Abstract
Background: Leptin has been proposed as a biomarker for endometriosis. Previous studies have shown mixed results. The aim of this study was to compare peritoneal fluid (PF) leptin concentrations between patients with and without endometriosis in a cohort of sufficient size to detect a significant difference.
Methods: Patients of reproductive age undergoing laparoscopic surgery for endometriosis or other benign indications in the Department of Gynecology, University of Bern between 2007 and 2018 were recruited. Peritoneal fluid was aspirated at laparoscopy and the concentration of leptin measured by Enzyme-linked immunosorbent assay (ELISA). Leptin concentrations were compared between patients with and without endometriosis by an analysis of covariance.
Results: 1054 patients were included in the analysis, of which 653 patients were diagnosed with endometriosis. Leptin concentrations strongly correlated with body mass index (BMI) (R²=0.313; F (1,1033)=470.73, p<0.001). After correcting for BMI, no difference was found in leptin concentrations between patients with and without endometriosis (p=0.051).
Conclusion: Peritoneal fluid leptin concentrations correlated with BMI, but did not significantly differ between patients with and without endometriosis. This suggests leptin does not represent a viable biomarker for endometriosis.
Keywords
Leptin, Endometriosis, Peritoneal fluid, Biological marker, BMI, Cycle phase
Abbreviations
ANOVA: Analysis of variance; ANCOVA: Analysis of Covariance; BMI: Body Mass Index; CA 125: Cancer Antigen 125; COC: Combined Oral Contraceptives; CP: Cycle Phase; DIE: Deep Infiltrating Endometriosis; DF: Degrees of Freedom; ELISA: Enzyme-Linked Immunosorbent Assay; GnRHa: Gonadotrophin-Releasing Hormone agonist; IUD: Intrauterine Device; LNG: Levonorgestrel; MS: Mean Square; PF: Peritoneal Fluid; POP: Progestin-Only Pill; rASRM: Revised classification of the American Society of Reproductive Medicine; SD: Standard Deviation; SS: Sum of Squares.
Introduction
Endometriosis is a chronic disease affecting approximately 10% of women in their reproductive years, often leading to pelvic pain and infertility. It is characterized by endometrial-like tissue, which grows outside the uterus [1]. A thorough medical history and gynecological examination including transvaginal ultrasound, are the primary diagnostic tools [2], although due to their low sensitivity, visualization during laparoscopy remains the gold standard. However, given its invasiveness, it is no longer recommended as a primary diagnostic procedure and according to previous guidelines hormonal therapy can be started empirically to treat endometriosis-associated pain [3]. Hormonal therapy in turn is not tolerated or inefficient in about a third of the patients [4,5]. Hence, endometriosis treatment may be delayed for up to ten years [6].
In the search for a more effective and less invasive diagnostic tool, many biomarkers have been proposed, but none is sufficiently effective to be adopted into clinical practice [7]. Some biomarkers have also been implicated in pathogenesis and represent potential therapeutic targets, as they are elevated in the peritoneal cavity in patients with endometriosis [8,9].
Leptin, a 16-kilodalton (kDa) protein, is an adipokine mainly produced in adipocytes and involved in the lipid metabolism, regulating hunger and food intake [10]. There is also increasing evidence that leptin is linked to immune function and plays a role in autoimmune disorders [11]. Furthermore, leptin may play a role in fertility. Higher serum leptin levels have been found in women with unexplained fertility [12]. Moreover, the leptin receptors have been shown to be expressed in endometrial cells and leptin is assumed to be produced by human ovarian follicles and stimulates Gonadotrophinreleasing hormone (GnRH)-secretion in the hypothalamus [ 13,14].
An association between leptin and endometriosis has also been previously suggested. A significantly elevated PF leptin concentration in women with endometriosis was identified in a study of 60 women with compared to 40 women without endometriosis. This study also showed leptin concentrations positively correlated with the stage of endometriosis [15]. Another study analyzing 60 patients with endometriosis and 18 controls confirmed elevated leptin levels in patients with endometriosis but found an inverse association between leptin concentrations and endometriosis stage, suggesting a role of leptin especially in the formation of peritoneal endometriosis lesions [16]. In contrast, a study using women with ovarian endometriosis found lower PF leptin levels in the endometriosis patients [17]. Other studies, however, did not reveal any difference in PF leptin concentrations between women with and without endometriosis [18,19].
