Abstract
Background. COVID-19 patients in intensive care units suffer from bacterial/fungal superinfections. However, the incidence and cause of such superinfections in high-altitude hospitals remain poorly investigated.
Objectives. The aim of this study was to evaluate the frequency of bacterial/fungal superinfection in patients with COVID-19 hospitalized in the intensive care unit (ICU) of the Hospital Universitario San José de Bogotá, Colombia, located at an altitude of 2,651 meters above sea level (high altitude). The impact of corticosteroids on the development of infection was also evaluated.
Methods. The cohort included 279 patients, of which 188 (67.4%) were male, 116 (42.3%) were treated with dexamethasone, and 48 (17.2%) were diagnosed with superinfection. A retrospective descriptive cohort study was performed to evaluate the association between bacterial/fungal superinfection frequency, corticosteroid treatment, mechanical ventilation, and mortality rate.
Results. Our results showed that bacteremia was the most frequent diagnosis (n=20; 41.6%) of patients with superinfection, followed by pulmonary superinfection (n=17; 35.4%). The most frequently identified causative agents of superinfection were K. pneumoniae (n=23; 26.1%), C. albicans (n=10; 11.4%) and P. aeruginosa (n=8; 9.1%). Moreover, our results showed no association between corticosteroid treatment (or the use of empiric antibiotic treatment) and mortality. However, we found a significant association between bacterial/fungal superinfection and the number of days on mechanical ventilation. However, bacterial/fungal superinfection showed no impact on the mortality rate.
Conclusions. We conclude that bacterial/fungal superinfection in ICU highland patients with SARS-CoV-2 treated at Hospital Universitario San José in Bogotá, Colombia, increases mainly in proportion to the time required for mechanical ventilation.
Keywords
SARS-CoV-2, ICU, Corticosteroids, Co-infection, Bacterial, Fungal
Introduction
The COVID-19 pandemic is associated with a large number of patients spending long periods of time in intensive care units (ICUs) [1], many of them suffering bacterial/fungal superinfections [2]. However, both the incidence and cause of bacterial/fungal superinfections in COVID-19 critically ill patients remain poorly investigated in hospitals located at high altitudes (above 2500 meters). At sea level, studies have reported secondary infections in the range of 5–27% in the severe acute respiratory syndrome (SARS)-CoV-2 [3]. Despite this low incidence of superinfection, but high mortality of critically ill patients with COVID-19, the extensive use of antibiotics (more than 70% of cases) has been motivated by the suspicion of bacterial pneumonia [4]. On the other hand, the incidence of a second lower respiratory tract infection among mechanically ventilated critically ill patients with COVID-19 was reported to be 50.5% [3-6]. Although it is presumed that these patients have a higher risk of secondary infection due to the need for mechanical ventilation, central venous catheters or prolonged hospital stays cannot be precluded [5]. Several common associated risk factors are linked to superinfection, such as advanced age (over 60 years), ICU admission, mechanical ventilation, renal failure requiring hemodialysis and pharmacological immunosuppression, among others. Despite this, unexpectedly low bacterial/fungal superinfection rates have been reported in patients participating in clinical trials of immunosuppressive therapies to treat SARS-CoV-2 pneumonia [7]. Moreover, the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial demonstrated that dexamethasone reduced mortality in COVID-19 patients, especially in mechanically ventilated patients [8]. However, this study did not report on the impact of corticosteroids on the development of secondary infections. Corticosteroids in other viral pneumonias have been shown to delay viral clearance, decrease immune system function, and contribute to secondary infections [9-11].
In this work, we evaluated the association between the frequency of bacterial/fungal superinfection, corticosteroid treatment, mechanical ventilation and mortality rate at the Hospital Universitario San José de Bogotá, Colombia, located at an altitude of 2651 meters. Pointing out the geographical altitude is of clinical importance since previous studies have shown that high altitude barometric hypoxia is associated with significant decreases in infection rate, severe symptoms, and mortality from COVID-19 compared to those at low altitude [12-14]. We found that bacterial/fungal superinfection in ICU patients with SARS-CoV-2 is mainly associated with the duration of mechanical ventilation required by the patients.
