Abstract
Introduction: Proton pump inhibitors (PPIs) are among the most commonly prescribed medications, and are often prescribed for unnecessarily extended courses. A variety of adverse outcomes have been attributed to prolonged PPI use. Outpatient deprescribing strategies aimed at decreasing the burden of these adverse effects have been infrequently described.
Materials and Methods: This was a retrospective before-after observational cohort study of a quality improvement program conducted at an internal medicine residency continuity clinic. The intervention was two-tiered. The first part of our intervention was a 15-minute presentation explaining the indications for discontinuing PPI therapy and the methods by which to do so (tapering versus on-demand). Secondly, an alert prompting the consideration of a PPI Discontinuation Protocol was attached to electronic medical record (EMR) patient records with ICD 10 codes commonly associated with PPI prescriptions. Appropriate PPI indication was based on published guidelines, and patients without an appropriate indication or exceeding the recommended course were targeted for discontinuation. Data collected one year prior to and six months after intervention implementation were compared. All data included patients’ demographics, ICD 10 diagnoses which indicated PPI therapy, whether or not the patient was on a PPI, have they ever attempted discontinuation, as well as other data regarding type of PPI with dosages, adverse effects, duration of therapy and whether or not the PPI was ultimately discontinued.
Results: Of the 210 patient records reviewed, 115 patients were prescribed PPIs. Comparing pre intervention to post intervention data, a Pearson Chi Square analysis was performed which did not reveal a statistical difference between the rate of PPI discontinuation attempts pre and post intervention (p-value > 0.05).
Conclusions: Education and raising awareness in the outpatient setting of the adverse effects resulting from prolonged use of and over prescription of PPIs could possibly increase the rate of reevaluation for continued use and de-prescription as seen in our study.
Keywords
Proton pump inhibitors, PPI-induced, Acid reflux, Esophagitis, Peptic ulcer, Helicobacter pylori, Gerd, Gastritis, Dyspepsia
Abbreviations
C. Diff: Clostridium Difficile Infection; EGD: Esophagogastroduodenoscopy; EMR: Electronic Medical Record; GERD: Gastroesophageal Reflux Disease; GI Bleed: Gastrointestinal Bleed; H. Pylori: Helicobacter Pylori; Mg: Milligrams; OTC: Over the Counter; PPI: Proton Pump Inhibitors; PUD: Peptic Ulcer Disease
Background
Since 1989, PPIs have become among the top-selling drug classes in the country. It is estimated that in the United States alone, about a quarter of the country suffers from acid related conditions [1]. Their versatility and popularity have prompted the World Health Organization to add PPIs to their list of essential medications [2]. The low cost of PPIs and over the counter (OTC) availability of these medications have made their use ubiquitous in outpatient care.
There are numerous indications for starting and continuing PPIs including gastroesophageal reflux disease (GERD), Barrett esophagus, peptic ulcer disease (PUD), etc. [1]. Indications for initiation of treatment as well as duration of therapy varies between diagnoses. Typically, a PPI is prescribed for a 4-to- 8-week course when the patient suffers from dyspepsia, mild GERD and uncomplicated PUD. For more extensive disease such as Barrett esophagus, severe GERD, erosive esophagitis and Zollinger-Ellison syndrome long-term maintenance therapy is typically recommended, and the course varies depending on numerous factors determined by the clinician. In patients who have received continuous therapy for over 6 months, some experts gradually taper therapy until discontinuation to avoid worsening or rebound symptoms, but there is no single agreed upon discontinuation strategy.
Although PPIs are well tolerated, there is mounting evidence about adverse events associated with prolonged use. These adverse events include increased risk of infections such as pneumonia or Clostridium difficile, or absorptive consequences such as impaired medication uptake, electrolyte abnormalities, vitamin deficiencies and increased fracture risk [3,4]. There also have been more serious, less commonly reported, adverse events including acute interstitial nephritis and cutaneous lupus erythematosus [5].
Moreover, in community practice, PPIs are often taken for unnecessarily long courses [5]. In patients presenting with an abundance of chronic conditions, it is not uncommon that a problem such as GERD is overlooked or pushed off. This, in turn, may result in unknowingly and unnecessary extended treatment courses which may complicate their other complex, chronic medical problems. Further awareness, education and strategic planning may aid in recognizing this problem before it progresses to a possible adverse event.