Given the need to improve diagnostic options for women with endometriosis, we aimed to use a cohort of sufficient size and power to assess the difference in PF leptin concentrations between women with and without endometriosis and determine its potential as a diagnostic biomarker or therapeutic target for endometriosis.
Materials and Methods
The study followed the “Strengthening the reporting of observational studies in epidemiology” guidelines [20]. This is a retrospective analysis of prospectively collected data. All women of reproductive age undergoing laparoscopic intervention for endometriosis or other benign gynecological conditions in the Department of Obstetrics and Gynecology, University of Bern (Switzerland) between 2007 and 2018 were recruited for the study. Written informed consent, detailed information on hormonal treatment usage, cycle phase and demographic biomarkers were obtained from all participants prior to surgery. The relevant Ethical committee (Project-ID 2020-00937) approved the project. Exclusion criteria were patients suffering from other inflammatory diseases, pregnancy, malignancy and surgery performed in an emergency situation. Surgery took place after at least 8 hours (h) of fasting. The peritoneal fluid (PF), that was present in the abdominal cavity at the beginning of the operation (between 0.5-40 ml), was aspirated from the pouch of Douglas at the beginning of the laparoscopy before performing any surgical procedure. For the laparoscopy, Karl Storz instruments and devices were used (distributed by Anklin AG, 4153 Reinach, Switzerland). Intraoperative findings and revised American Fertility Society (rASRM) stage of endometriosis were assessed and documented. Menstrual cycle was determined by selfreported cycle day, or serum progesterone measurement in nanomols per liter (nmol/L). If the surgery took place between the 10th and 19th cycle day or if the date of the last menstruation was not available, the menstrual cycle phase was determined according to the progesterone concentration in PF (cut-off 25 nmol/L)). Patients taking hormonal treatments were considered amenorrheic. In patients with no hormonal therapy, the cycle phase was marked as unknown if the cycle day was between the 10th and 19th cycle day without available progesterone concentration or if the cycle day and progesterone concentration in PF were not available or provided conflicting results. The cycle phase was also marked as unknown if the history of hormonal treatment was unknown.
Peritoneal fluid samples were clarified by centrifugation (10 minutes (min) at 1800 times gravity) and stored undiluted at −70°C prior to assay. Hemolyzed PF or samples with insufficient PF volume were excluded. The total protein content in PF was determined using a micro-bicinchoninic assay (Quanti-Pro® BCA, Sigma-Aldrich, St. Louis, 140 Missouri, United States of America) to ascertain absence of dilution with abdominal flushing medium under the procedure. Samples with a protein concentration below 10 ng/ml were excluded. Peritoneal fluid leptin concentrations were measured by ELISA as previously described [17], using a Duoset® microplate sandwich protocol (DY398; R&D Systems, Abingdon, England). The diluent (Cat. No. DY995) was obtained from the same provider, the standard range was from 2000 to 31.25 pg/ml in serial 1:2 steps as recommended, and the sample dilution was 1:50. All incubations were run at a constant temperature of 25°C, in DY995 and for the duration of the recommended in the protocol.
