NO-VATS in terms of sampled nodes and resulted in a significant reduction in the OR occupation. Patients who underwent VATS had a reduction in postoperative pain level and a lower request for breakthrough pain medications. VATS technique resulted in a significant reduction in mean hospital stay and a lower postoperative complication rate. Our study confirmed that VATS had higher surgical costs than lateral thoracotomy but it assessed that VATS yielded a significant reduction in hospital stay costs and this resulted in a reduction of the overall cost for VATS compared to NO-VATS.
In conclusion, being VATS a technique that reduces postoperative pain, it would grant a better quality of life so that patients would be more responsive to a fast recovery in order to go back to their everyday activities and their jobs, reducing the indirect healthcare costs. In the current epidemiological panorama, this data appears to be noteworthy because lung cancer is diagnosed earlier and earlier, meaning a younger population that has to undergo surgery.
This study recognizes some limitations: first of all, it is not a randomized, retrospective study so, nevertheless the ethical issue, it would be helpful to lead a prospective study to assure and confirm these evidence and to prevent selection bias; we had a limited sample size, due to the initial stage of our experience with VATS lobectomy; in our economic analysis we did not included outpatient clinical related costs and this element could enhance the advantage of performing VATS rather than open surgery.
Conflict of Interest Statement
The authors declare no conflict of interest.
Author Contributions Statement
PC and GT designed the study. Data acquisition was carried out by GiT, FL, FC. Data analysis, interpretation and statistical analysis were performed by PM, GiT, LF, SS and FaC. Manuscript editing and review were performed by GT and FaC. Due to initials similarity, the corresponding author specifies that GT stands for Giuseppe Tonini, GiT for Giovanni Tacchi, FC for Filippo Carannante and FaC for Fabrizio Citarella.
References
2. Zhao Y, Chen H. Direct comparison between videoassisted thoracoscopic surgery and muscle-sparing minithoracotomy in the era of minimally invasive thoracic surgery. The Journal of Thoracic and Cardiovascular Surgery. 2018 Mar;155(3):1307-8.
3. Boffa DJ, Dhamija A, Kosinski AS, Kim AW, Detterbeck FC, Mitchell JD, Onaitis MW, Paul S. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. The Journal of Thoracic and Cardiovascular Surgery. 2014 Aug 1;148(2):637-43.
4. Cao C, Manganas C, Ang SC, Peeceeyen S, Yan TD. Videoassisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interactive Cardiovascular and Thoracic Surgery. 2013 Mar 1;16(3):244-9.
5. Falcoz PE, Puyraveau M, Thomas PA, Decaluwe H, Hurtgen M, Petersen RH, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. European Journal of Cardio-Thoracic Surgery. 2016 Feb;49(2):602-9.
6. Murakawa T, Ichinose J, Hino H, Kitano K, Konoeda C, Nakajima J. Long-term outcomes of open and videoassisted thoracoscopic lung lobectomy for the treatment of early stage non-small cell lung cancer are similar: a propensity-matched study. World Journal of Surgery. 2015 May 1;39(5):1084-91.
7. Scott WJ, Matteotti RS, Egleston BL, Oseni S, Flaherty JF. A comparison of perioperative outcomes of videoassisted thoracic surgical (VATS) lobectomy with open thoracotomy and lobectomy: results of an analysis using propensity score based weighting. Annals of Surgical Innovation and Research. 2010 Dec 1;4(1):1.
8. Smith CB, Kale M, Mhango G, Neugut AI, Hershman DL, Mandeli JP, Wisnivesky JP. Comparative outcomes of elderly stage I lung cancer patients treated with segmentectomy via video-assisted thoracoscopic surgery versus open resection. Journal of Thoracic Oncology. 2014 Mar 1;9(3):383-9.
9. Yang HX, Woo KM, Sima CS, Bains MS, Adusumilli PS, Huang J, Finley DJ, Rizk NP, Rusch VW, Jones DR, Park BJ. Long-term survival based on the surgical approach to lobectomy for clinical stage I non-small cell lung cancer: comparison of robotic, video assisted thoracic surgery, and thoracotomy lobectomy. Annals of Surgery. 2017 Feb;265(2):431.
10. Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy—video-assisted thoracic surgery versus muscle-sparing thoracotomy: a randomized trial. The Journal of Thoracic and Cardiovascular Surgery. 1995 May 1;109(5):997-1002.
11. Sugi K, Kaneda Y, Esato K. Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World Journal of Surgery. 2000 Jan 1;24(1):27-31.
12. Craig SR, Leaver HA, Yap PL, Pugh GC, Walker WS. Acute phase responses following minimal access and conventional thoracic surgery. European Journal of Cardio-Thoracic Surgery. 2001 Sep 1;20(3):455-63.
13. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. The Lancet Oncology. 2016 Jun 1;17(6):836-44.
14. Cao C, Manganas C, Ang SC, Yan TD. A meta-analysis of unmatched and matched patients comparing videoassisted thoracoscopic lobectomy and conventional open lobectomy. Annals of Cardiothoracic Surgery. 2012 May;1(1):16.
15. Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. The Annals of Thoracic Surgery. 2008 Dec 1;86(6).
16. Taioli E, Lee DS, Lesser M, Flores R. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. European Journal of Cardio-Thoracic Surgery. 2013 Oct 1;44(4):591-7.