Methods
Ethics declaration
The project was approved by the corresponding Research and Ethics Committee and was considered a risk-free study since only clinical histories were collected.
Study design and inclusion/exclusion criteria
A retrospective descriptive cohort study was performed. Inclusion criteria were PCR-confirmed COVID-19 patients older than 18 years, admitted to the ICU of the Hospital Universitario de San José during April and December 2020, corresponding to the first wave of COVID-19 infection. Exclusion criteria were pregnant patients, patients with confirmed bacterial and/or fungal co-infections prior to admission, and patients with immunosuppression. Convenience sampling was performed and included all patients who met the aforementioned selection criteria.
Diagnosis of superinfections
A superinfection, or secondary infection, was defined as such when there was evidence of: 1) clinical deterioration, 2) positive sample obtained 2 or more days after hospital admission, and 3) finding of pathogenic microorganisms (bacterial, fungal, or viral other than SARS-CoV-2), through the use of culture media, galactomannan antigen assay (GM-EIA) or PCR. Findings of coagulase-negative staphylococci in a single blood culture and Candida spp. in the respiratory tract they were considered contaminants of the commensal flora and not superinfections. Patients with microbial infections between days 0 and 2 after admission were excluded from this study.
Patients
A total of 279 patients were studied, including 188 men (67.4%) and 91 (32.6%) women. Median age was 62 years (interquartile range [IQR] 52-69). The clinical history of patients showed that 144 (51.6%) had arterial hypertension, 89 (31.9%) had diabetes mellitus, and 51 (18.3%) were smokers. In addition, 100 (35.8%) had hyperglycemia, and 33 (11.8%) had lower gastrointestinal bleeding. Furthermore, 86 patients (30.8%) were overweight, and 79 (28.3%) were grade II obese. The median hospitalization time was 8 days (IQR 4-14). The APACHE index at admission had a median of 10 points (IQR 6-15) and the SOFA index had a median of 4 points (IQR 2-6) (Table 1).
|
VARIABLE |
TOTAL PATIENTS |
SUPERINFECTION |
NO SUPERINFECTION |
p-value |
|
N:279 |
N:48 |
N: 231 |
||
|
Age |
62 (52-69) |
60.5 (51-66.75) |
62 (52-69) |
0.350* |
|
Apache - median (IQR) |
10 (6-15) |
12 (9-17.5) |
10 (6-14) |
0.039* |
|
SEX Men |
91(32.6) |
31 (64.6) |
157 (68.0) |
0.01** |
|
SEX Women |
188 (67.4) |
17 (35.4) |
74 (32.0) |
0.001** |
|
SOFA - Median (IQR) |
4 (2-6) |
5 (3-7.7) |
3 (2-6) |
0.085* |
|
Comorbidities |
176 (63.1) |
33 (68.8) |
143 (61.9) |
0.328** |
|
Hypertension |
144 (51.6) |
30 (62.5) |
114 (49.4) |
0.554** |
|
Diabetes Mellitus |
89 (31.9) |
20 (41.7) |
70 (30.3) |
1.000** |
|
Smoking |
51(18.3) |
8 (16.7) |
43 (18.6) |
1.000** |
|
Obesity (IMC) |
104 (40.9) |
25 (52.1) |
89 (38.5) |
0.003** |
|
Use of corticoids |
118 (42.3) |
25 (52.1) |
93 (40.3) |
1.000** |
|
Days of corticoids |
7 (4-10) |
7 (3-10) |
7 (4-10) |
0.644* |
|
Days of antibiotics |
7 (5-11) |
15 (7.2-25. 7) |
7 (4-8) |
0.000* |
|
Days of IMV - median (IQR) |
9 (4-15) |
17 (12-29) |
7 (4-10) |
0.001* |
|
PaFi02 to admission |
125 (85-192) |
130 (85.5-173-7) |
122 (85-198) |
0.083* |
|
Days of HNFC - median (IQR) |
4 (3-7) |
5 (2.2-13.7) |
4 (3-7) |
0.664* |
|
Pneumothorax Spontaneous |
15 (5.4) |
5 (10.4) |
10 (4.3) |
0.286** |
|
Tracheostomy |
19 (6.8) |
14 (29.2) |
5 (2.2) |
0.292** |
|
Failed extubation |
12 (4.3) |
11(22.9) |
1(0.4) |
- |
|
Days of hospitalization |
9 (4-15) |
19 (14-35) |
4 (4-12) |
0.083* |
|
Mortality |
135 (48.4) |
24 (50.0) |
111(48.1) |
1.000** |
Oxygen therapy