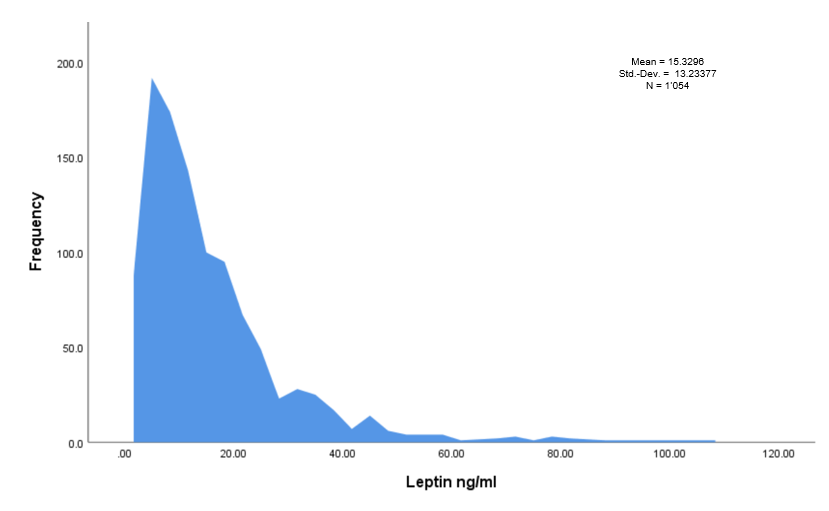
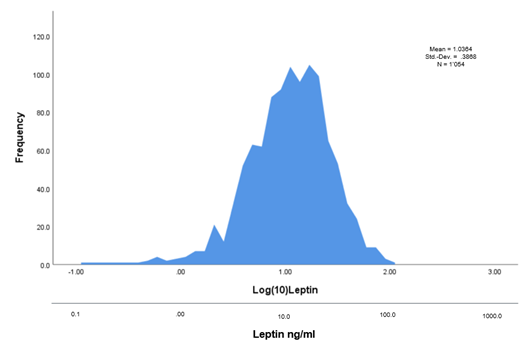
Raw data were imported in IBM SPSS (Statistical Package for the Social Sciences) statistics for Windows (Version 25.0. IBM Corp: Armonk, New York, United States of America) for statistical analysis. Mean values and standard deviations (SD) were calculated for continuous variables and percentages for qualitative variables. Body mass index (BMI) and age between patients with and without endometriosis were compared by t test while the influence of cycle phase was assessed via a χ2 test. Data normality was tested using the Kolmogorov– Smirnov test. Due to a deviation from a normal distribution (p=0.000), we performed a logarithmic transformation log(10) to obtain log transformed leptin levels corresponding to a normal distribution (Figures 1 and 2).
Figure 1: Histog ram representing the frequency dist ribution of the leptin values (ng/ml). Ng/ml: Nanograms/ml; Std.-Dev.: Standard Deviation; N: Number of patients.
Figure 2: Histogram after logarithmic transformation of the leptin values. Ng/ml: Nanograms/ml; Std.-Dev.: Standard Deviation; N: Number of patients.
Linear regression analysis was used to study the relationship between leptin concentrations and BMI in all patients as well as in patients based on endometriosis status. Differences between regression lines were analyzed using ANOVA. Differences in logarithmic leptin concentrations between patients with and without endometriosis (fixed factor, two levels: yes, no) and between menstrual cycle phases (fixed factor, four levels: proliferative, secretory, unknown, hormonal treatment) were tested using analysis of covariance (ANCOVA; general linear model, full factorial). The potential confounders age and Body Mass Index were included as covariates in the ANCOVA model to reduce sources of bias. The obligatory assumptions as a prerequisite for carrying out this analysis (Levene’s test for homogeneity of variance, homogeneity of regression, normality check, and independence) were checked and met.
Results
A total of 1054 patients were included in the analysis after excluding cases with protein levels lower than 10 ng/ml and three extreme outliers with very low leptin levels of 0.1 ng/ ml. Body mass index was available for 1035 patients (98.2%). 19 patients had no BMI described. 653 patients (62.0%) were diagnosed with endometriosis, of which 276 (42.3%) patients with mild stage (rASRM stage I or II) and 377 patients (57.7%) with advanced stage (rASRM stage III or IV) endometriosis. 401 patients did not have any intraoperative finding of endometriosis and were counted as controls (38.0%). 433 patients (41.4%) had a hormonal treatment at the time of the surgery, of which 313 (72.3%) patients with endometriosis and 120 (27.7%) patients without endometriosis. The cycle phase was available in 424 (40.2%) patients when excluding patients with hormonal treatment from which 291 (27.6%) of the patients underwent surgery in the proliferative, 133 (12.6%) in the secretory cycle phase.
Body mass index was statistically significantly lower (p<0.001) in patients with endometriosis (23.0 ± 4.0 kg/m2) compared to patients without endometriosis (24.5 ± 4.8 kg/m2). Patients with endometriosis were significantly younger (33.0 ± 6.2 years) than control patients (35.1 ± 8.2 years), p≤0.000. The detailed patient`s characteristics are summarized in Table 1.