We implemented an internal medicine residency outpatient clinic-based quality project in order to improve the awareness of PPI over prescription and increase the rate of indicated PPI deprescription. Six months after the implementation of the project we conducted a retrospective before-after observational cohort study in order to measure the impact of our intervention on the following outcomes of interest: duration of therapy, indication of therapy, discontinuation attempts, dose reduction attempts and adverse events.
Methods
Study Design, Setting and Population
This is a retrospective before-after observational cohort study of a quality improvement program conducted at an internal medicine residency continuity clinic in South Florida. We examined data from visits between September 25, 2017 and February 26, 2018. The collected data was checked manually for completeness and entered in Research Electronic Data Capture System (REDCap version 10.6.22) for data tracking.
The first part of our intervention was a 15-minute presentation explaining the indications for discontinuing PPI therapy and the methods by which to do so (tapering versus on-demand). Next, we developed a protocol for considering and executing PPI discontinuation. Lastly, an alert prompting the consideration of the PPI Discontinuation Protocol was attached to electronic medical record patient records with ICD 10 codes commonly associated with PPI prescriptions. Appropriate PPI indication was based on published guidelines, and patients without an appropriate indication or exceeding the recommended course were targeted for intervention. Data was collected one year prior to and six months after intervention implementation.
Enrolled patients were identified by EMR query based on the attribution of ICD 10 codes commonly associated with PPI prescription. This included all genders, races and age groups above 18 years of age. Exclusion criteria included patients who were not on a PPI and those which were prescribed histamine blocker instead of a PPI.
All data included patients’ demographics, ICD 10 diagnoses which indicated PPI therapy, whether or not the patient was on a PPI, have they ever attempted discontinuation, as well as other data regarding type of PPI with dosages, adverse effects, duration of therapy and whether or not the PPI was ultimately discontinued. Adverse events were listed as pneumonia, Clostridium difficile infection (C. diff), vitamin B 12 deficiency, hypomagnesemia and osteoporosis.
Data analysis
SPSS software ver. 26.0 was used for statistical analyses. Pearson chi-squared tests of independence and Analysis of Variance (ANOVA) testing were used for categorical and continuous variables respectively. Means were used for continuous variables, and categorical variables were described as percentages. Our primary analysis assessed whether the number of PPI discontinuation attempts was higher prior to or after our intervention.
Results
Population characteristics
One hundred and sixteen patients were included in the study. The sample included 75 females and 41 male’s total. The average age was 54.86 years (SD=15.42, Range=20 to 94). Patients self-reported as Asian (n=7), Black or African American (n=9), Hispanic/Latin (n=49), White (n=38), More than one race (n=3), or not reported (n=10). There were no differences between the treatment and control groups in sex, race/ethnicity, and age (p>0.05) as shown in Table 1.