17. Zhang Z, Zhang Y, Feng H, Yao Z, Teng J, Wei D, Liu D. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. European Journal of Cardio-Thoracic Surgery. 2013 Sep 1;44(3):407-14.
18. Chen FF, Zhang D, Wang YL, Xiong B. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: a meta-analysis. European Journal of Surgical Oncology (EJSO). 2013 Sep 1;39(9):957-63.
19. Li Z, Liu H, Li L. Video-assisted thoracoscopic surgery versus open lobectomy for stage I lung cancer: A metaanalysis of long-term outcomes. Experimental and therapeutic medicine. 2012 May 1;3(5):886-92.
20. Cai YX, Fu XN, Xu QZ, Sun W, Zhang N. Thoracoscopic lobectomy versus open lobectomy in stage I non-small cell lung cancer: a meta-analysis. PloS one. 2013;8(12).
21. Yan TD, Cao C, D’amico TA, Demmy TL, He J, Hansen H, Swanson SJ, Walker WS, International VATS Lobectomy Consensus Group, Casali G, Dunning J. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. European journal of cardio-thoracic surgery. 2014 Apr 1;45(4):633-9.
22. Burfeind Jr WR, Jaik NP, Villamizar N, Toloza EM, Harpole Jr DH, D’Amico TA. A cost-minimisation analysis of lobectomy: thoracoscopic versus posterolateral thoracotomy. European journal of cardio-thoracic surgery. 2010 Apr 1;37(4):827-32.
23. Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: can we afford it?. European journal of cardiothoracic surgery. 2009 Mar 1;35(3):423-8.
24. Sugi K, Kaneda Y, Nawata K, Fujita N, Ueda K, Nawata S, Esato K. Cost analysis for thoracoscopy: thoracoscopic wedge resection and lobectomy. Surgery today. 1998 Jan 1;28(1):41-5.
25. Lewis RJ, Caccavale RJ, Sisler GE, Bocage JP, O’Brien K, Warner S. Is video-assisted thoracic surgery cost effective?. New Jersey medicine: the journal of the Medical Society of New Jersey. 1996 Dec;93(12):35-41.
26. Liu HP, Wu YC, Liu YH, Hsieh MJ, Cheng KS, Chu JJ, Lin PJ. Cost-effective approach of video-assisted thoracic surgery: 7 years experience. Chang Gung medical journal. 2000 Jul;23(7):405-12.
27. Nakajima J, Takamoto S, Kohno T, Ohtsuka T. Costs of videothoracoscopic surgery versus open resection for patients with of lung carcinoma. Cancer. 2000 Dec 1;89(S11):2497-501.
28. Bendixen M, Kronborg C, Jørgensen OD, Andersen C, Licht PB. Cost–utility analysis of minimally invasive surgery for lung cancer: a randomized controlled trial. European Journal of Cardio-Thoracic Surgery. 2019 Oct 1;56(4):754-61.
29. Farjah F, Backhus LM, Varghese TK, Mulligan MS, Cheng AM, Alfonso-Cristancho R, Flum DR, Wood DE. Ninety-day costs of video-assisted thoracic surgery versus open lobectomy for lung cancer. The Annals of thoracic surgery. 2014 Jul 1;98(1):191-6.
30. Gonzalez-Rivas D, Paradela M, Fernandez R, Delgado M, Fieira E, Mendez L, Velasco C, de la Torre M. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. The Annals of thoracic surgery. 2013 Feb 1;95(2):426-32.
31. Crucitti P, Gallo IF, Santoro G, Mangiameli G. Lung cancer screening with low dose CT: experience at Campus Bio-Medico of Rome on 1500 patients. Minerva chirurgica. 2015 Dec;70(6):393-9.
32. Mangiameli G, Longo F, Grasso RF, Iacopino A, Rocco R, Quintarelli F, Crucitti P. Focus on lung cancer screening program at Campus Bio-Medico of Rome: update on over 3250 patients. Minerva chirurgica. 2017 Aug;72(4):361-3.
33. Rocco R, Incalzi RA, Pennazza G, Santonico M, Pedone C, Bartoli IR, Vernile C, Mangiameli G, La Rocca A, De Luca G, Rocco G. BIONOTE e-nose technology may reduce false positives in lung cancer screening programmes. European Journal of Cardio-Thoracic Surgery. 2016 Apr 1;49(4):1112-7.
34. Rocco G, Pennazza G, Santonico M, Longo F, Rocco R, Crucitti P, Incalzi RA. Breathprinting and early diagnosis of lung cancer. Journal of thoracic oncology. 2018 Jul 1;13(7):883-94.
35. Cho S, Do YW, Lee EB. Comparison of costs for videoassisted thoracic surgery lobectomy and open lobectomy for non-small cell lung cancer. Surgical endoscopy. 2011 Apr 1;25(4):1054-61.
36. Ramos R, Masuet C, Gossot D. Lobectomy for earlystage lung carcinoma: a cost analysis of full thoracoscopy versus posterolateral thoracotomy. Surgical endoscopy. 2012 Feb 1;26(2):431-7.
37. Onaitis MW, Petersen RP, Balderson SS, Toloza E, Burfeind WR, Harpole Jr DH, D’Amico TA. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Annals of surgery. 2006 Sep;244(3):420.
38. Burfeind WR, D’Amico TA. Thoracoscopic lobectomy. Operative techniques in thoracic and cardiovascular surgery. 2004 Jun 1;9(2):98-114.