| Characteristics | All Patients N=1054 | Endometriosis N=653 | No endometriosis N=401 | p-value |
| Age (mean ± SD) | 33.8 ± 7.1 | 33.0 ± 6.2 | 35.1 ± 8.2 | <0.001 |
| BMI in kg/m2 (mean ± SD, N=1035) |
23.6 ± 4.4 | 23.0 ± 4.0 | 24.5 ± 4.8 | <0.001 |
| Stages rASRM I-II (n, %) | 276 (42.3) | |||
| Stages rASRM III-IV (n, %) | 377 (57.7) | |||
| Proliferative phase (n, %) | 291 (27.6) | 167 (25.7) | 124 (30.9) | ns |
| Secretory phase (n, %) | 133 (12.6) | 70 (10.7) | 63 (15.7) | ns |
| Cycle phase unknown (n, %) | 197 (18.7) | 103 (15.8) | 94 (23.4) | <0.001 |
| Hormonal treatment (n, %) | 433 (41.1) | 313 (47.9) | 120 (29.9) | <0.001 |
| No horm. therapy (n, %) | 489 (46.4) | 289 (44.3) | 200 (49.9) | <0.001 |
| Hormonal therapy (n, %) | 433 (41.1) | 313 (48.0) | 120 (30) | <0.001 |
| COC (n, %) | 106 (10.1) | 68 (10.4) | 38 (9.5) | |
| POP, LNG-IUD (n, %) | 254 (24.1) | 176 (27.0) | 78 (19.5) | |
| Zoladex (n, %) | 73 (6.9) | 69 (10.6) | 4 (1.0) | |
| Hormonal therapy unknown (n, %) | 132 (12.5) | 51 (7.8) | 81 (20.2) | |
| SD: Standard Deviation; N: Number | ||||
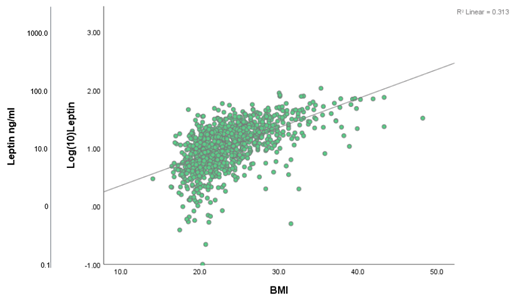
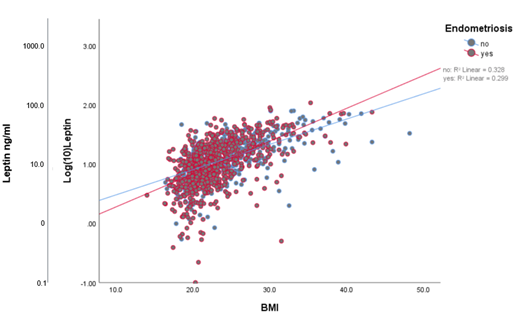
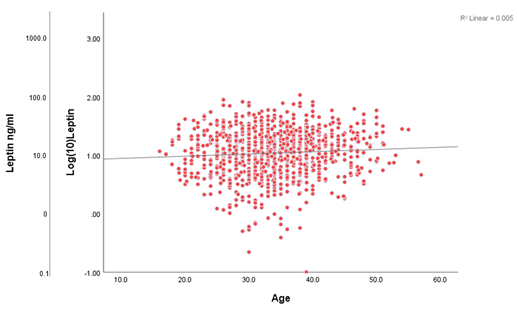
A simple linear regression analysis with leptin concentrations as the dependent variable and BMI as the explanatory variable showed that 31.3% of the variance of leptin was attributable to the patient’s BMI (β= 0.050, R2= 0.313; F (1,1033)=470.728, p<0.001) (Figure 3). Splitting patients by endometriosis status showed similar results (with endometriosis: β=0.055, R2=0.299; F (1,644)=274.471, p=0.000 (Figure 4); without endometriosis: β=0.043, R2=0.328; F (1,387)=188.787, p<0.001) (Figure 4). The same analysis performed with age as the explanatory variable resulted in a minimal effect on the leptin variance by the patient’s age (β=0.004, R²=0.005; F (1,1052)=5.175, p=0.023) ( Figure 5).
Figure 3:Scatter plot demonstrating linear regression of leptin levels according to BMI. β=0.050, R2=0.313; F (1,1033)=470.728, p<0.001. Ng/ml: Nanograms/ml; BMI: Body Mass Index in kg per meter square (kg/m²).
Figure 4: Scatter plot demonstrating leptin levels according to BMI and endometriosis status (no/yes) with linear adjustment lines of the mean values. This figure represents the performed linear regression analysis. With endometriosis: β=0.055, R2=0.299; F (1,644)=274.471, p=0.000; Without endometriosis: β=0.043, R2=0.328; F (1,387)=188.787, p< 0.001. Ng/ml: Nanograms/ml; BMI: Body Mass Index.
