Abstract
Background: Crystalloid bolus administration remains a cornerstone of sepsis management. Studies indicate that geriatric patients with sepsis benefit from guideline-recommended bundles. The effect of fluid timeliness on patient-centered outcomes in geriatric patients with sepsis is unknown. We conducted a retrospective cohort study to assess association between time-to-crystalloid and composite mortality and discharge to hospice. Hospital length of stay (LOS), ICU LOS, ICU admission and intubation rate were investigated secondarily. These outcomes were compared in prespecified prompt (< 60 minutes), early (60-180 minutes), and late ( >180 minutes) resuscitation groups, and within the cohort as a whole.
Results: 225 patients were included in this study. 46.2% received crystalloid before 60 minutes, while 40.9% and 12.9% of patients were resuscitated within 60-180 minutes and greater than 180 minutes, respectively. Baseline characteristics in all three groups were similar. There was no difference in composite in-hospital mortality or hospice discharge [27 (26.2%) vs. 24 (26.4%) vs. 10 (34.5%); p=0.653] between the three groups. The late crystalloid administration group had statistically significant longer ICU LOS [2.35 (3.99) vs. 3.82 (6.42) vs 6.14 (7.31); p=0.014] and increased intubation rate [6 (5.8) vs. 6 (6.5) vs. 6(20.7); p=0.026]. Regression analyses indicated that time-to-crystalloid directly correlated with hospital LOS, ICU LOS and intubation rate.
Conclusions: In this hypothesis-generating cohort study of geriatric patients with sepsis, there was no association between time-to-crystalloid and composite in-hospital mortality or hospice discharge.
Keywords
Sepsis, Hemodynamics, Geriatric, Elderly, Crystalloid, Bolus, Fluid, Resuscitation, Timeliness
Background
Sepsis is a key driver of worldwide mortality, representing close to 20% of global deaths in 2017 [1]. Approximately half of patients hospitalized with sepsis are 65 years and older [2]. Delivery of 30ml/kg of crystalloid fluid bolus within three hours of diagnosing sepsis remains a cornerstone of the Surviving Sepsis Campaign (SSC) recommendations [3]. Implementation of these guidelines have demonstrated improved mortality, as well as decreased hospital and ICU length of stay in the general population, and in older adults [4,5]. The more recent 2018 SCCM Sepsis guidelines include a 1-hour bundle, which recommends the initiation of this resuscitation within an hour of diagnosing sepsis [6]. The optimal timing of fluid administration, however, remains controversial due to the limited quality of existing evidence.
The landmark study by Rivers et al. and subsequent analyses of early goal-directed therapy indicated that early antibiotics and crystalloid administration decreases mortality in patients with sepsis [7-10]. Observational studies have produced discordant results on the effect of fluid timeliness on patient outcomes [11-13]. Optimal time-to-fluid has also been investigated within a variety of subgroups, and contradictory results were found [14]. Accordingly, investigative teams have postulated that demographic variables, such as older age and obesity, and comorbidities could alter the effect of crystalloid administration in sepsis. Patients with comorbidities including chronic respiratory failure, end stage renal disease, cirrhosis, and heart failure have been identified as subgroups that are less likely to receive 30 mL/kg of crystalloid within 3 hours of sepsis diagnosis [15-16]. Despite evidence supporting the use of the bundle treatments for sepsis in the elderly, they remain among the subgroups most likely to receive less aggressive resuscitation.
Importantly, the decreased in-hospital mortality signal observed in recent sepsis studies could have been biased by the exclusion of patients discharged to hospice [17]. We conducted a hypothesis-generating cohort study to assess for an association between crystalloid timeliness and composite mortality or discharge to hospice. The secondary aim of the study was to detect associations with hospital length of stay (LOS), ICU LOS, ICU admission and intubation rate. These outcomes were compared in the prespecified prompt (< 60 minutes), early (60-180 minutes), and late (>180 minutes) resuscitation groups and within the cohort as a whole.
Methods
Study design, setting and population
This is a retrospective cohort study conducted on patients admitted to the emergency department (ED) of a 400-bed urban tertiary care center in South Florida. We examined data from visits between September 25, 2017 and February 26, 2018; this period represents the time period for which the IRB approved this study. Power analyses were not conducted a priori. The collected data was checked manually for completeness and entered in Research Electronic Data Capture System (REDCap version 10.6.5) for data tracking. Informed consent was waived and study approval was granted by the Institutional Review Board and hospital research committee. The study followed “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies”.
