Abstract
A 59-year-old man presents to the hospital following a syncopal episode after a low-speed car accident without visible injuries. He was diagnosed with stress cardiomyopathy and severe triple-vessel coronary artery disease. The case was complicated by complete heart block that culminated in refractory cardiogenic shock. Literature and current approach are reviewed.
Keywords
Takotsubo, Stress induced cardiomyopathy, Complete heart block, Cardiogenic shock, Coronary artery disease, Impella, IABP
Abbreviations
IABP: Intra-Aortic Balloon Pump; SIC: Stress Induced Cardiomyopathy; CS: Cardiogenic Shock; CAD: Coronary Artery Disease, PPM: Permanent Pacemaker; HAD: Hemodynamic Assisting Devices
History of Presentation
A 59-year-old man was found diaphoretic and transiently unresponsive in his recliner. A few hours prior to this episode, the patient had a low-speed car accident on an icy road that did not result in airbag deployment. He was the restrained driver and did not sustain any visible injuries. However, he felt distraught by the accident and became nauseated and sweaty shortly after. The patient denies chest pain, shortness of breath, palpitations, or dizziness and a review of systems did not illicit any additional complaints. Upon evaluation, he was afebrile, hypotensive (91/62 mmHg), tachycardic (112 beats/minute), and saturated comfortably on ambient room air. He was diaphoretic and lethargic but appropriately responsive with a GCS of 15/15. The rest of the physical exam did not reveal any signs of acute trauma and was otherwise unrevealing.
Past Medical History
Non-insulin-dependent diabetes, hypertension, hyperlipidemia, and ischemic stroke with residual dysphagia requiring percutaneous gastrostomy tube for feeding.
Differential Diagnosis
Acute coronary syndrome, cardiac contusion, pneumothorax, aortic dissection, acute heart failure, pulmonary embolism, arrhythmia, and stroke.
Investigations