Figure 5: Scatter plot demonstrating linear regression o f leptin levels according to age. β=0.004, R²=0.005; F (1,1052)=5.175, p=0.023. Age in years.
The mean leptin concentration in patients with and without endometriosis was Log(10) Leptin 1.020 ± .015 and 1.065 ± .017, respectively. A one-way ANCOVA was conducted to compare leptin concentrations between patients with and without endometriosis and between patients in proliferative and secretory menstrual cycle phases whilst controlling for BMI (covariate) and age (covariate) (Table 2). 1035 patients with available information for all variables were included in the model. No significant difference was found in the mean leptin levels between patients with and without endometriosis (F(1,1026)=3.828, p=.051) (Table 2a). Patients in the proliferative cycle phase without hormonal therapy had significantly lower leptin concentrations compared to patients under hormonal therapy (p=.003) (Table 2b). In patients with advanced stages of endometriosis (rASRM III-IV), leptin concentrations were significantly lower (Log(10)Leptin 1.016 ± .020) compared to patients without endometriosis (Log(10) Leptin 1.073 ± .017) (F(1,752)=4.733, p=0.030) (Tables 3a, 3b).
| Table 2a: Results from the one-way ANCOVA. | |||||||
| Dependent Variable | Independent Variable | Sum of squares | df | Mean square | F | p-value | Partial Eta squared |
| Log(10)Leptin | Endometriosis | 0.388 | 1 | 0.388 | 3.838 | 0.051 | 0.004 |
| Cycle phase | 2.432 | 3 | 0.811 | 7.994 | <0.001 | 0.023 | |
| BMI (covariate) | 46.579 | 1 | 46.579 | 459.329 | <0.001 | 0.309 | |
| Age (covariate) | 0.103 | 1 | 0.103 | 1.019 | 0.313 | 0.001 | |
| R-squared = .333 (corrected R-squared=.327); df: Degrees of freedom; BMI: Body Mass Index | |||||||
| Table 2b: Post hoc tests. | |||||||
| Dependent Variable | Independent Variable | Regression coefficient B | Std. Error | Sig. | 95% confident interval | Partial Eta Squared | |
| Lower bound | Upper bound | ||||||
| Log(10) Leptin | BMI | 0.05 | 0.002 | <0.001 | 0.046 | 0.055 | 0.309 |
| Endometriosis | -0.035 | 0.035 | 0.284 | -0.105 | 0.031 | 0.001 | |
| Reference: no endometriosis | |||||||
| Proliferative CP | -0.122 | 0.041 | 0.003 | -0.203 | -0.041 | 0.008 | |
| Secretory CP | 0.049 | 0.051 | 0.339 | -0.051 | 0.149 | 0.001 | |
| Unknown CP | -0.054 | 0.045 | 0.232 | -0.141 | -0.034 | 0.001 | |
| Reference: hormonal treatment | |||||||
| BMI: Body Mass Index; CP: Cycle Phase | |||||||
| Table 3a: Results from the one-way ANCOVA. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Independent Variable | Sum of squares | df | Mean square | F | p-value | Partial Eta squared | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High grade Endometriosis | 0.456 | 1 | 0.456 | 4.733 | 0.03 | 0.006 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cycle phase | 2.084 | 3 | 0.695 | 7.209 | <0.001 | 0.028 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI (covariate) | 33.236 | 1 | 33.236 | 344.832 | <0.001 | 0.324 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Age (covariate) | 0.004 | 1 | 0.004 | 0.04 | 0.842 | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| R-squared = .354 (corrected R-squared=.346); df: Degrees of Freedom; BMI: Body Mass Index | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Table 3b: Post hoc tests. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BMI: Body Mass Index; CP: Cycle Phase | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
In this study, we found that after correcting for BMI and age there was no significant difference in PF leptin concentrations between patients with and without endometriosis (Table 2a). On the contrary, leptin tended to be lower in patients with compared to without endometriosis with even statistically significantly lower concentrations in patients with high-stage endometriosis (Tables 3a, 3b).