Data collection
All primary data was extracted from the electronic medical record by physician-investigators using a standardized protocol for examining charts. Source data verification by an additional investigator occurred for all included records. Discordant reviewer evaluation negated chart selection.
Inclusion and exclusion criteria
Patients greater than or equal to 65 years old were included. Patients with confounding etiologies for acute organ dysfunction such as anaphylaxis, acute myocardial infarction, adverse medication reaction, autoimmune disease flare or pancreatitis were excluded. If hospice evaluation or newly ordered do-not-resuscitate status occurred while in the emergency department, exclusion occurred. For patients with multiple encounters, only the first encounter was included.
Cohort selection and sepsis definitions
Charts were identified by electronic medical record query based on the attribution of International Classification of Diseases-10 (ICD-10) codes for sepsis, severe sepsis and septic shock while in the ED. Sepsis was defined using the 2016 Society of Critical Care Medicine/European Society of Intensive Care Medicine (SCCM/ESICM) sepsis-3 definition. A standardized protocol for chart review was used to identify the presence of clinical suspicion of infection in the ED. An increase of two or more points on the Sequential Organ Failure Assessment (SOFA) score was used as a criterion for organ dysfunction [18]. In patients that did not have an arterial blood gas while in the ED, an SaO2/FiO2 ratio was used in place of PaO2/FiO2 to calculate the SOFA score (Supplementary Table 2). For patients with missing SOFA variables, a modified version of SOFA was used to identify organ dysfunction. A score of one or more in the modified version of SOFA corresponds to an increase of two or more points in the SOFA score Supplementary Table 3) [19,20].
Three cohorts based on clinically significant time-to-fluid intervals were constructed. Prompt crystalloid administration was defined as initiation of a fluid bolus in less than 60 minutes. Early administration was defined as 60 to 180 minutes and late administration was defined as greater than 180 minutes. The prompt and early administration groups were selected to maintain consistency with the 1 hour and 3 hour bundle recommendations by SCCM [6,21]. Crystalloid bolus administration was characterized by delivery of either lactated ringers or normal saline at a rate of less than or equal to 500 mL in 30 minutes.
Statistical analysis and outcomes
SPSS software ver. 26.0 was used to conduct statistical analyses. Patient demographics and characteristics were reported using means and standard deviations for continuous variables, and categorical variables were described as proportions and percentages. To assess the difference between the three groups, we used chi-squared tests of independence and Analysis of Variance (ANOVA) testing for categorical and continuous variables, respectively.
Our primary analysis investigated the association of time-to-fluid administration on the following patient-centered outcomes: (1) composite in-hospital mortality or hospice discharge, (2) hospital length of stay (LOS), (3) ICU LOS, (4) ICU admission, and (5) intubation. LOS was only calculated for patients who did not experience in-hospital mortality or hospice discharge.
Two statistical series were conducted. In the first series, outcomes within the clinically relevant prompt, early and late crystalloid administration groups were compared. ANOVA testing was conducted for continuous variables hospital LOS and ICU LOS. The independent variable was time-to-crystalloid with three levels (<60 minutes, 60-180 minutes, >180 minutes), while the dependent variable was length of stay in days. When ANOVA resulted in statistically significant between-group differences, post-hoc analysis using Least Square Difference (LSD) was conducted to determine where the variance lies within the model. Chi-square testing was used for categorical variables of composite in-hospital mortality or discharge to hospice, ICU admission and intubation. For chi-square tests with statistical significance, Cramer’s V was computed in order to detect effect size. In the second series, regression analyses were conducted to detect an association between time-to-crystalloid in minutes and the outcomes of interest. Using time-to-crystalloid as a predictor variable, linear regressions were performed for continuous outcomes and binomial logistic regressions were performed for dichotomous categorical variables.