A plain chest radiograph revealed increased interstitial markings, but no acute fractures or pneumothorax. Electrocardiography shown below (Figure 1). Labs were notable for leukocytosis 19.0 10ˆ3/μL, high sensitivity troponin was 2,810 and later 33,119 pg/mL, brain natriuretic peptide was 90 pg/dL, and lactate level was 3.2 mMol/L. CT scan of the head was negative for acute events. CT angiography of the chest visualized an intact aorta and demonstrated no evidence of pulmonary embolism, pneumothorax, pulmonary contusion, or other acute traumatic injuries. Imaging of the abdomen and pelvis did not show solid organ injuries or free intra-abdominal fluid.
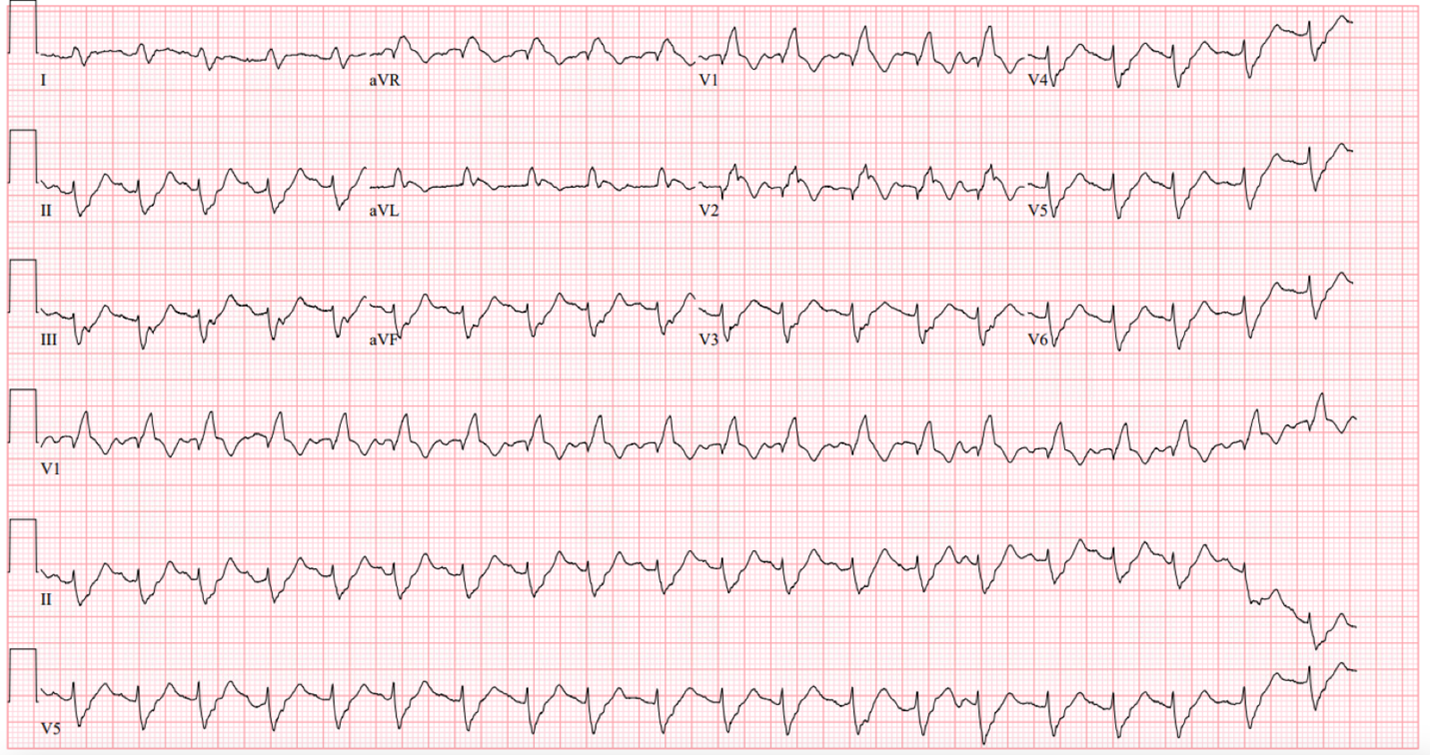
Figure 1: EKG on presentation: SVT (Supraventricular Tachycardia) with aberrancy, right bundle branch block, left axis deviation resembling bifasicular block.
Management
A left heart catheterization revealed a pattern of apical ballooning consistent with stress cardiomyopathy (Figure 2) and a severely depressed left ventricular ejection fraction of 10%. Significant obstructive triple-vessel coronary artery disease was noted with poor collateral circulation (Figures 3-4). No evident left ventricular outflow obstruction. An intra-aortic balloon pump (IABP) was placed, while coronary interventions were held at that time. On the second day of hospitalization, the patient developed intermittent high-grade AV block with hemodynamic instability which progressed to persistent complete heart block (CHB) (Figure 5). Medical management and transcutaneous pacing were initiated until transvenous pacing was achieved. The IABP was later exchanged for an Impella device due to refractory cardiogenic shock and worsening vasopressor requirements.
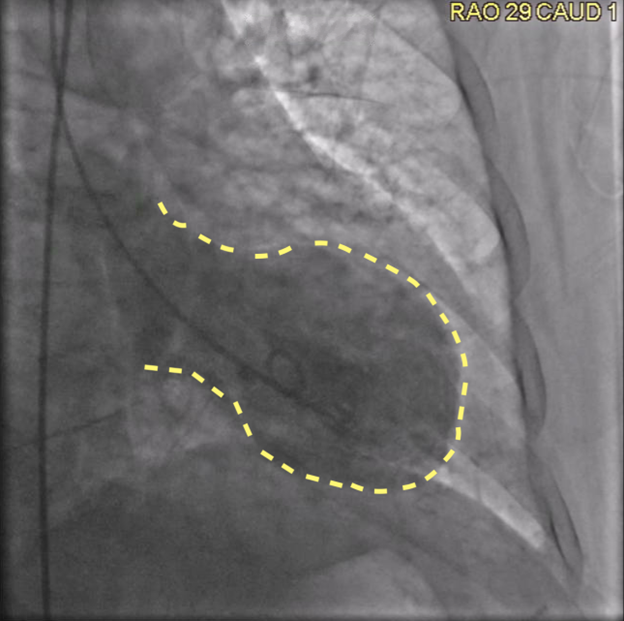
Figure 2: Coronary angiogram, right anterior oblique, caudal view revealing classical apical ballooning, as outlined with yellow striped line.