Noninvasive ventilation (NIV) was used to treat patients with acute hypoxemic respiratory failure (AHRF) who did not respond adequately to high-flow nasal oxygen therapy (HFNO). NIV was administered as bilevel positive airway pressure (BiPAP) with a mechanical ventilator (Puritan Bennett 840R). The initial NIV settings were IPAP 12 cm H2O and EPAP 8 cm H2O. If required, IPAP was adjusted to 16 cm H2O to relieve the work of breathing. Subsequently, the criteria for intubation (mechanical ventilation) included signs of persistent or worsening respiratory failure, hemodynamic instability, or altered consciousness. Optiflow (Fisher & Paykel Healthcare, Auckland, New Zealand), Airvo 2 (Fisher & Paykel Healthcare, Auckland, New Zealand) and Hi-Flow Star (Dräguer, Lübeck, Germany) high-flow devices were used for this therapy. Treatment was initiated with flow parameters between 50 and 60 ltr/min and inspired oxygen fraction (FiO2) of 1, with progressive titration to maintain saturation between 90% and 92%. The parameters were adjusted according to the clinical judgment of the attending physician and the patient's needs. The main objective of mechanical ventilation was maintaining a lung protective strategy for all patients with acute respiratory distress syndrome (ARDS), defined as a tidal volume of 4 to 8 mL/kg predicted body weight (PCP), and a plateau pressure less than 30 cmH2O. The prone position was applied to all patients with invasive mechanical ventilation and patients with a high-flow nasal cannula, according to patient need and tolerance.
Corticosterone and antibiotics administration
Routine corticosteroid administration for patients with ARDS due to SARS-CoV-2 is dexamethasone 6 mg/day for 10 days. Patients with septic shock receive equivalent doses with an infusion of 200 mg of hydrocortisone over 24 hours or 40 mg/12 hours of methylprednisolone. The diagnosis of superinfection was defined as the microbiological isolation of infectious agents different from the viral etiology of SARS- CoV-2 during the patient's stay in the ICU. Empirical antibiotic treatment included beta-lactams 12-18 gr/24 hrs and macrolides 500 mg c/12 hrs for patients with suspected community-acquired co-infection. For patients with nosocomial superinfections, treatment with carbapenems (meropenem 2 gr c/8 hrs) and macrolides (clarithromycin 500 mg c/12 hrs) was used. If necessary, these treatments were modified according to the results of the corresponding microbial cultures.
Data and statistical analysis
Data were collected directly from digital medical records and included the following variables: age, sex, APACHE index, SOFA index, patient's final vital status, pre-existing comorbidities (hypertension, diabetes mellitus, smoking), administration of corticosteroids (type, dose and days of treatment), administration of antimicrobials (type, dose and days of treatment), confirmed superinfection, culture site, isolated microorganism, resistance pattern, presence of hyperglycemia, epistaxis, pneumothorax or local infection, mortality at 28 days, and tracheostomy or failed extubation. Data analysis was performed with Stata©17 software. Qualitative variables were represented by absolute and relative frequencies, and quantitative variables were represented by medians and interquartile ranges. Finally, Qualitative variables were compared using the Chi-square test, or Fisher's exact test when the approximation to the Chi-square distribution was not correct. To study which variables could be independently related to a given response, multivariate linear regression models were used. These models were summarized with the estimation of the coefficients, together with their 95% confidence intervals and their p-values.