Three systematic reviews and meta-analyses have previously reported elevated PF leptin concentrations in patients with endometriosis compared to controls. In the meta-analysis by Tian et al., elevated PF but not serum leptin concentrations were found in patients with endometriosis. In subgroup analyses, significantly elevated PF leptin levels were shown both for early and advanced stage endometriosis. In this meta-analysis 13 studies on PF leptin concentrations were included, consisting of 376 patients with endometriosis and 246 controls [21]. Kalaitzopoulos et al., assessed 18 studies on 531 women with endometriosis and 331 controls and reported similar results. Among them are all 13 studies included by Tian et al. as well as five additional studies [22]. Zhao et al. reported both elevated PF and serum leptin levels in endometriosis in their recently published meta-analysis [23]. They included 13 studies on PF leptin concentrations, whereby some of the studies included in the other meta-analyses were omitted but two additional studies were included. In these meta-analyses however, most studies included had sample sizes of less than 50 patients. To the best of our knowledge, the largest study on leptin levels in PF until now included 58 patients with endometriosis and 40 controls [17]. Studies with very small sample sizes are prone to be less representative of the population, have less statistical power and tend to publication bias. Our study has a larger sample size than each of the three mentioned meta-analyses, resulting in greater statistical power.
Five studies have shown lower or similar PF leptin levels in endometriosis, which is in line with our findings. Interestingly, three of these studies included a relatively high number of patients (56, 72 and 98 patients) compared to other studies evaluating PF leptin in endometriosis [17-19,24-26].
Elevated serum leptin concentrations have been reported in obese patients [11,27] and a correlation of BMI and leptin concentration has also been shown in follicular fluid [28]. In line with these findings, our study reports a strong influence of the patient’s BMI on the variance of PF leptin levels. Moreover, endometriosis has been associated with a lower average BMI than women without endometriosis [29,30], which was confirmed in our cohort (Table 1). Therefore, we included BMI as a covariate in the ANCOVA analysis. Although most studies included in the above-mentioned meta-analyses have taken the patients’ BMI into account, BMI was not included as a covariate. Instead, patient groups were either matched according to BMI or a leptin-BMI ratio was applied. Some studies have not considered the BMI in the methodology or statistical analysis at all [25,26]. A confounding bias has to be assumed in these studies. In the present study, we logarithmically transformed the initial leptin values in order to obtain a normal distribution ( Figures 3 and 4) and perform parametric statistical tests. To the best of our knowledge, this has not been performed by any of the previously published studies. Previous studies mostly used non-parametric tests, which apply to non-normally distributed data but have weaker statistical power. Certain studies nevertheless performed parametric tests but did not describe the distribution of the leptin values [25,31-33]. The normal distribution and consequently performed parametric tests in our study represent an advantage in our opinion.
Our observation of lower leptin values in the proliferative compared to the secretory cycle phase is consistent with previously published studies [34,35]. Pathophysiologically, higher leptin expression in the secretory endometrium is assumed, although this remains unclear in detail. Leptin receptors have been found expressed in human endometrium, whereas contradictory findings exist concerning leptin expression by endometrial cells [14,36,37]. Increased leptin expression in ectopic lesions of patients with endometriosis compared to eutopic endometrium has been reported [32,38,39], whereas another study showed no significant elevation in leptin concentration in ovarian tissue affected by endometrioma compared to normal ovarian tissue [24]. Therefore, due to the controversial findings, no conclusive statement regarding leptin expression in the ectopic endometrium could be made yet. Our results suggest no higher expression of leptin in ectopic endometrium.
If higher leptin levels were true in endometriosis, we would also expect higher leptin levels in higher stages of endometriosis and lower leptin levels in patients under hormonal therapy. This was however not shown in our study, which additionally speaks against a positive correlation between endometriosis and PF leptin levels.
Possible limitations also need to be elucidated. First, the possible batch effect due to the duration of the study (11 years), which could mask differences in leptin levels between groups. On the other hand, it is the duration of the study, which allowed a large sample size and sufficient statistical power. Moreover, the retrospective design of the study has to be mentioned. Due to some missing data on hormonal treatment or cycle day as well as progesterone value, some patients could not be included in the ANCOVA analysis. Finally, the determination of the cycle phase could be subject to a certain bias. Although we determined the cycle phase by means of cycle day and, in the mid-cycle period, by means of the progesterone value, as described under paragraph 4, it is possible that individual cases were assigned to an incorrect cycle phase, especially in the case of longer cycles of 35 days and more and short cycles of less than 24 days.
