Keywords
C1P, Ceramdie, DKD, Podocyte, S1P, Sphingolipid
Commentary
Diabetic kidney disease (DKD) is a common complication of diabetes [1], characterized by kidney damage. Podocytes are specialized, terminally differentiated cells in the kidney’s filtration barrier that are key responders to the metabolic and environmental changes that occur in diabetes. Change in the function and in the number of podocytes is the main signature of the development and progression of DKD. However, the exact causes of podocyte injury and detachment in DKD are not fully understood. Recent research has shed light on the intricate role that sphingolipid metabolism may play in podocyte injury and its relevance to DKD progression, as discussed in this commentary.
Sphingolipids belong to a large class of lipid molecules which are involved in various cellular processes, including cell membrane structure, cell signaling and proliferation, inflammation, oxidative stress, or apoptosis. The most critical role sphingolipids play is in the lipid raft domains, the sphingomyelin-, cholesterol- and glycosyl-phosphatidylinositol (GPI)-rich microdomains of the plasma membrane. The slit diaphragm of podocytes is a specialized structure that resembles a lipid raft and organized into a complex network of transmembrane proteins such as nephrin, podocin, alpha-actinin-4, signaling adaptors CD2AP and ion channels like TRPC6. The proper localization and function of slit diaphragm proteins depend largely on bioactive sphingolipids. Changes in the sphingolipid composition of lipid raft domains affect the biophysical properties of the cell plasma membrane, leading to changes in signaling properties and further podocyte injury (Reviewed in [2]). Ceramide, ceramide-1-phosphate (C1P), sphingosine-1-phosphate (S1P), glycosphingolipids (particularly, gangliosides) and galactosylceramides (GalCer) are the main sphingolipid metabolites that have been considered as bioactive signaling sphingolipids in podocytes (Figure 1).
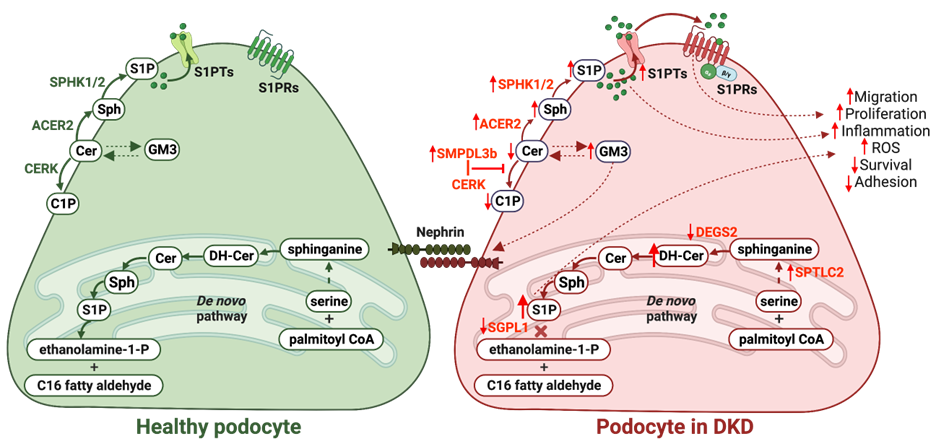
Figure 1. Sphingolipid metabolism dysregulation in podocytes in DKD. Increased activity of serine palmytioltransferase long chain subunit 2 (SPTLC2) and decreased activity of desaturase 2 (DEGS2) in the de novo sphingolipid pathway leads to the accumulation of dihydroceramides (DH-Cer), which causes reactive oxygen species (ROS) accumulation. In turn, decreased activity of sphingosine-1-phosphate lyase 1 (SGPL1) results in the accumulation of sphingosine-1-phosphate (S1P), which contributes to disturbance in all intracellular processes. Disturbance of sphingolipid composition at the plasma membrane due to increased activity of alkaline ceramidase 2 (ACER2) increases levels of sphingosine (Sph), which, together with increased activity of sphingosine kinases 1 and 2 (SPHK1/2), leads to increased production of S1P. Overproduction of S1P results in increased S1P efflux via S1P transporters (S1PTs). Further, effluxed S1P acts as a paracrine factor and activates S1P receptors (S1PRs), which may also contribute to apoptosis. Overexpression of sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b) blocks ceramide kinase (CERK) activity and results in decreased ceramide to ceramide-1-phosphate (C1P) production. However, accumulation of ganglioside GM3 due to sphingolipid metabolism disturbance results in nephrin stabilization at the plasma membrane. This image was created using BioRender software (www.biorender.com).