| Variable | Before Intervention N=53 (%) | After Intervention N=63 (%) |
| Demographics | ||
| Male | 20 (37.7%) | 22 (34.9%) |
| Female | 33 (62.3%) | 41 (65.1%) |
| Race | ||
| Asian | 0 (0%) | 7 (11.1%) |
| Black or African American | 5 (9.4%) | 4 (6.3%) |
| Hispanic/Latin | 19 (35.8%) | 30 (47.6%) |
| White | 20 (37.7%) | 18 (28.5%) |
| More than one race | 3 (5.6%) | 0 (0%) |
| Unknown/not reported | 6 (11.3%) | 4 (6.3%) |
| ICD 10 diagnosis | ||
| Dyspepsia | 3 (5.6%) | 4 (6.3%) |
| Esophagitis | 1 (1.8%) | 1 (1.6%) |
| Gastritis | 7 (13.2%) | 2 (3.2%) |
| GERD | 27 (50.9%) | 44 (69.8%) |
| GI bleed | 2 (3.7%) | 2 (3.2%) |
| H. Pylori | 3 (5.6%) | 6 (9.5%) |
| PUD | 2 (3.7%) | 0 (0%) |
| Not listed | 5 (9.4%) | 4 (6.3%) |
| EGD Findings | ||
| Barrett’s Esophagus | 2 (20%) | 0 (0%) |
| Gastritis | 7 (70%) | 5 (100%) |
| Ulcer Forrest Class Ia | 1 (10%) | 0 (0%) |
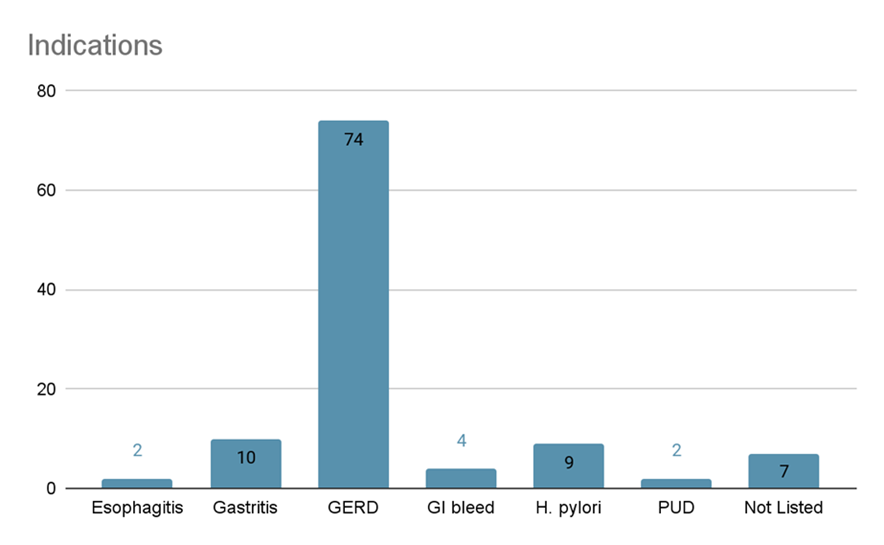
The primary ICD10 diagnosis was GERD K21.9 with 62.9% (n=73) of the sample, while Gastritis K29 accounted for 8.6% (n=10), Dyspepsia R10.13 accounted for 7.8% (=9) and H. pylori A04.8 accounted for 7.8% (n=9). The remaining diagnoses of Esophagitis K20.9, GI bleed K92.2, and PUD K27.9 each accounted for less than 3.5% of the overall sample.
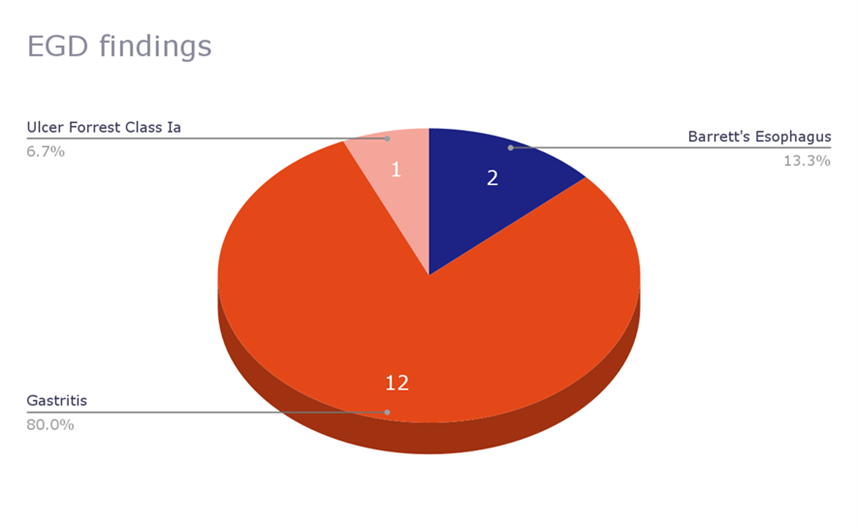
Out of the 116 subjects, 29 (25.2%) had a documented esophagogastroduodenoscopy (EGD) in the EMR, 15 of which included the EGD findings as shown in Table 1. Majority of the patients in the pre and post intervention groups had documented gastritis (70% vs 100%, respectively).