The secondary analysis assessed for the presence of effects on patient outcomes attributable to previously described predictors of outcomes in patients with sepsis. Odds ratios were computed from contingency tables in order to explore the relationship between variables of interest, such as Obesity, ESRD and CHF, and categorical outcome variables. Linear regression and multiple regression were used for continuous outcomes variables hospital LOS and ICU LOS. An overall model was presented to explain the cumulative influence of these variables on the outcomes selected in the primary analysis. An additional regression analysis using a stepwise method was used to highlight variables with the strongest influence on these outcomes. A binomial logistic regression was performed to ascertain the effect of volume of crystalloid on the outcomes of interest. P values of <0.05 were considered significant, and analyses assumed the presence of a normal distribution.
Results
Demographics and patient characteristics
A total of 457 patient encounters were reviewed. After application of study criteria, a cohort of 225 patients remained (Supplementary Figure 1). Patients were divided into three groups based on the start time of crystalloid bolus administration: prompt fluid administration (<60 minutes), early fluid administration (60-180 minutes) and late fluid administration (>180 minutes). Overall, 46.2% of patients received prompt fluid administration, while 40.9% received early crystalloid administration, and 12.9% of patients received late crystalloid administration. Baseline characteristics were similar between the three groups, with the exception of obesity. A larger proportion of obese patients were observed within the delayed fluid administration group (Table 1). Time-to-antibiotics was longest in the delayed group, however the inter-group difference was not statistically significant (p=0.230). Volume of crystalloid was lowest in the delayed group (16.99 mL/kg) when compared to the prompt (27.36 mL/kg) and early (25.32 mL/kg)) fluid groups (p=0.001). Demographics, as well as sepsis and treatment variables, are displayed in Table 1.
| Total | Less than 60 minutes | 60 to 180 min- utes | Greater than 180 minutes | p value | |
|---|---|---|---|---|---|
| N (%) | 225 | 104 (46.2%) | 92 (40.9%) | 29 (12.9%) | |
| Demographics | |||||
| Male (%) | 137 (60.9) | 61 (58.7) | 57 (62) | 19 (65.5) | 0.770 |
| Female (%) | 88 (39.1) | 43 (41.3) | 35 (38) | 10 (34.5) | |
| Age (SD) | 82.74 (9.1) | 82.66 (9.64) | 82.66 (8.52) | 82.93 (8.82) | 0.908 |
| BMI (SD), kg/m² | 26.23 (6.29) | 25.61 (5.92) | 26.16 (5.99) | 27.01 ( 7.91) | 0.542 |
| Charlson Comorbidity Index (SD) | 5.84 (2.06) | 5.84 (2.13) | 5.72 (2.11) | 6.07 (1.73) | 0.809 |
| SpecialPopulations | |||||
| Age ≥ 80 Years (%) | 146 (64.9) | 64 (61.5) | 60 (65.2) | 22 (75.9) | 0.359 |
| Obesity (%) | 47 (20.9) | 16 (15.4) | 20 (21.7) | 11 (37.9) | 0.030 |
| Heart Failure (%) | 54 (24) | 22 (21.2) | 22 (23.9) | 10 (34.5) | 0.331 |
| End Stage Renal Disease (%) | 17 (7.6) | 6 (5.8) | 7 (7.6) | 4 (13.8) | 0.352 |
| COPD (%) | 33 (14.7) | 14 (13.5) | 13 (14.1) | 6 (20.7) | 0.612 |
| Shock (%) | 33 (14.7) | 16 (15.4) | 11 (12) | 6 (21.4) | 0.352 |
| Sepsis and Treatment Variables | |||||
| MAP<65 mmHg (%) | 88 (39.1) | 46 (44.2) | 32 (34.8) | 10 (34.5) | 0.345 |
| Lactate, mmol/L | 3.019 (2.117) | 3.031 (2.046) | 3.103 (2.189) | 2.739 (2.131) | 0.615 |
| SIRS | 2.61 (1.067) | 2.80 (1.031) | 2.52 (1.056) | 2.27 (1.106) | 0.005 |
| qSOFA | 1.72 (0.925) | 1.77 (0.862) | 1.61 (0.994) | 1.86 (0.915) | 0.317 |
| Time to Antibiotics (SD) | 194.56 (185.23) |
179.92 (183.64) | 194.5 (171.89) | 246.76 (225.32) | 0.230 |
| Volume of Crystalloid in mL/kg (SD) | 25.18 (12.83) | 27.36 (13.98) | 25.22 (9.97) | 16.99 (13.82) | 0.001 |
| Source of Infection | |||||
| Lung (%) | 79 (35.3) | 32 (30.8) | 35 (38.5) | 12 (41.4) | |
| Urine (%) | 82 (36.6) | 42 (40.4) | 32 (35.2) | 8 (27.6) | |
| Gastrointestinal (%) | 25 (11.2) | 15 (14.4) | 7 (7.7) | 3 (10.3) | |
| Skin and Soft Tissue (%) | 13 (5.8) | 4 (3.8) | 7 (7.7) | 2 (6.9) | |
| Osteomyelitis/Diskitis (%) | 2 (0.9) | 2 (1.9) | 0 (0) | 0 (0) | |
| Endocarditis (%) | 3 (1.3) | 1 (1) | 2 (2.2) | 0 (0) | |
| Undetermined (%) | 20 (8.9) | 8 (7.7) | 8 (8.7) | 4 (13.8) | |
SD: Standard Deviation; BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease Shock defined as requiring vasopressors while in the emergency department
Table 1. Patient demographics and characteristics.