It is important to note that hyperglycemia is the key determinant of podocyte injury in diabetes. Therefore, high glucose levels significantly contribute to disturbance in sphingolipid metabolism. Thus, excessive glucose has been shown to increase glycosphingolipid production by elevating the availability of ceramide [3]. Another study demonstrated that elevated levels of advanced glycation end-products lead to increased levels of gangliosides in renal cells [4]. Ceramides have also been implicated in insulin resistance via interfering with glucose uptake and impaired storage of glycogen or triglycerides (Reviewed in [5]). Increased production of ceramides is linked to elevated activity of serine palmitoyltransferase, the de novo ceramide synthesis pathway enzyme, in the presence of high glucose levels [6], while decreased activity of acid ceramidase, an enzyme responsible for degradation of ceramide, has been also reported in the hyperglycemia conditions in association with increased inflammatory response [7].
The knowledge about podocyte sphingolipid metabolism under physiological and pathophysiological conditions is scarce. Recent experimental studies however suggest that decreased levels of long- (C14:0, C16:0, C18:0) and very-long- (C24:0, C24:1) chain ceramide species in mice kidney cortices are associated with altered genes expression involved in ceramide synthesis and metabolism [8-10], while human studies suggest an inverse correlation between ceramide long- (C16:0) and very-long- (C24:0) chain species in plasma and kidney cortex [11] and elevated urinary ceramide species [12]. Ceramide accumulation in glomeruli mice with DKD is also associated with expression of junctional adhesion molecule-like protein (JAML) [13]. Importantly, not only ceramide synthesized via de novo pathway are toxic to podocytes, but ceramides produced by the action of ceramidases or sphingomyelinases have also been shown to be equally toxic. Thus, podocyte-specific acid ceramidase 1 (Asah1) deletion in mice leads to increased glomerular ceramide levels and development of nephrotic syndrome [14], which is ameliorated by the deletion of acid sphingomyelinase (Smpd1) gene [15]. Asah1 has also been shown to play an important role in podocyte injury by promoting nicotine adenine dinucleotide phosphate (NADPH) oxidase associated oxidative stress [16], suggesting an existence of a crosstalk between sphingolipids and NADPH oxidase signaling (as reviewed in [17]). However, many aspects of the role of ceramide metabolism in the podocyte remain to be discovered. One of the most important questions to be answered is the role of different ceramide species in DKD development, as short-chain ceramides are generally associated with pro-survival and anti-inflammatory effects, while long-chain ceramides are often linked to inflammation and apoptosis.
Ceramide can be further catabolized into sphingosine, which is then phosphorylated into S1P, a bioactive sphingolipid that has gained a lot of attention in recent years. Very often, S1P and ceramide antagonistically signal cell survival or death largely via shared mediators, and both can directly inhibit each other’s synthesis. S1P primarily acts as a ligand for high-affinity five cell-surface receptors (S1Pr1-S1Pr5). However, its intracellular actions may also possess signaling potential, despite the fact that its direct targets remain elusive and controversial [18]. Loss of S1P lyase, an enzyme that degrades S1P, has been shown to be associated with nephrotic syndrome [19,20]. In podocytes, this loss resulted in a reduction of nephrin, an important protein of a slit diaphragm, via a protein kinase C d-dependent mechanism [21]. Additionally, the levels of sphingosine kinase, an enzyme that catalyzes S1P formation, are increased in DKD, leading to increased renal S1P levels as shown in rodent models [22,23] and in patients with DKD [9,22]. Abnormalities in S1P receptors expression also contribute to DKD progression (Figure 1), where S1P signaling mediated by S1Pr2 is shown to be toxic to cells, while S1Pr1 signaling has a protective role [24]. While significant progress has been made in understanding S1P signaling in podocytes, several questions remain unanswered. The relative contribution of intracellular and extracellular S1P remains to be established. Furthermore, the relative expression and activation of S1P receptors in podocytes during DKD, and the downstream signaling pathways involved, remain largely unexplored. Finally, it also remains unclear what the intracellular resources of S1P are in the podocyte, as well as how S1P transport and distribution occurs within the cell. Elucidating S1P crosstalk with other signaling pathways, such as renin-angiotensin system, oxidative stress, or inflammation-related pathways, needs further investigation and may lead to the development of targeted therapeutic intervention.