Results
A total of 279 patients admitted to the ICU with a confirmed diagnosis of pneumonia due to SARS-CoV-2 infection were recruited for this study. Superinfection was evidenced in 17.2% (n=48) of the patients. The most frequent cause was bacteremia with 7.1% (n=20), followed by pulmonary superinfection with 6.1% (n=17) and bacteremia plus pulmonary superinfection with 2.1% (n=6). Among the causal agents of superinfection investigated (n=88), the most identified germ was K pneumoniae with 26.1% (n=23), followed by C. albicans with 11.4% (n= 10) and P. aeruginosa with 9 .1% (n=8) (Table 2) .Regarding the resistance profile, it was shown that the usual one predominated to the identified germs in 69.3% (n=61), while 14.8% was the producer of carbapenemases (n=13), 5, 7% were extended-spectrum beta. lactamase (n=5), 5.7% were resistant to methicillin (n=5) and 4.5% had AmpC-type β-lactamase (n=4).
|
Variable |
Men |
Women |
OR |
CI |
p value (Chi |
|
|
n (%) |
n (%) |
|||||
|
Corticosteroids |
45 (43.7) |
35 (62.5) |
0.96 |
0.31-2.93 |
0.943 |
|
|
Diagnosis of superinfection |
Negative |
74 (71.8) |
39 (69.6) |
- |
- |
- |
|
Bacteriemia |
13 (12.6) |
8 (14.3) |
26 |
1.30-5.17 |
<0.0001 |
|
|
Bacteriemia and fungemia |
1 (1.0) |
1 (1.8) |
- |
- |
<0.0001 |
|
|
Bacteriemia and pulmonar |
3 (2.9) |
3 (5.4) |
- |
- |
<0.0001 |
|
|
Bacteriemia. fungemia and |
- |
1 (1.8) |
- |
- |
- |
|
|
Fungemia |
- |
1 (1.8) |
- |
- |
- |
|
|
Pulmonar superinfection |
12 (11.7) |
3 (5.4) |
1.33 |
0.96-1.84 |
0.001 |
|
|
Clinical superinfection |
29 (28.2)
|
17 (30.4)
|
5.54
|
1.60-19.12
|
0.005
|
|
|
Mechanical Ventilation |
103 (100.0)
|
56 (100.0)
|
- |
- |
- |
|
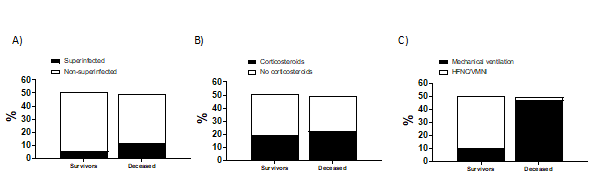
Our data revealed no significant differences between the groups of surviving or deceased patients who had (or did not have) superinfection (Figure 1A). Our results also showed no significant differences between the groups of surviving or deceased patients, treated or not treated with corticosterone (Figure 1B). However, the mortality rate of mechanically ventilated patients was significantly higher than that of patients who received oxygen through HFNC or NIV (Figure 1C). These results suggest that mechanical ventilation is the most important factor associated with patient mortality, rather than superinfection or corticosterone treatment.
Figure 1. A) No differences between surviving (or deceased) patients with (or without) superinfection. B) No differences between surviving (or deceased) patients treated (or not) with corticosterone. C) The mortality rate of mechanically ventilated patients is higher than those oxygenated by HFNC or NIV.
Bacterial/fungal superinfection does not increase the risk of death
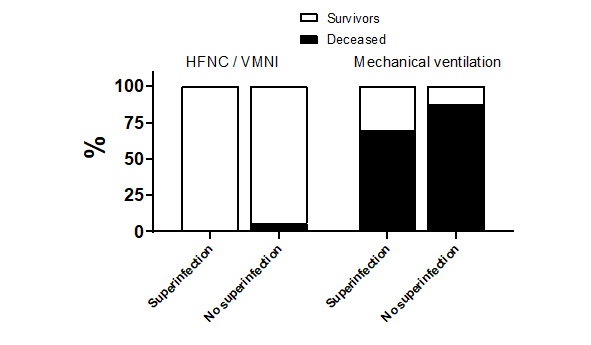
Analysis of mortality as a function of infection (superinfection vs. non-superinfection) showed no significant difference between patients who received HFNC/NIV oxygenation or were mechanically ventilated (non-superinfected vs. superinfected: 135 patients; 48.4% vs. 111 patients; 48.1%; p=1.000) (Figure 2).