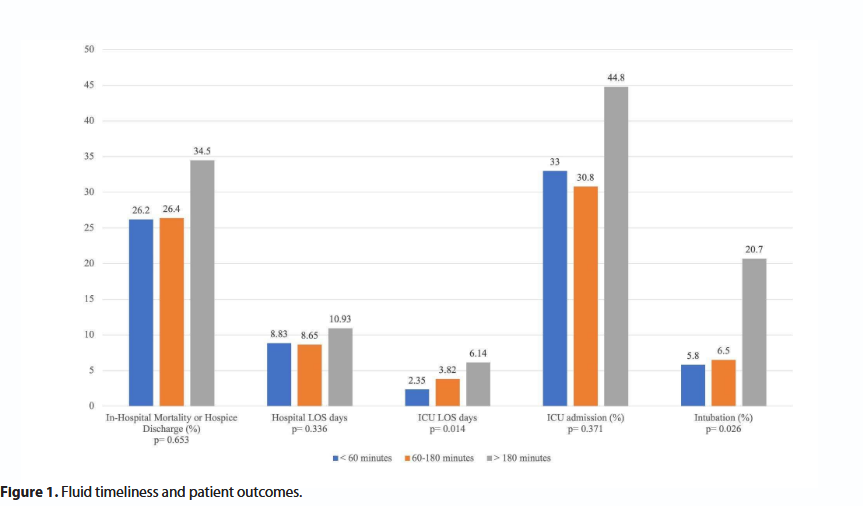
Fluid timeliness and patient outcomes
When comparing the prompt (<60 minutes), early (60-180 minutes), and late ( >180 minutes) resuscitation groups in series 1, there was no difference in the proportion of patients with composite in-hospital mortality or hospice discharge [27 (26.2%) vs. 24 (26.4%) vs. 10 (34.5%); p=0.653]. There was no statistically significant difference between the three cohorts with regards to Hospital LOS (p=0.336) or ICU admission (p=0.371). We did, however, detect a difference in ICU LOS and intubation rate. The late crystalloid administration group experienced longer ICU LOS [2.35 (3.99) vs. 3.82 (6.42) vs 6.14 (7.31); p= 0.014] and an increased intubation rate [6 (5.8) vs. 6 (6.5) vs. 6(20.7); p=0.026] (Figure 1). Post-hoc analysis using Least Square Difference (LSD), revealed a significant difference between prompt and late administration groups (p=0.005).
Regression analyses from series 2 revealed that time-to-crystalloid was predictive of hospital LOS and ICU LOS. More specifically, results revealed that time-to-crystalloid predicted hospital LOS [F(1,223)=5.818, p=0.017, R²=0.025] (Table 2). Time-to-crystalloid accounted for 2.5% of the variation in hospital LOS with an adjusted R²=2.1%, which reflects a relatively small effect size [22]. The following regression equation can be created from our results:
hospital LOS = 8.271 + (.007 x time-to-crystalloid).