Ceramide can also be phosphorylated into C1P by ceramide kinase (CERK), another bioactive sphingolipid involved in diverse intracellular processes including survival, proliferation, migration through an unknown receptor-initiated signaling or inflammation. The function of C1P in the podocyte remains largely unknown. We recently demonstrated decreased levels of C1P in kidney cortices of mice with DKD [8]. This was associated with increased expression of sphingomyelin phosphodiesterase acid-like 3b (SMPDL3b), an enzyme of the sphingolipid pathway that modulates insulin receptor signaling and that regulates C1P availability in podocytes via interfering with CERK expression [25]. Given the fact that C1P is heavily involved in the cellular processes, as shown in other cell types, its role in podocytes remains largely unknown and requires further investigation.
In addition, the role of glucosylceramides (glucosylcerebrosides, lactocylceramides and gangliosides) in podocyte biology and in DKD has recently gained some attention. Thus, glucosylceramide C18:0 is increased in plasma of mouse models of DKD, while glucosylceramide C16:0 is decreased in the DKD kidney [10]. In previous studies, ganglioside GM3, the most abundant sphingolipid in podocytes, has been found to be up-regulated in DKD and implicated in insulin resistance [26], while a recent study demonstrated that increased GM3 levels stabilize nephrin and prevent podocyte loss and albuminuria [27]. Increased levels of hexocyl-, glucosyl-, galactosyl- and lactosylceramides were observed in kidney cortices of mouse model of DKD [3]. Another study reported an association between hexosylceramide C18:1 and DKD, while very-long-chain lactosylceramide species are associated with microalbuminuria development in patients with type 1 diabetes [28].
Understanding the intra- or extracellular factors that drive sphingolipid pathophysiology in podocytes, as well as the contribution of specific sphingolipid species to podocyte physiology and pathophysiology is likely to result in the development of novel diagnostic biomarkers and novel therapeutic opportunities. Numerous studies have investigated the potential of sphingolipids as diagnostic biomarkers for DKD. These studies have identified specific changes in the levels of sphingolipids in the blood or urine of DKD patients compared to individuals without DKD or with other types of kidney disease. For example, recent research has established links between urinary ceramides and the various stages of diabetic nephropathy. In this study, certain ceramides such as Cer d18:1/16:0, Cer d18:1/18:0, Cer d18:1/20:0, Cer d18:1/22:0, and Cer d18:1/24:0 were found to be elevated in patients with stage 3 of diabetic nephropathy. Furthermore, the levels of these ceramides in urine were positively associated with urinary clinical biomarkers such as albumin and N?acetyl?β?d?glucosaminidase, as well as the sediment score [12]. In addition, a clinical study demonstrated increased levels of C16 and C18 ceramides in the plasma of patients with type 2 diabetes (T2D) [29]. Similarly, elevated levels of C16 and C18 ceramides were found in the urine of DKD patients, and these levels were correlated with urinary biomarkers such as albumin [10]. Conversely, another study revealed that lower plasma levels of very long chain ceramide species (C20–C26) were associated with the progression of proteinuria in DKD [30]. These findings indicate that sphingolipids, particularly ceramides, may not only serve as diagnostic biomarkers for DKD but also as indicators of disease progression and prognosis. In addition to their potential as diagnostic biomarkers, the identification of sphingolipids as key players in DKD has led to the exploration of sphingolipid-targeted therapeutic interventions. Activation of sphingosine-1-phosphate receptor 1 (S1P1R) has been shown to have protective effects on renal function and histology in an STZ-induced diabetic rat model [31]. Furthermore, we have previously demonstrated that supplementation with exogenous C1P may represent a lipid therapeutic strategy to treat DKD [8]. However, further research is needed to better understand the complex sphingolipid signaling network and validate the efficacy and safety of sphingolipid-targeted therapies in clinical settings.
Therefore, our rudimentary understanding of the role of sphingolipid metabolism in podocytes in health and disease is expanding and paves the way for further investigations.