Figure 2. Infection-related mortality (superinfection vs. non-superinfection) among patients with oxygenated HFNC/NIV or mechanical ventilation showed no significant differences.
Bacterial/fungal superinfection increases with time of exposure to mechanical ventilation.
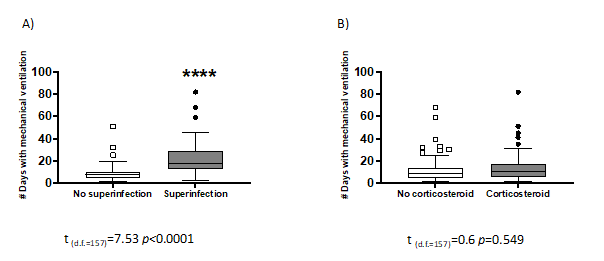
We then evaluated whether exposure time to mechanical ventilation is associated with increased bacterial/fungal infection. Our results clearly show that superinfection significantly increases with mechanical ventilation exposure time (t(d.f.=157) = 7.53 p<0.0001) (Figure 3A). On the other hand, no significant difference was found between mechanically ventilated patients who were administered corticosteroids or not (t(d.f.=157) = 0.6 p<0.0549) (Figure 3B). These results demonstrate that corticosteroids do not increase the risk of superinfection in patients on mechanical ventilation for longer than 20 days.
Figure 3. A) Superinfection increases significantly with time of exposure to mechanical ventilation. B) No significant differences between mechanically ventilated patients, administered or not with corticosteroids.
The administration of corticosteroids is not associated with superinfection
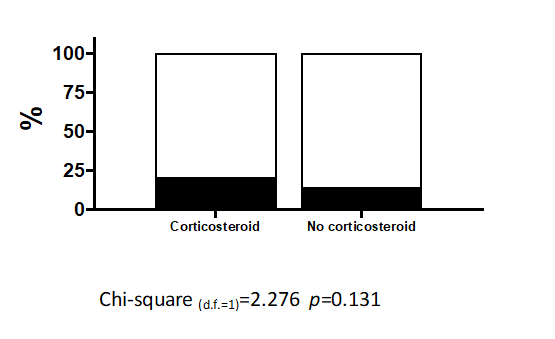
The most frequent type of infection in our cohort was bacteremia with 41.6% (n=20), followed by pulmonary infection with 35.4% (n=17) (Table 3). Diagnoses related to superinfection were found in 48 patients. Of this group, 25 subjects were treated with corticosteroids. We performed an analysis to determine the efficacy of corticosteroid therapy in treating COVID-19 infection and the associated superinfection. Our results show that there is no association between superinfection and corticosteroid treatment (Chi-square(d.f.=1) = 2.276 p<0.131) (Figure 4). This clinically relevant result demands the attention of physicians and pharmacists to find more effective strategies for treating mechanically ventilated COVID-19 patients.