Conclusion
This study reports on peritoneal fluid leptin concentrations in patients with and without endometriosis in the largest sample size to date. In contrast to the existing literature, patients with endometriosis had no difference in PF leptin concentrations compared to patients without endometriosis. This contradictory finding questions whether leptin plays a role in endometriosis pathogenesis and its utility as a biomarker for the disease.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
We would like to thank Marie Roumet, PhD (University of Bern) for statistical consulting.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Bern (Kantonale Ethikkommission für die Forschung). Project-ID 2020-00937, date of approval: 07/23/2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
References
2. Falcone T, Flyckt R. Clinical management of endometriosis. Obstetrics & Gynecology. 2018 Mar 1;131(3):557-71.
3. Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Human Reproduction. 2014 Mar 1;29(3):400-12.
4. Becker CM, Gattrell WT, Gude K, Singh SS. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertility and Sterility. 2017 Jul 1;108(1):125-36.
5. Nirgianakis K, Vaineau C, Agliati L, McKinnon B, Gasparri ML, Mueller MD. Risk factors for non‐response and discontinuation of Dienogest in endometriosis patients: A cohort study. Acta Obstetricia et Gynecologica Scandinavica. 2021 Jan;100(1):30-40.
6. Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: a call to action. American Journal of Obstetrics and Gynecology. 2019 Apr 1;220(4):354-e1.
7. Nisenblat V, Bossuyt PM, Shaikh R, Farquhar C, Jordan V,Scheffers CS, et al. Blood biomarkers for the non‐invasive diagnosis of endometriosis. Cochrane Database of Systematic Reviews.2016(5):CD012179.
8. Nirgianakis K, McKinnon B, Ma L, Imboden S, Bersinger N, Mueller MD. Peritoneal fluid biomarkers in patients with endometriosis: a cross-sectional study. Hormone Molecular Biology and Clinical Investigation. 2021 Jun 1;42(2):113-22.
9. Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Human Reproduction. 2002 Feb 1;17(2):426-31.
10. Houseknecht KL, Baile CA, Matteri RL, Spurlock ME. The biology of leptin: a review. Journal of Animal Science. 1998 May 1;76(5):1405- 20.
11. de Candia P, Prattichizzo F, Garavelli S, Alviggi C, La Cava A, Matarese G. The pleiotropic roles of leptin in metabolism, immunity, and cancer. Journal of Experimental Medicine. 2021 Apr 15;218(5):e20191593.
12. Kumari P, Jaiswar SP, Shankhwar P, Deo S, Ahmad K, Iqbal B, et al. Leptin as a predictive marker in unexplained infertility in north indian population. Journal of Clinical and Diagnostic Research: JCDR. 2017 Mar;11(3):QC28.
13. Childs GV, Odle AK, MacNicol MC, MacNicol AM. The importance of leptin to reproduction. Endocrinology. 2021 Feb;162(2):bqaa204.
14. González RR, Caballero-Campo P, Jasper M, Mercader A, Devoto L, Pellicer A, et al. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. The Journal of Clinical Endocrinology & Metabolism. 2000 Dec 1;85(12):4883-8.
15. Bedaiwy MA, Falcone T, Goldberg JM, Sharma RK, Nelson DR, Agarwal A. Peritoneal fluid leptin is associated with chronic pelvic pain but not infertility in endometriosis patients. Human Reproduction. 2006 Mar 1;21(3):788-91.
16. Mahutte NG, Matalliotakis IM, Goumenou AG, Vassiliadis S, Koumantakis GE, Arici A. Inverse correlation between peritoneal fluid leptin concentrations and the extent of endometriosis. Human Reproduction. 2003 Jun 1;18(6):1205-9.
17. Kocbek V, Vouk K, Bersinger NA, Mueller MD, Rižner TL. Panels of cytokines and other secretory proteins as potential biomarkers of ovarian endometriosis. The Journal of Molecular Diagnostics. 2015 May 1;17(3):325-34.
18. Wertel I, Gogacz M, Polak G, Jakowicki J, Kotarski J. Leptin is not involved in the pathophysiology of endometriosis-related infertility. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2005 Apr 1;119(2):206-9.
19. Barcz E, Milewski Ł, Radomski D, Dziunycz P, Kamiński P, Roszkowski PI, et al. A relationship between increased peritoneal leptin levels and infertility in endometriosis. Gynecological Endocrinology. 2008 Jan 1;24(9):526-30.
20. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-7.
21. Tian Z, Wang Y, Zhao Y, Chang XH, Zhu HL. Serum and peritoneal fluid leptin levels in endometriosis: a systematic review and metaanalysis. Gynecological Endocrinology. 2021 Aug 3;37(8):689-93.