| In-Hospital Mortality or Hospice Discharge (%) | Hospital LOS days (SD) | ICU LOS days (SD) | ICU admission (%) | Intubation (%) | |
|---|---|---|---|---|---|
| Series 1. | |||||
| <60 minutes | 27 (26.2) | 8.83 (7.55) | 2.35 (3.99) | 34 (33) | 6 (5.8) |
| 60 - 180 minutes | 24 (26.4) | 8.65 (5.85) | 3.82 (6.42) | 28 (30.8) | 6 (6.5) |
| >180 minutes | 10 (34.5) | 10.93 (10.99) | 6.14 (7.31) | 13 (44.8) | 6 (20.7) |
| P value | p=0.653 | p=0.336 | p=0.014 | p=0.371 | p=0.026 |
| Series 2. | |||||
| Variable | OR | B | B | OR | B |
| Time to crystalloid (minutes) | 1.001 (0.999 to 1.003) | 0.007 (0.001 to 0.013) |
0.008 (0.004 to 0.013) | 1.001 (0.999 to 1.003) | 1.002 (1.000 to 1.004) |
| P value | 0.312 | 0.017 | 0.001 | 0.244 | 0.047 |
Table 2. Fluid timeliness and patient outcomes.
As an example, for a time-to-crystalloid of 30 minutes, estimated hospital LOS would be 8.48 days. For a time-to-crystalloid of 90 minutes, estimated hospital LOS would be 8.90 days.
The model from series 2 also showed that time-to-crystalloid also predicted ICU LOS [F(1,169)=12.182, p=0.001, R²=0.067]. Time-to-crystalloid accounted for 6.7% of the variation in ICU LOS with adjusted R²=6.2%; again this was a relatively small effect size [22]. The resultant regression equation would be:
ICU LOS = 2.477 + (.008 x time-to-crystalloid)
Accordingly, for a times-to-crystalloid of 30 minutes and 90 minutes, estimated ICU LOS would be 2.71 days and 3.20 days respectively.
The model explained 3.3.% (Nagelkerke R2) of the variance in intubation. Further, for each minute increase time-to-crystalloid, the odds of being intubated increase by a factor of 1.002.
Independent predictors of outcomes
Multivariate regression results are listed in Table 3. The presence of heart failure was associated with a longer hospital LOS. Shock in the ED, as might be expected, was associated with increased intubation, ICU admission and composite in-hospital mortality or hospice discharge. The odds of in-hospital mortality or hospice discharge was 3.384 times higher for patients in shock, while the odds of being intubated was 6.489 higher. Hospital and ICU LOS was also longer in patients with shock. Our model revealed that 5.8.% (Nagelkerke R2) of the variance in ICU admission could be attributed to crystalloid volume. Specifically, for each mL/kg increase in volume of crystalloid, the odds of being admitted to the ICU increased by a factor of 1.565.
| In-Hospital Mortality or Hospice Discharge | Hospital LOS | ICU LOS | ICU Admission | Intubation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | OR | p | B | p | B | p | OR | p Value | OR | p Value |
| Age ≥ 80 Years | 1.266 (0.794 to 2.017) | 0.196 | 0.282 (-1.671 to 2.234) | 0.777 | 1.164 (-0.404 to 2.731) | 0.145 | 0.759 (0.527 to 1.092) | 0.094 | 0.515 (0.288 to 0.921) | 0.021 |
| Male Sex | 0.825 (0.468 to 1.453) | 0.3 | -0.284 (-2119 to 1551) | 0.761 | 0.829 (-0.648 to 2.306) | 0.270 | 1.12 (0.652 to 1.924) | 0.393 | 1.034 (0.511 to 2.090) | 0.538 |
| Obesity | 0.63 (0.305 to 1.305) | 0.14 | 1.151(-1.085 to 3.386) | 0.312 | 0.980 (-0.883 to 2.843) | 0.301 | 1.17 (0.623 to 2.197) | 0.368 | 1.55 (0.716 to 3.356) | 0.181 |
| ESRD | 1.115 (0.315 to 2.551) | 0.537 | 2.575 (-0.731 to 5.880) | 0.126 | 2.003 (-0.515 to 4.521) | 0.118 | 0.718 (0.285 to 1.807) | 0.316 | 0.708 (0.225 to 2.233) | 0.371 |
| HF | 1.204 (0.645 to 2.250) | 0.333 | 2.996 (0.874 to 5.117) | 0.006 | 0.936 (-0.752 to 2.624) | 0.275 | 1.326 (0.736 to 2.389) | 0.215 | 1.028 (0.470 to 2.247) | 0.543 |
| Shock | 3.384 (1.621 to 7.065) | 0.001 | 2.737 (0.058 to 5.416) | 0.045 | 5.265 (3.332 to 7.197) | 0.001 | 38.222 (11.218 to 130.235) | 0.001 | 6.489 (2.926 to 14.392) | 0.001 |
| Volume of Crystalloid, mL/kg | 1.286 (0.988 to 1.674) | 0.062 | 0.007 (-0.072 to 0.058) | 0.839 | 0.035 (-0.029 to 0.078) | 0.371 | 1.565 (1.201 to 2.039) | 0.001 | 0.983 (0.722 to 1.337) | 0.911 |
Table 3. Multivariate analyses.