|
Variable |
n (88) |
|
K pneumoniae, n (%) |
23 (26.1) |
|
C albicans, n (%) |
10 (11,4) |
|
P Aeruginosa, n (%) |
8 (9.1) |
|
S epidermidis, n (%) |
7 (7,5) |
|
P mirabillis, n (%) |
7 (7,5) |
|
E faecalis, n (%) |
6 (6.8) |
|
S aureus, n (%) |
5 (5.7) |
|
E cloacae, n (%) |
4 (4.5) |
|
E coli, n (%) |
2 (2.8) |
|
C tropicalis, n (%) |
2 (2,8) |
|
K aerogenes, n (%) |
2 (2,8) |
|
E faecium, n (%) |
1 (1,1) |
|
S haemolyticus, n (%) |
1 (1,1) |
|
E Hominis, n (%) |
1 (1,1) |
|
S mutans, n (%) |
1 (1,1) |
|
E raffiosus, n (%) |
1 (1,1) |
|
B cepacia, n (%) |
1 (1,1) |
|
C indologenes, n (%) |
1 (1,1) |
|
S marscences, n (%) |
1 (1,1) |
|
M morganii, n (%) |
1 (1,1) |
|
k oxytoca, n (%) |
1 (1,1) |
|
S simulans, n (%) |
1 (1,1) |
|
S intermedius, n (%) |
1 (1,1) |
|
Resistance Profiles, n (88) |
|
|
Multisensitive, n (%) |
61 (69.3) |
|
Carbapenemase resistance, n (%) |
13 (14.8) |
|
Extended spectrum beta-lactamase resistance, n (%) |
5 (5.7) |
|
Stafilococcus meticilin- resistance, n (%) |
5 (5.7) |
|
β-lactamase AmpC Resistance, n (%) |
4 (4.5) |
Figure 4. The administration of corticosteroids is not associated with superinfection.
Sex dimorphism
Because COVID-19 infection was reported to be a sex-dependent pathology, we analyzed the association between sex, corticosteroid use, mechanical ventilation and mortality after 28 days in the ICU. Our results showed that the risk of developing bacteremia is 2.6 times lower in men than in women (95% confidence interval [CI], 1.30-5.17), and that superinfection is 5.54 times lower in men than in women (95% CI, 1.60-19.12). However, despite these differences, mortality was not statistically significant between men and women (Table 2).
Discussion
To our knowledge, this is the first study to report data on bacterial/fungal superinfection in patients with COVID-19 in the ICU in a hospital located at a high altitude. The results of this study show that: 1) bacterial/fungal superinfection is associated with the number of days on mechanical ventilation; but has no impact on the mortality rate, 2) corticosteroid treatment does not alter the frequency of bacterial/fungal superinfection or mortality, and 3) there is no association between mortality, sex and corticosteroids with empirical antibiotic treatment.
In our high-altitude hospital, superinfection in patients with COVID-19 in the ICU was mostly caused by gram-negative enterobacteria (21%) followed by gram-positive bacteria (17%), and fungi (14%). Of these pathogens, K. pneumoniae was the most frequent (26.1%). These results differ from that reported by Falcone et al. in Italy at sea level, who also identified gram-negative, gram-positive, fungal, and enterobacter, but in different order of importance [15]. However, as in our study, K. pneumoniae was the most frequently isolated pathogen (31.2%) [15]. Prior to the pandemic, the multiresistant agents most often isolated in the ICUs of high-altitude hospitals in Latin America were K. pneumoniae and E. coli. On the contrary, studies carried out in hospitals at sea level show that P. aeruginosa was the most frequent microorganism in both bacteremia and pneumonia associated with mechanical ventilation; 34.7% in Italy [16], 14% in France [17], and 10.7% in Spain [18]. Likewise, it was reported that 87.7% of fungal co-infections occurred in superinfected patients in Switzerland, with C. albicans representing 67.7% of cases [19]. Taken together, these results suggest that there are important differences in the populations of dominant microorganisms in hospitals, most probably depending on the geographical region and possibly also the altitude level. altitude level is known to influence certain physiological traits for instance, angiotensin-converting enzyme-2 (ACE-2) expression has been reported to be decreased in high-altitude patients. Since ACE-2 expression modulates the overexpression of proinflammatory cytokines IL-6, IL-7, TNF-α, IL-2, and IL-1β, among others [20], this mechanism may explain (at least in part) our results. Moreover, as mentioned above, high-altitude patients are chronically exposed to slightly elevated levels of inflammatory factors and cytokines [20], suggesting a higher tolerance and immune adaptation. From a clinical point of view, these important results suggest that individual measurement of inflammatory biomarkers should not be limited to just when initiating anti-inflammatory therapy (i.e. steroids, anti-IL-6, anti-IL-1). This recommendation is especially relevant in high-altitude patients, where complex adaptation to inflammatory factors is less well understood [20].