Disclosure
A.M. and R.N. declare nothing to disclose. A.F. is one of the inventors on pending (PCT/US2019/032215; US 17/057,247; PCT/US2019/041730; PCT/US2013/036484; US 17/259,883; US17/259,883; JP501309/2021, EU19834217.2; CN-201980060078.3; CA2,930,119; CA3,012,773; and CA2,852,904) or issued patents (US10,183,038 and US10,052,345) aimed at preventing and treating renal disease. A.F. stands to gain royalties from their future commercialization. A.F. is a vice president of L&F Health LLC and a consultant for ZyVersa Therapeutics, Inc. ZyVersa Therapeutics, Inc. has licensed worldwide rights to develop and commercialize hydroxypropyl-beta-cyclodextrin for the treatment of kidney disease from L&F Research, which was partially funded by L&F Health LLC. A.F. also holds equities in Renal 3 River Corporation.
Funding
A.M. is supported by Chernowitz Medical Research Foundation (GR021608), Carl W. Gottschalk Research Scholar Grant, American Society of Nephrology (GR018262) and CTSI Pilot Award Program by University of Miami Miller School of Medicine (PG015117). A.F. is supported by National Institutes of Health grants R01DK104753 and R01CA227493. A.F. is also supported by U54DK083912, UM1DK100846, U01DK116101, and UL1TR000460 (Miami Clinical Translational Science Institute).
References
2. Mitrofanova A, Drexler Y, Merscher S, Fornoni A. Role of sphingolipid signaling in glomerular diseases: focus on DKD and FSGS. Journal of Cellular Signaling. 2020 Sep;1(3):56-69.
3. Subathra M, Korrapati M, Howell LA, Arthur JM, Shayman JA, Schnellmann RG, et al. Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. American Journal of Physiology-Renal Physiology. 2015 Aug 1;309(3):F204-15.
4. Tsuboi N, Utsunomiya Y, Kawamura T, Kawano T, Hosoya T, Ohno T, et al. Ganglioside as an endogenous growth suppressor for glomerular mesangial cells. Kidney International. 2001 Oct 1;60(4):1378-85.
5. Chaurasia B, Summers SA. Ceramides–lipotoxic inducers of metabolic disorders. Trends in Endocrinology & Metabolism. 2015 Oct 1;26(10):538-50.
6. Imierska M, Zabielski P, Roszczyc-Owsiejczuk K, Sokołowska E, Pogodzińska K, Kojta I, et al. Serine palmitoyltransferase gene silencing prevents ceramide accumulation and insulin resistance in muscles in mice fed a high-fat diet. Cells. 2022 Mar 26;11(7):1123.
7. Yuan X, Bhat OM, Lohner H, Zhang Y, Li PL. Endothelial acid ceramidase in exosome-mediated release of NLRP3 inflammasome products during hyperglycemia: Evidence from endothelium-specific deletion of Asah1 gene. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2019 Dec 1;1864(12):158532.
8. Mitrofanova A, Mallela SK, Ducasa GM, Yoo TH, Rosenfeld-Gur E, Zelnik ID, et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nature Communications. 2019 Jun 19;10(1):2692.
9. Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. Journal of the American Society of Nephrology: JASN. 2015 Jan;26(1):133-47.
10. Sas KM, Nair V, Byun J, Kayampilly P, Zhang H, Saha J, et al. Targeted lipidomic and transcriptomic analysis identifies dysregulated renal ceramide metabolism in a mouse model of diabetic kidney disease. Journal of Proteomics & Bioinformatics. 2015 Oct.
11. Yun H, Sun L, Wu Q, Zong G, Qi Q, Li H, et al. Associations among circulating sphingolipids, β-cell function, and risk of developing type 2 diabetes: A population-based cohort study in China. PLoS Medicine. 2020 Dec 9;17(12):e1003451.
12. Morita Y, Kurano M, Sakai E, Nishikawa T, Nishikawa M, Sawabe M, et al. Analysis of urinary sphingolipids using liquid chromatography‐tandem mass spectrometry in diabetic nephropathy. Journal of Diabetes Investigation. 2020 Mar;11(2):441-9.
13. Fu Y, Sun Y, Wang M, Hou Y, Huang W, Zhou D, et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metabolism. 2020 Dec 1;32(6):1052-62.
14. Li G, Kidd J, Kaspar C, Dempsey S, Bhat OM, Camus S, et al. Podocytopathy and nephrotic syndrome in mice with podocyte-specific deletion of the Asah1 gene: role of ceramide accumulation in glomeruli. The American journal of pathology. 2020 Jun 1;190(6):1211-23.