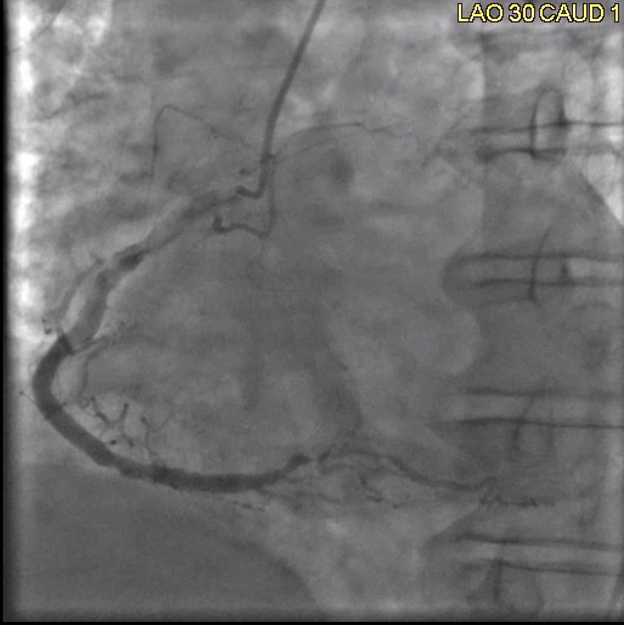
Figure 3: Coronary angiogram, left anterior oblique view, mild RCA beading, severely diseased RPDA.
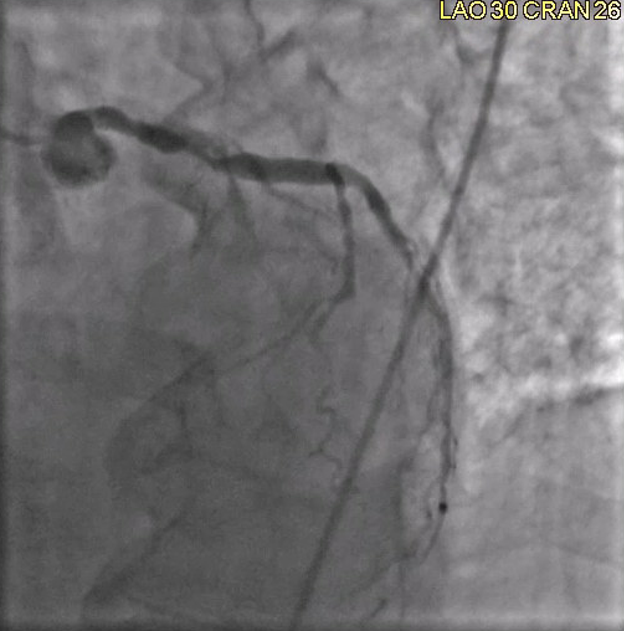
Figure 4. Left anterior oblique view showing severe stenosis at the ostium of LAD.
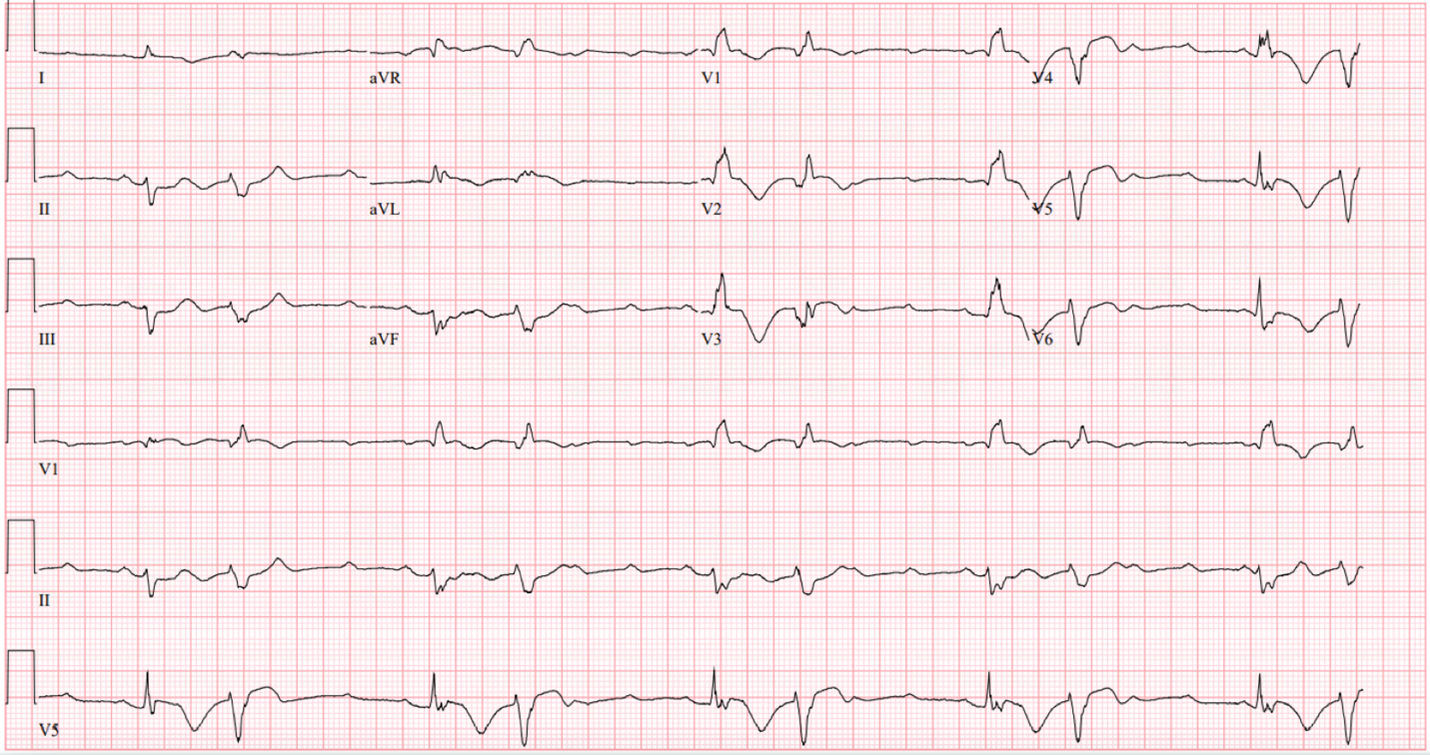
Figure 5. EKG on day 2, complete heart block, ventricular rate 30 bpm, PVCs (PREMATURE VENTRICULAR CONTRACTIONS) burden 50%.
Discussion
Takutsobo; the octopus pot, or stress-induced cardiomyopathy (SIC) is a form of collateral damage striking cardiac compliance after excessive emotional or physical event. Generally, the regional systolic dysfunction does not follow a specific coronary territory, and thus is believed to occur independently. Though previously considered self-limiting, the recognition of more severe presentations with cardiogenic shock (CS), arrhythmia, and coexistent coronary artery disease (CAD) has necessitated a reassessment of current approach.
Concomitant CAD in patients with SIC is not uncommon. Though few cases reported successful intervention in the acute phase, mortality risk remained higher in combined SIC-CS than CAD related CS [1,2]. It is believed that the high surge of catecholamines nicking the stunned myocardium may generate shock state in 11.4% of cases [3]. Albeit, using inotropes should be carefully considered, especially if an outflow tract obstruction along with anterior motion of mitral flap was present. Herein, bridging hemodynamic assisting devices (HAD) such as IABP, or impella was suggested to unload refractory CS. Though the resultant picture appears gloomy, current literature does not classify SIC induced CS as an independent mortality factor as in CAD related CS [2,3]. It is important to note that prior studies selected their population per original Mayo clinic criteria, where the presence of CAD is excluded.
Bradyarrhythmia, and particularly complete heart block, are rare complications of SIC. Prior work estimated the prevalence as 0.7-2.2% of all cases per Mayo Clinic criteria [4,5]. Most of these individuals were identified at older ages, and the presence of degenerative conduction disease couldn’t be ruled out. The decision regarding implanting a permanent pacemaker (PPM) is clinically justified. Most of the reported cases acquired PPM despite the recovery of SIC [5-7]. Our patient was relatively young with no known history of conductive or infiltrative diseases. However, implanting a transvenous pacer along with HAD could not help resolve CS, but rather composed multi-organ failure. This case highlights the importance of investigating SIC related arrhythmia at large scale in light of existing CAD and CS.