Regarding the characteristics of the patients, the median age in our study was 62 years (IQR: 52-69), similar to Ferrando et al. [21], who reported a median age of 64 years (IQR 56-72) in 663 patients, but lower than that of Falcone, 71 years (IQR 60-78.5) [15]. On the other hand, the predominant nutritional status in our study was overweight (30.8%), a risk factor associated with mortality during viral infection [17]. Obesity also predominated in France, Italy, Spain, Croatia, and Switzerland [16,19,21-23]. About 52% of patients had a history of arterial hypertension, and 18% had diabetes, similar to the rates reported in an Italian study (arterial hypertension: 43.5%, diabetes mellitus: 20.3%). Other investigations reported rates of 57% and 32% [17], 49.62% and 22.7% [21], 53% and 27% [24], 52.6% and 36.8% [19] for hypertension and diabetes, respectively, as well as a higher prevalence of both pathologies in the Czech Republic [25]. These results suggest that age, nutritional status, and associated pathologies may influence the incidence of superinfection in the ICU.
Treatments to relieve critically ill patients are challenging. In COVID-19, the direct viral attack on the alveolocapillary membranes produces increasing hypoxia. This effect generates insufficient hyperventilation as the lung surface cannot ventilate sufficiently to reduce it. In these cases, oxygen administration by mechanical ventilation is the last resort to overcome life-threatening desaturation [26,27]. In our study, mechanical ventilation, vasopressors, hemodialysis, and septic shock were associated with a more extended hospital stay. Thus, our results showed a greater likelihood of superinfection with the time spent under mechanical ventilation. Hospitalization in our hospital ICU lasted a median of 8 days (IQR 4.5-14), similar to that reported by Bartovská et al. (8 days) [25], and close to that found by Signorini et al. (5 days, IQR 1.5-8.5), but lower than that reported in a 19-day study conducted in Italy (IQR 11-29.75), which identified that the stay was longer in superinfected patients (30 days vs. 11 days, p <0.001) [15]. On the other hand, the use of corticosteroids in our study population was 42.3%, lower than that reported in the Italian studies, 73.9% [15] and 98% [16] and the Swiss study (68%) [19]. But much higher than that mentioned by Ferrando et al. (76%) in Spain [21]. Differences in these percentages are perhaps due to the type of patient included, the diagnostic method used for diagnosing superinfection, etc.
Conclusion
Bacterial/fungal superinfection is a variable event in patients with COVID-19 viral infection that may be directly associated with geographic altitude but not with corticosteroid use. Furthermore, this study did not show an impact of corticosteroid use on mortality but did show an increased need for mechanical ventilation. Our results warrant further research to identify, among others, subgroups of critically ill patients with defined inflammatory profiles in whom immunosuppressive treatments (such as corticosteroids) have clinical benefits.
Conflict of Interest
The authors have no conflict of interest in this study.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial or non-profit sectors. This research was funded by the authors' own resources.
References
2. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York city. New England Journal of Medicine. 2020 Jun 11;382(24):2372-4.
3. Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clinical Infectious Diseases. 2020 Nov 1;71(9):2459-68.
4. Langford BJ, So M, Raybardhan S, Leung V, Soucy JP, Westwood D, Daneman N, MacFadden DR. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clinical Microbiology and Infection. 2021 Apr 1;27(4):520-31.
5. Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest. 2007 Apr 1;131(4):954-63.
6. Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. The Lancet Respiratory Medicine. 2020 Mar 1;8(3):267-76.
7. Remap-Cap Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. New England Journal of Medicine. 2021 Apr 22;384(16):1491-502.
8. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. New England Journal of Medicine. 2021 Feb 25;384(8):693-704.
9. Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial. JAMA. 2020 Oct 6;324(13):1317-29.