15. Li G, Huang D, Camus S, Li N, Li PL. Amelioration of Podocytopathy by Smpd1 Gene Deletion in Podocyte‐Specific Asah1 Gene Knockout Mice. The FASEB Journal. 2020 Apr;34(S1):1.
16. Boini KM, Xia M, Li C, Zhang C, Payne LP, Abais JM, et al. Acid sphingomyelinase gene deficiency ameliorates the hyperhomocysteinemia-induced glomerular injury in mice. The American Journal of Pathology. 2011 Nov 1;179(5):2210-9.
17. Njeim R, Alkhansa S, Fornoni A. Unraveling the Crosstalk between Lipids and NADPH Oxidases in Diabetic Kidney Disease. Pharmaceutics. 2023 Apr 28;15(5):1360.
18. Drexler Y, Molina J, Mitrofanova A, Fornoni A, Merscher S. Sphingosine-1-phosphate metabolism and signaling in kidney diseases. Journal of the American Society of Nephrology: JASN. 2021 Jan;32(1):9-31.
19. Lovric S, Goncalves S, Gee HY, Oskouian B, Srinivas H, Choi WI, et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. The Journal of Clinical Investigation. 2017 Mar 1;127(3):912-28.
20. Prasad R, Hadjidemetriou I, Maharaj A, Meimaridou E, Buonocore F, Saleem M, et al. Sphingosine-1-phosphate lyase mutations cause primary adrenal insufficiency and steroid-resistant nephrotic syndrome. The Journal of Clinical Investigation. 2017 Mar 1;127(3):942-53.
21. Imeri F, Stepanovska Tanturovska B, Manaila R, Pavenstädt H, Pfeilschifter J, Huwiler A. Loss of S1P Lyase Expression in Human Podocytes Causes a Reduction in Nephrin Expression That Involves PKCδ Activation. International Journal of Molecular Sciences. 2023 Feb 7;24(4):3267.
22. Klein RL, Hammad SM, Baker NL, Hunt KJ, Al Gadban MM, Cleary PA, et al. Decreased plasma levels of select very long chain ceramide species are associated with the development of nephropathy in type 1 diabetes. Metabolism. 2014 Oct 1;63(10):1287-95.
23. Ren S, Babelova A, Moreth K, Xin C, Eberhardt W, Doller A, et al. Transforming growth factor-β2 upregulates sphingosine kinase-1 activity, which in turn attenuates the fibrotic response to TGF-β2 by impeding CTGF expression. Kidney International. 2009 Oct 2;76(8):857-67.
24. Sui J, He M, Wang Y, Zhao X, He Y, Shi B. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Experimental and Therapeutic Medicine. 2019 Nov 1;18(5):3603-14.
25. Mallela SK, Mitrofanova A, Merscher S, Fornoni A. Regulation of the amount of ceramide-1-phosphate synthesized in differentiated human podocytes. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2019 Dec 1;1864(12):158517.
26. Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, et al. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proceedings of the National Academy of Sciences. 2007 Aug 21;104(34):13678-83.
27. Kawashima N, Naito S, Hanamatsu H, Nagane M, Takeuchi Y, Furukawa JI, et al. Glycosphingolipid GM3 prevents albuminuria and podocytopathy induced by anti-nephrin antibody. Scientific Reports. 2022 Sep 26;12(1):16058.
28. Lopes-Virella MF, Baker NL, Hunt KJ, Hammad SM, Arthur J, Virella G, et al. Glycosylated sphingolipids and progression to kidney dysfunction in type 1 diabetes. Journal of Clinical Lipidology. 2019 May 1;13(3):481-91.
29. Chew WS, Torta F, Ji S, Choi H, Begum H, Sim X, et al. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight. 2019 Jul 7; 4;5(13):e126925.
30. Liu JJ, Ghosh S, Kovalik JP, Ching J, Choi HW, Tavintharan S, et al. Profiling of plasma metabolites suggests altered mitochondrial fuel usage and remodeling of sphingolipid metabolism in individuals with type 2 diabetes and kidney disease. Kidney International Reports. 2017 May 1;2(3):470-80.
31. Awad AS, Rouse MD, Khutsishvili K, Huang L, Bolton WK, Lynch KR, et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney International. 2011 May 2;79(10):1090-8.