Further research endeavors could be directed towards exploring the concurrent presence of valvular heart disease, heart failure, structural abnormalities, and conduction defects in individuals with implanted cardiac devices, particularly in the context of stress-induced cardiomyopathy (SIC) cases. It would be valuable to develop novel protocols for the effective management of emergency medical conditions, with a specific focus on SIC. Conducting comprehensive clinical and observational studies would contribute to a deeper understanding of critical emergencies, facilitating the development of more refined approaches in the future. Additionally, investigating the efficacy of conventional interventions in controlling stress-related triggers that may contribute to the recurrence of SIC cases holds promise for further advancements in this field.
Follow-up
The patient continued to have refractory cardiogenic shock despite maximal therapy (Table 1). The clinical course was, unfortunately, exacerbated by multi-organ dysfunction. Due to the futility of additional therapy, the family elected to de-escalate care and the patient subsequently passed away.
|
Hospital Day |
Vasopressor |
Inotropes |
Hemodynamic assisting device |
Transvenous pacing |
Mechanical Ventilation |
EF |
|
Day 1 |
- |
- |
IABP |
None |
None |
19% |
|
Day 2 |
Phenylephrine, Epinephrine Norepinephrine, Vasopressin, |
Dobutamine |
IABP |
None |
Yes |
- |
|
Day 3 |
Phenylephrine, Dopamine, Epinephrine, Norepinephrine, Vasopressin |
Milrinone |
Impella |
Yes |
Yes |
- |
|
Day 4 |
Phenylephrine, Epinephrine, Dopamine, Norepinephrine Vasopressin |
Milrinone |
Impella |
Yes |
Yes |
- |
|
Day 5 |
Phenylephrine, Epinephrine, Norepinephrine Dopamine, Vasopressin |
Dobutamine Milrinone |
Impella |
Yes |
Yes |
24% |
|
Day 6 |
Phenylephrine, Epinephrine, Norepinephrine Dopamine, Vasopressin |
Milrinone |
Impella |
Yes |
Yes |
- |
Conclusion
Though SIC is no longer considered benign, the ominous triad of CHB, obstructive CAD and CS creates a significant challenge navigating staged medical versus interventional approaches.
Learning Objectives
- SIC-induced CHB is rare but could be fatal, the decision regarding PPM is clinically justified.
- This case highlights the importance of investigating SIC related arrhythmia at large scale in light of existing CAD.
- The interplay between SIC-CS, severe CAD and CHB creates a significant challenge navigating medical versus interventional approaches.
Disclosures
The authors have nothing to disclose.
Funding
None.
References
2. Napp LC, Cammann VL, Jaguszewski M, Szawan KA, Wischnewsky M, Gili S, et al. Coexistence and outcome of coronary artery disease in Takotsubo syndrome. Eur Heart J. 2020;41(34): 3255-3268.
3. Almendro-Delia M, Núñez-Gil IJ, Lobo M, Andrés M, Vedia O, Sionis A, et al. Short- and Long-Term Prognostic Relevance of Cardiogenic Shock in Takotsubo Syndrome: Results From the RETAKO Registry. JACC Heart Fail. 2018;6(11):928-936.
4. Patil SP, Gonuguntla K, Rojulpote C, Kumar M, Chen KG. Takotsubo cardiomyopathy related complete heart block: a nationwide cohort analysis. Eur Heart J. 2020;41(Supplement_2):ehaa946.0697.
5. Stiermaier T, Eitel C, Denef S, Desch S, Schuler G, Thiele H, et al. Prevalence and Clinical Significance of Life-Threatening Arrhythmias in Takotsubo Cardiomyopathy. J Am Coll Cardiol. 2015;65(19):2148-50.
6. Terui T, Iwai-Takano M, Watanabe T. Permanent Pacemaker Implantation in a Patient with Takotsubo Cardiomyopathy and Complete Atrioventricular Block. Case Rep Cardiol. 2021;2021:6637720.
7. McGee MJ, Yu W, McCarthy J, Barlow M, Hackworthy R. Complete Heart Block Complicating Takotsubo Syndrome: Case Report and Literature Review. Case Rep Cardiol. 2020;2020:7614836.