Discussion
In our single-center retrospective cohort of geriatric patients with sepsis, no association between time-to-crystalloid and composite in-hospital mortality or discharge to hospice was observed. Delayed crystalloid resuscitation (>180 minutes) was associated with increased ICU length of stay, and increased intubation rate when compared with prompt resuscitation (< 60 minutes). Time-to-crystalloid was predictive of hospital LOS, ICU LOS and intubation rate across all patients in our cohort.
It is notable that the delayed crystalloid resuscitation group had a higher prevalence of obesity than the prompt and early resuscitation groups. For this reason, it is possible that obesity acted as an effect modifier for fluid resuscitation timeliness in sepsis. The secondary analysis in Table 3 demonstrated that obesity across all three subgroups was not associated with the increase in intubation rate or ICU LOS, implying that obesity does not represent a confounding variable. Additionally, patients in the late resuscitation group had received lesser fluid volume than those in the prompt and early groups. Our secondary analysis showed that ICU admission rate was positively correlated with fluid volume, suggesting that crystalloid volume did not act as a confounder in our primary analysis. It also must be recognized that the retrospective design of our study raises the possibility that unmeasured confounders influenced the results. Additionally, the single-center sampling further increases the risk of selection biases; collider bias in particular must be considered given the conditioning of the patient population to the syndrome of sepsis. For example, conditioning the sample to geriatric patients with sepsis could have unintentionally selected for patients with dysphagia and consequent dehydration which predisposed this group to the benefits of more timely crystalloid therapy.
Existing literature on the impact of crystalloid resuscitation timeliness on mortality in patients with sepsis is largely observational, with incongruent results. A large cohort study by Seymour et al. indicated that time to crystalloid bolus completion had no influence on mortality [11]. However, the arbitrary use of bundle initiation time as time-zero is a possible source of bias. Notably, our analysis used ED registration time as time-zero, which is a more clinically useful starting point. Two smaller series suggested that fluid administration within 30 minutes of sepsis identification was associated with decreased mortality [12,23].
Longer ICU LOS has been associated with increased 1-year mortality in elderly patients [24]. A study comparing patients who received 30 mL/kg within three hours to those who received fluid after three hours found ICU LOS was approximately 2 days longer in the delayed resuscitation group [15]. In a large cohort study comparing crystalloid initiation time within 30 minutes, 31-120 minutes and greater than 120 minutes found shorter ICU LOS in patients in the < 30 and 31-120 minutes groups [23]. A similar group of authors found that fluid initiation within 30 minutes was associated with 12% shorter hospital LOS in patients with sepsis [12]. Our findings in geriatric patients also indicate that prompt resuscitation is associated with shorter hospital LOS.
Khan et al. studied 208 patients with sepsis or septic shock and heart failure, ESRD or cirrhosis which found no difference in the intubation rate between restricted (<30 ml/kg) and standard (≥ 30 ml/kg) fluid resuscitation group [25]. However, the use of a 6-hour window for resuscitation after the diagnosis of sepsis in this study may have obscured the impact of fluid timeliness. Our study suggests that prompt resuscitation may be associated with a decreased intubation rate.