Adverse events
Out of the 116 patients, only a few experienced adverse events associated with prolonged PPI use overall, results are demonstrated in Table 2.
| Before Intervention | After Intervention N (%) | |
| Pneumonia on PPIs (%) | 0 (0%) | 0 (0%) |
| C. Diff Colitis on PPIs (%) | 1 (1.9%) | 0 (0%) |
| B12 Deficiency (%) | 1 (1.9%) | 1 (2.3%) |
| Hypomagnesemia (%) | 0 (0%) | 0 (0%) |
| Osteoporosis (%) | 3 (5.7%) | 1 (2.3%) |
Outcomes
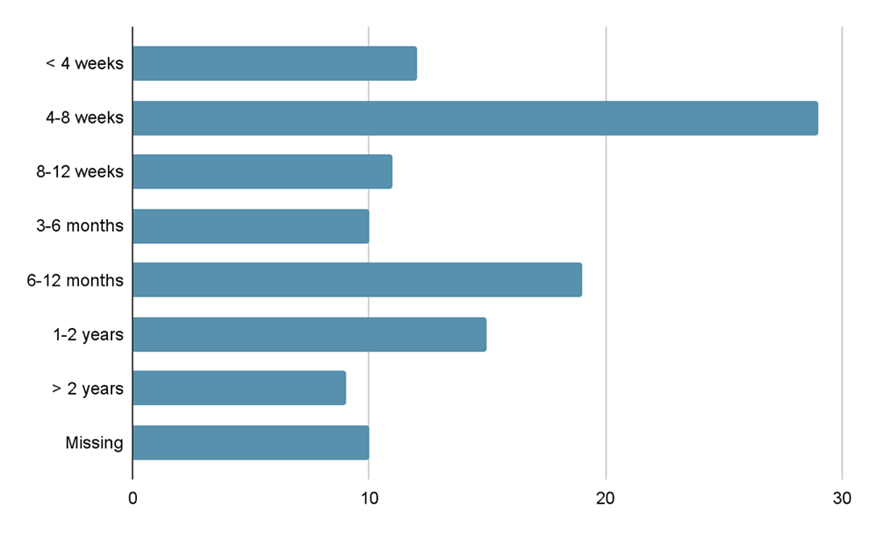
Length of therapy at time of visit varied between <4 weeks up to >2 years, with the majority of patients on therapy for 4-8 weeks, 28.3% in the pre intervention group vs 22.2% in the post intervention group as shown in Table 3. A total of 45.7% (n=52) of the patients had been taking a PPI for less than 12 weeks, 8.6% (n=10) for 3 to 6 months, 16.4% (n=19) for 6 to 12 months, 13.8% (n=16) for 1 to 2 years, and 7.8% (n=9) for over 2 years.
| Before Intervention N (%) | After Intervention N (%) | |
| Discontinuation attempts, % | 26% | 46% |
| > 8 weeks of therapy | 33 (62.2%) | 40 (63.4%) |
| Limited course prescription | 12 (22.6%) | 18 (28.5 %) |
| Duration of PPI Therapy | ||
| < 4 weeks | 6 (11.3%) | 6 (9.5%) |
| 4-8 weeks | 15 (28.3%) | 14 (22.2%) |
| 8-12 weeks | 4 (7.5%) | 7 (11.1%) |
| 3-6 months | 4 (7.5%) | 6 (9.5%) |
| 6-12 months | 8 (15%) | 11 (17.4%) |
| 1-2 years | 5 (9.4%) | 11 (17.4%) |
| > 2 years | 6 (11.3%) | 3 (4.8%) |
| Not listed | 5 (9.4%) | 5 (7.9%) |
| aIQR =interquartile range bIndependent-Sample Median |
||
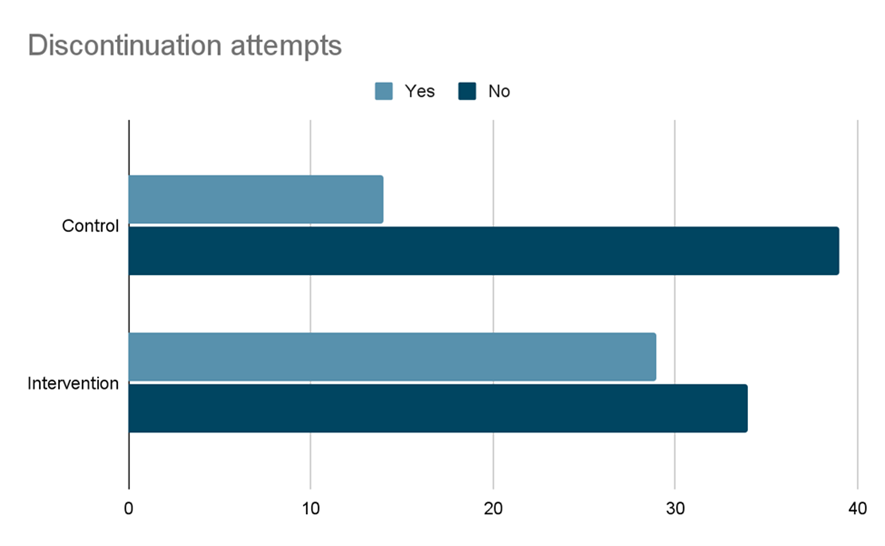
Discontinuation of PPI attempts in the pre intervention group versus the post intervention group are shown in Figure 1. From the total of patients (n=63) who received discontinuation consultation (treatment), 29 attempted discontinuations. From the total of patients (n=53) who did not receive discontinuation consultation (control), 14 attempted discontinuations. There were also only 10 patients (8.6%) overall in which dose reduction attempts were made prior to any discontinuation attempts in both groups. The odds ratio of attempting discontinuation in those who received discontinuation consultation vs those not receiving discontinuation consultation is 2.376, 95% CI [1.082, 5.216]. Specifically, individuals who received discontinuation consultation were 2.376 times more likely to attempt discontinuation than those who did not receive discontinuation consultation.