10. Brun-Buisson C, Richard JC, Mercat A, Thiébaut AC, Brochard L. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2011 May 1;183(9):1200-6.
11. Hughes S, Troise O, Donaldson H, Mughal N, Moore LS. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clinical Microbiology and Infection. 2020 Oct 1;26(10):1395-9.
12. Arias-Reyes C, Zubieta-DeUrioste N, Poma-Machicao L, Aliaga-Raduan F, Carvajal-Rodriguez F, Dutschmann M, et al. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir Physiol Neurobiol. 2020 Jun;277:103443.
13. Lei S, Jiang F, Su W, Chen C, Chen J, Mei W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020 Apr 5;21:100331.
14. Ortiz-Prado E, Simbaña-Rivera K, Gómez-Barreno L, Rubio-Neira M, Guaman LP, Kyriakidis NC, et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the Coronavirus Disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020 Sep;98(1):115094.
15. Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. Journal of Antimicrobial Chemotherapy. 2021 Apr;76(4):1078-84.
16. Signorini L, Moioli G, Calza S, Van Hauwermeiren E, Lorenzotti S, Del Fabro G, et al. Epidemiological and Clinical Characterization of Superinfections in Critically Ill Coronavirus Disease 2019 Patients. Critical Care Explorations. 2021 Jun;3(6).
17. Fekkar A, Lampros A, Mayaux J, Poignon C, Demeret S, Constantin JM, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. American Journal of Respiratory and Critical Care Medicine. 2021 Feb 1;203(3):307-17.
18. Ferrando C, Mellado-Artigas R, Gea A, Arruti E, Aldecoa C, Bordell A, et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: a prospective, cohort, multicentre study. Revista Española de Anestesiología y Reanimación (English Edition). 2020 Oct 1;67(8):425-37.
19. Buehler PK, Zinkernagel AS, Hofmaenner DA, Garcia PD, Acevedo CT, Gómez-Mejia A, et al. Bacterial pulmonary superinfections are associated with longer duration of ventilation in critically ill COVID-19 patients. Cell Reports Medicine. 2021 Apr 20;2(4):100229.
20. Del Valle-Mendoza J, Tarazona-Castro Y, Merino-Luna A, Carrillo-Ng H, Kym S, Aguilar-Luis MA, et al. Comparison of Cytokines Levels Among Covid-19 Patients Living at Sea Level and High Altitude. International Journal of Infectious Diseases. 2022 Mar 1;116:S58.
21. Cortellazzi P, Lamperti M, Minati L, Falcone C, Pantaleoni C, Caldiroli D. Sedation of neurologically impaired children undergoing MRI: a sequential approach. Pediatric Anesthesia. 2007 Jul;17(7):630-6.
22. Kukoč A, Mihelčić A, Miko I, Romić A, Pražetina M, Tipura D, et al. Clinical and laboratory predictors at ICU admission affecting course of illness and mortality rates in a tertiary COVID-19 center. Heart & Lung. 2022 May 1;53:1-0.
23. Oliva A, Ceccarelli G, Borrazzo C, Ridolfi M, D’Ettorre G, Alessandri F, et al. Comparison of clinical features and outcomes in COVID-19 and influenza pneumonia patients requiring intensive care unit admission. Infection. 2021 Oct;49(5):965-75.
24. Bartovská Z, Andrle F, Beran O, Zlámal M, Řezáč D, Murinova I, et al. Data from the first wave of Covid-19 from the Central Military Hospital, Prague, Czech Republic. Epidemiologie, Mikrobiologie, Imunologie: Casopis Spolecnosti pro Epidemiologii a Mikrobiologii Ceske Lekarske Spolecnosti JE Purkyne. 2020 Jan 1;69(4):164-71.
25. Ehrenreich H, Weissenborn K, Begemann M, Busch M, Vieta E, Kamilla W. Erythropoietin as candidate for supportive treatment of severe COVID-19. Molecular Medicine. 2020;26:58.
26. Yuan M, Wu NC, Zhu X, Lee CD, So RTY, Lv H, et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020 May 8;368(6491):630-633.