Geriatric patients with sepsis are thought to present and respond uniquely to resuscitation because of age-related physiologic changes [5]. Decreased chronotropic response to stress, decreased arterial compliance, impaired humoral and cell-mediated immunity and increased circulating inflammatory cytokines (TNF-α, IL-1, and IL-6) have been identified in the elderly [5,26]. A study by a group from the Netherlands suggests that patients above the age of 70 with suspected infection experience under-rescucitation, and this was correlated to increased mortality [27]. An inverse correlation between mortality and initial systolic blood pressure (SBP) was noted in the same age group at SBPs of 100-140mm Hg [28]. This is somewhat contrary to a study of mostly geriatric patients with sepsis who received early resuscitation upon paramedic contact that found mortality benefit was largely limited to patients with SBP below 100 mmHg [14]. These conflicting findings are difficult to explain and raise clinically relevant concerns of whether presenting SBP in geriatric patients with sepsis correlates with benefit of early crystalloid resuscitation.
Unfortunately, the small sample size of our study precludes the ability to draw conclusions on the impact of crystalloid timeliness on mortality in geriatric patients with sepsis. The relationship between reduced time-to-crystalloid and consistent reductions in the key surrogate outcomes of hospital LOS, ICU LOS and intubation rate in geriatric patients with sepsis are novel findings. Knowledge from this study can be used to influence center-specific quality improvement investigations or future research endeavors. Conducting a prospective randomized control trial to pinpoint the effect size of time-to-crystalloid in geriatric patients would be ideal but both logistically and ethically fraught. A larger prospective cohort study investigating the effect of fluid timeliness on 28-day mortality represents an opportunity for further research.
Limitations
There are key limitations of our investigation that must be highlighted. As a retrospective study, it is impossible to account for all unmeasured potential confounders. It is conceivable that patients who received fluid later had more severe disease and that the delay to diagnosis of sepsis itself could have worsened outcomes. Moreover, the relationships presented that suggest causality must be treated as correlations and are not sufficient evidence to establish causality. The inability to account for fluid administered by paramedic services is also a limitation of our study. The use of a modified version of SOFA score increased the sensitivity of our inclusion criteria, but is not widely adopted and well-verified as the SOFA score. Therefore, the external validity of our data can be questioned.
Conclusion
Our hypothesis-generating study found no association between time-to-crystalloid and composite in-hospital mortality or hospice discharge in geriatric patients with sepsis. Timely fluid administration may be associated with shorter ICU LOS and a lower intubation rate in these patients.
List of Abbreviations
mL: milliliters; kg: kilogram; SSC: Surviving Sepsis Campaign; ICU: Intensive Care Unit; SCCM: Society of Critical Care Medicine; LOS: Length of Stay; ED: Emergency Department; REDCap: Research Electronic Data Capture System; STROBE: Strengthening the Reporting of Observational Studies in Epidemiology; ICD-10: International Classification of Diseases-10; ESICM: European Society of Intensive Care Medicine; SOFA: Sequential Organ Failure Assessment; SD: Standard Deviation; ANOVA: Analysis of Variance; LSD: Least Square Difference; ESRD: End Stage Renal Disease; CHF: Congestive Heart Failure; BMI: Body Mass Index; COPD: Chronic Obstructive Lung Disease; MAP: Mean Arterial Pressure; SIRS: Systemic Inflammatory Response Syndrome; qSOFA: Quick Sequential Organ Failure Assessment Score; SBP: Systolic Blood Pressure
Ethics Approval and Consent to Participate
IRB and ethics approval were acquired following hospital research committee review of the research protocol and plan. Written, informed consent was waived given the nature of the study.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets used in the production of this study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
There was no institutional funding in the production of this work.
Author’s Contributions
ZS, KI and PN designed the study, participated in literature review, study design, data collection, and manuscript writing. KR, DW, and CG were involved in study planning and data collection. SF participated in study planning and electronic medical record queries. MD completed data collection and participated in manuscript writing. SS was involved in study supervision and planning.
References
2. Yoshikawa, TT, Reyes, BJ, Ouslander, JG. Sepsis in Older Adults in Long-Term Care Facilities: Challenges in Diagnosis and Management. J Am Geriatr Soc. 2019;67:2234-2239.
3. Dellinger R, Levy M, Carlet L, Bion J, Parker M, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36:296–327.
4. Levy MM, Rhodes A, Phillips GS, Townsend SR, Schorr CA, Beale R, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med. 2015 Jan;43(1):3-12.
5. Nasa P, Juneja D, Singh O. Severe sepsis and septic shock in the elderly: An overview. World J Crit Care Med. 2012;1(1): 23–30.
6. Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Int Care Med. 2018;44(6):925–928.
7. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N Engl J Med. 2001;345(19):1368–77.
8. ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-93.
9. Investigators A, Group ACT, Peake SL, Delaney A, Bailey M, Bellomo R, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-506.
10. Mouncey PR, Osborn TM, Power GS, Harrison DA , Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-11.
11. Seymour CW, Gesten F, Prescott HC, Friedrich ME, Iwashyna TJ, Phillips GS, et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017;376(23):2235-2244.
12. Leisman D, Wie B, Doerfler M, Bianculli A, Ward MF, Akerman M, et al. Association of Fluid Resuscitation Initiation Within 30 Minutes of Severe Sepsis and Septic Shock Recognition With Reduced Mortality and Length of Stay. Ann Emerg Med. 2016;68(3):298-311.
13. Andrews B, Semler BW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, et al. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA. 2017;318(13):1233-1240.
14. Lane DJ, Wunsch H, Saskin R, Cheskes S, Lin S, Morrison LJ, et al. Association Between Early Intravenous Fluids Provided by Paramedics and Subsequent In-Hospital Mortality Among Patients With Sepsis. JAMA Netw Open. 2018;1(8):e185845.
15. Kuttab H, Lykins JD , Hughes MD, Wroblewski K, Eric P Keast EP, Kukoyi O et al. Evaluation and Predictors of Fluid Resuscitation in Patients With Severe Sepsis and Septic Shock. Intensive Care Med. 2017;43(5):625-632.
16. Palomba H, Corrêa TD, Silva E, Pardini A, Cesar de Assuncao MS. Comparative Analysis of Survival Between Elderly and Non-elderly Severe Sepsis and Septic Shock Resuscitated Patients. Einstein (São Paulo). 2015;13(3):357-363.
17. Kadri SS, Rhee C, Strich JR, Morales MK, Hohmann S, Menchaca J, et al . Estimating ten-year trends in septic shock incidence and mortality in United States academic medical centers using clinical data. Chest. 2017;151(2):278-285.
18. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810.
19. Grissom CK, Brown SM, Kuttler KG, Boltax JP, Jones J, Jephson AR, et al. A Modified Sequential Organ Failure Assessment Score for Critical Care Triage. Disaster Medicine and Public Health Preparedness. Cambridge University Press; 2010;4(4):277–84.
20. Wilson B, Tran D, Dupuis JY, McDonald B. 126: VALIDATION OF A MODIFIED SOFA SCORE AS A MORTALITY RISK PREDICTION MODEL IN CARDIAC SURGICAL ICU.Crit Care Med. 2016;44(12):109.
21. Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016.Crit Care Med. 2017;45(3):486–552.
22. Cohen J. (1988). Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic.
23. Leisman DE, Goldman C, Doerfler ME, Masick KD, Dries S, Hamilton E, et al. Patterns and Outcomes Associated With Timeliness of Initial Crystalloid Resuscitation in a Prospective Sepsis and Septic Shock Cohort. Crit Care Med. 2017;45(10):1596-1606.
24. Moitra VK , Guerra C, Linde‐Zwirble WT ,et al. Relationship between ICU length of stay and long‐term mortality for elderly ICU survivors.Crit Care Med.2016;44(4):655-62.
25. Khan R, Khan N, Bauer S, Li M, Duggal A, Wang X, Wunsch H. Association Between Volume of Fluid Resuscitation and Intubation in High Risk Septic Patients with Heart Failure, End Stage Renal Disease and Cirrhosis. Chest. 2019;157(2).
26. Girard TD, Opal SM, Ely EW. Insights into Severe Sepsis in Older Patients: From Epidemiology to Evidence-Based Management.Clin Infect Dis.2005;40(5):719-27.
27. Ko SY, Cuevas LME, Willeboer L, Ansems A, Blomaard LC, Lucke JA, et al. The association between intravenous fluid resuscitation and mortality in older emergency department patients with suspected infection. Int J Emerg Med. 2019;12(1):1.
28. Warmerdam M, Baris L, Liebergen MV, Ansems A, Cuevas LE, Willeboer M, et al. The association between systolic blood pressure and in-hospital mortality in older emergency department patients who are hospitalised with a suspected infection. Emerg Med J.2018;35(10):619-622.