Figure 1. Indications of PPI therapy, combined data. GERD: Gastroesophageal Reflux Disease; H. pylori: Helicobacter pylori; PUD: Peptic Ulcer Disease; GI bleed: Gastrointestinal bleed.
Figure 2. EGD findings.
Figure 3. Length of PPI therapy, combined data.
Figure 4. Discontinuation attempts of PPIs in the pre intervention group vs the post intervention group.
Discussion
The rate of PPI discontinuation in our study, although not statistically significant, supports the need for awareness and education in the outpatient and specifically residency run clinic setting. Different means of intervention may aid in the success of PPI discontinuation. Additionally, assessment for symptom return following dose decrease or discontinuation should be taken into consideration. Further information regarding means by which patients are obtaining the medications, prescription rate versus patient driven self-treatment, could also aid in identifying further root sources of PPI overuse.
A study conducted by Boster et al. had similar interventions at a different internal medicine residency clinic, but they implemented a tapering method rather than discontinuation [6]. They reported 44% of their patients were either decreased on PPI dosage or discontinued but it was unclear whether or not patients ultimately required up titration of their PPI following the completion of the study, similarly in our case.
Literature reports an abundance of adverse effects from PPI use, including those mentioned above in addition to gastric neoplasia, liver disease and even dementia [6-8]. Although relatively rare when compared to the widespread use of PPIs, considerations should be made on the necessity of unnecessary treatments when no longer warranted. Some studies have shown genetic variation in CYP2C19 that may contribute to differences in the inhibitory activity of PPIs [9-11]. About 15–20% of Asians and 2–6% of Caucasians are known to be slow or poor metabolizers, which in turn may put them at a higher risk for adverse events [12-14]. Clinicians also may need to weigh risk versus benefit of prolonged use, even when the patient still has a diagnosis warranting ongoing treatment [15-17].
A survey study conducted by Khan et al. reported that 44.6% of their sample population endorsed concerns about the adverse effects of using PPIs and 30.6% endorse having tried to reduce the dosage or stop PPIs in the past without the recommendation of their physician (28.2%). This is similar to what our study found, with initially about 26% of patients attempting dose reductions or discontinuation prior to intervention.