22. Kalaitzopoulos DR, Lempesis IG, Samartzis N, Kolovos G, Dedes I, Daniilidis A, et al. Leptin concentrations in endometriosis: A systematic review and meta-analysis. Journal of Reproductive Immunology. 2021 Aug 1;146:103338.
23. Zhao Z, Wu Y, Zhang H, Wang X, Tian X, Wang Y, et al. Association of leptin and adiponectin levels with endometriosis: a systematic review and meta-analysis. Gynecological Endocrinology. 2021 Jul 3;37(7):591-9.
24. Zendron C, Gonçalves HF, Cavalcante FS, Pereira TR, Evangelista A, Ramos CF, et al. Increased expression of the leptin receptor in human ovaries affected by endometrioma and detection of high levels of leptin in the ovarian endometriomal fluid. Journal of Ovarian Research. 2014 Dec;7(1):1-7.
25. Gonçalves HF, Zendron C, Cavalcante FS, Aiceles V, Oliveira MA, Manaia JH, et al. Leptin, its receptor and aromatase expression in deep infiltrating endometriosis. Journal of Ovarian Research. 2015 Dec;8(1):1-7.
26. Tao Y, Zhang Q, Huang W, Zhu H, Zhang D, Luo W. The Peritoneal Leptin, MCP‐1 and TNF‐α in the Pathogenesis of Endometriosis‐ Associated Infertility. American Journal of Reproductive Immunology. 2011 Apr;65(4):403-6.
27. Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertility and Sterility. 2017 Apr 1;107(4):840-7.
28. Wunder DM, Mueller MD, Birkhäuser MH, Bersinger NA. Steroids and protein markers in the follicular fluid as indicators of oocyte quality in patients with and without endometriosis. Journal of Assisted Reproduction and Genetics. 2005 Jun;22(6):257-64.
29. Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, et al. Risk for and consequences of endometriosis: a critical epidemiologic review. Best practice & research Clinical Obstetrics & Gynaecology. 2018 Aug 1;51:1-5.
30. Shah DK, Correia KF, Vitonis AF, Missmer SA. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Human Reproduction. 2013 Jul 1;28(7):1783-92.
31. Milewski Ł, Barcz E, Dziunycz P, Radomski D, Kamiński P, Roszkowski PI, et al. Association of leptin with inflammatory cytokines and lymphocyte subpopulations in peritoneal fluid of patients with endometriosis. Journal of Reproductive Immunology. 2008 Oct 1;79(1):111-7.
32. Wu MH, Chuang PC, Chen HM, Lin CC, Tsai SJ. Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Molecular Human Reproduction. 2002 May 1;8(5):456-64.
33. De Placido G, Alviggi C, Carravetta C, Pisaturo ML, Sanna V, Wilding M, et al. The peritoneal fluid concentration of leptin is increased in women with peritoneal but not ovarian endometriosis. Human Reproduction. 2001 Jun 1;16(6):1251-4.
34. Ajala OM, Ogunro PS, Elusanmi GF, Ogunyemi OE, Bolarinde AA. Changes in serum leptin during phases of menstrual cycle of fertile women: relationship to age groups and fertility. International Journal of Endocrinology and Metabolism. 2013;11(1):27-33.
35. Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clinical Endocrinology. 1997 Jul;47(1):101-6.
36. Kitawaki J, Koshiba H, Ishihara H, Kusuki I, Tsukamoto K, Honjo H. Expression of leptin receptor in human endometrium and fluctuation during the menstrual cycle. The Journal of Clinical Endocrinology & Metabolism. 2000 May 1;85(5):1946-50.
37. Lima-Couy I, Cervero A, Bonilla-Musoles F, Pellicer A, Simon C. Endometrial leptin and leptin receptor expression in women with severe/moderate endometriosis. Molecular Human Reproduction. 2004 Nov 1;10(11):777-82.
38. Nácul AP, Lecke SB, Edelweiss MI, Morsch DM, Spritzer PM. Gene expression of leptin and long leptin receptor isoform in endometriosis: a case-control study. Obstetrics and Gynecology International. 2013 Jan 1;2013:879618.
39. Oh HK, Choi YS, Yang YI, Kim JH, Leung PC, Choi JH. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. MHR: Basic Science of Reproductive Medicine. 2012 Nov 25;19(3):160-8.