Limitations
There are key limitations of our investigation that must be highlighted. As a retrospective study, it is impossible to account for all unmeasured potential confounders. Accounting for confounding medications or conditions that could also result in adverse effects similar to PPI use may better demonstrate the true rate of adverse effects to PPI therapy alone. The diversity of the population studied also lacked an even distribution, as the predominant population in our study was Hispanic/Latin females. It is unclear whether this population is at a higher risk for disorders necessitating PPI therapy or other factors such as geographic location limited our diversity. Finally, alert fatigue should be taken into consideration, which arises from an overwhelming number of alerts or alarms that desensitizes the providers tasked with responding to them. This may have led to missed or ignored alerts which in turn may have missed patients which would have qualified for medication reduction or discontinuation.
Conclusions
Our study found no change in the rate of PPI discontinuation after intervention with education, EMR prompts and increasing overall awareness of prolonged PPI use. Our study can be used for further research on more efficacious interventions, in larger more diverse populations.
Ethics Approval and Consent to Participate
Ethics approval was acquired following the hospital research committee review of the research protocol and plan. Written, informed consent was waived given the nature of the study.
Availability of Data and Materials
The datasets used in the production of this study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Author’s Contributions
PG, ZS, SP, NK designed the study, participated in literature review, data collection, and manuscript writing. ZS, EA, JF were involved in study planning and data collection. ZS, SP, NK, JF participated in study planning and electronic medical record queries. MD completed data collection and participated in manuscript writing. PG, ZS was involved in study supervision and planning.
References
2. Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017 Jul 1;153(1):35-48.
3. Corley DA. Safety and complications of long-term proton pump inhibitor therapy: getting closer to the truth. Gastroenterology. 2019 Sep 1;157(3):604-7.
4. Freedberg DE, Kim LS, Yang YX. The risks and benefits of longterm use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017 Mar 1;152(4):706-15.
5. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Internal Medicine. 2015 May 1;175(5):784-91.
6. Boster J, Lowry LE, Bezzant ML, Kuiper B, Surry L. Reducing the inappropriate use of proton pump inhibitors in an internal medicine residency clinic. Cureus. 2020 Jan;12(1): e6609.
7. Tang HL, Li Y, Hu YF, Xie HG, Zhai SD. Effects of CYP2C19 loss-offunction variants on the eradication of H. pylori infection in patients treated with proton pump inhibitor-based triple therapy regimens: a meta-analysis of randomized clinical trials. PLoS One. 2013 Apr 30;8(4):e62162.
8. Khan RS, Hadi Y, Gayam S. “Get Me Off This Medication!”: A Comparison Between Gastroenterology and Primary Care Regarding Patient’s Perceptions of Proton Pump Inhibitor Therapy. Cureus. 2020 Oct;12(10): e11158.
9. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999- 2012. Jama. 2015 Nov 3;314(17):1818-30.
10. Zhou B, Huang Y, Li H, Sun W, Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporosis International. 2016 Jan;27(1):339-47.
11. Fohl AL, Regal RE. Proton pump inhibitor-associated pneumonia: not a breath of fresh air after all?. World Journal of Gastrointestinal Pharmacology and Therapeutics. 2011 Jun 6;2(3):17-26.
12. Lee C, Lo A, Ubhi K, Milewski M. Outcome after discontinuation of proton pump inhibitors at a residential care site: quality improvement project. The Canadian Journal of Hospital Pharmacy. 2017 May;70(3):215-233.
13. Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019 Sep 1;157(3):682-91.
14. Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton pump inhibitors: review of emerging concerns. InMayo Clinic Proceedings 2018 Feb 1 (Vol. 93, No. 2, pp. 240-246). Elsevier.
15. Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. International Journal of Molecular Sciences. 2019 Jan;20(20):5203.
16. Centers for Medicare and Medicaid Services. Proton pump inhibitors: Food and Drug Administration-approved indications and dosages for use in adults. Department of Health and Human Services. 2013. www.cms.gov/Medicare-Medicaid-Coordination/
17. Wintemute K. (2019, May). Bye-bye PPI. Choosing wisely Canada. https://choosingwiselycanada.org/wp-content/uploads/2017/07/ CWC_PPI_Toolkit_v1.2_2017-07-12.pdf