Abstract
Background: Neuromyelitis Optica (NMO) is a serious condition associated with inflammation. Early diagnosis and detection are critical for early intervention. In this systematic review, we investigate the role of the neutrophil to lymphocyte ratio (NLR) as an important biomarker for NMO.
Methods: Ten studies were selected that were sufficiently high quality and then checked for quality. The studies were organized by English language and selective inclusion criteria.
Results: NLR was significantly increased in NMO patients compared to controls. The ratio was specifically proportional to severity of disease. More severe disease had a higher ratio.
Conclusion: NLR offers a reliable and affordable method for early detection of disease severity. This can help guide appropriate treatment selection and monitor treatment response.
Keywords
Neuromyelitis optica spectrum disorder, Neutrophil to lymphocyte ratio, Systematic review
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a neurodegenerative disease and defined as an inflammatory autoimmune disease of the central nervous system (CNS) which clinically presents as acute optic neuritis (ON), transverse myelitis (TM), and brain and/or spinal cord syndromes and follows a relapsing/remitting course [1-3]. Based on epidemiological investigations, NMOSD mostly affects late-middle-aged women [4], and the prevalence of the disease is 0.5-4 per 100,000 people, which can be higher (up to 10/100,000) in some ethnic groups [5]. However, the real prevalence is probably underestimated since 30-40% of NMO cases have been mistaken as Multiple Sclerosis (MS) patients [3]. Until recently, it had been a matter of controversy whether or not NMOSD can be considered a more serious form of MS, but the identification of NMOSD-specific aquaporin 4 (AQP4) antibody as a specific marker autoantibody distinguished NMOSD from MS [6]. Although not all NMOSD patients are seropositive for NMO-IgG, myelin oligodendrocyte glycoprotein (MOG)-antibodies were detected in some negatively tested NMO-IgG patients [7].
NLR is a widely used reliable biomarker to assess the prognosis of many diseases, including systemic lupus erythematosus [8], Behçet’s disease [9], primary Sjogren’s syndrome [10], Guillain-Barré syndrome [11], acute coronary syndrome [12], breast cancer [13] and intracerebral hemorrhage [14]. This ratio is a novel index that demonstrates systemic inflammation and is correlated with autoimmune disorders such as MS [15-18].
In addition, a few researchers have reported the association between NLR and NMOSD. However, all the research up to now has been descriptive in nature. Also, literature has emerged that offers contradictory findings about the relationship. In addition, most studies in this context have only been carried out in a small number of areas and a limited number of patients. These indicate a need for a systematic review which consolidates the available data to help guide further clinical management with the predictive capabilities of the NLR. The aim of this study is to systematically review the evidence on the association between NLR and NMOSD diagnosis, prognosis, and severity. This significantly aids in improving treatment. To the best of our knowledge, our manuscript is the first systematic review in this context.
Material and Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria were used to report this systematic review. We conducted a systematic search in three main databases, including Scopus, PubMed, and Web of Science. This was done on the 28th of November 2021 using the following terms: (“neutrophil to lymphocyte ratio” NLR) AND (NMOSD OR "neuromyelitis optica spectrum disorder") (Table 1). Manually screening the references of the retrieved publications for intact relevant studies was also done.
|
Database |
Search terms |
Number of articles |
|
PubMed |
(" Neuromyelitis Optica Spectrum Disorder " [All Fields] OR "NMOSD" [All Fields]) AND (("neutrophil"[All Fields] AND "lymphocyte"[All Fields] AND "ratio"[All Fields]) OR "NLR"[All Fields]) |
9 |
|
Scopus |
(TITLE-ABS-KEY ("Neuromyelitis Optica Spectrum Disorder " OR "NMOSD" ) ) AND ( ALL ("neutrophil to lymphocyte ratio" OR NLR ) ) |
76 |
|
Web of Science |
(All=("neutrophil to lymphocyte ratio" OR NLR)) AND (All=("Neuromyelitis Optica Spectrum Disorder " OR "NMOSD")) |
11 |
Selection of studies
Figure 1 presents the process of search and study selection. Two reviewers individually assessed all potentially relevant papers. All the studies found through the search were examined in title and abstract, and all candidate publications were obtained in full text. The consensus was used to settle disagreements. We identify eligible studies according to the PICOS principle as outlined below:
(a) Population: Patients with NMOSD
(b) Intervention: NLR
(c) Control: Healthy control patients
(d) Outcomes. The diagnostic and prognostic performance of NLR OR association of NLR with other factors
(e) Study design: cross-sectional, case-control, and cohort studies
When studies met all the above requirements, they were considered eligible. There were no restrictions on article language or publishing date. Articles were not accepted if they fit into any of the following categories: (1) overlapping or duplicating data; (2) case reports, conference proceedings, reviews, and letters to the editor. When numerous publications or data analyses were presumably from the same data source, and no confirmation on potentially duplicate data was available, the study with the most patients was kept, and other relevant studies were eliminated. Corresponding authors were contacted to explain incomplete or ambiguous data, but no further data was obtained.
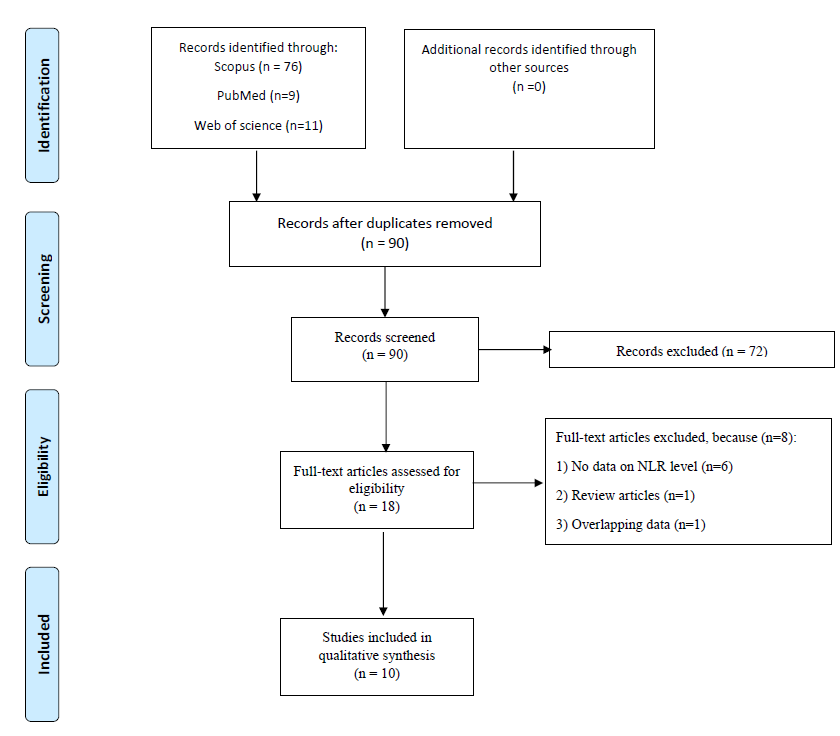
Figure 1. Flow chart of search and study selection.
Extraction of data
The following data was extracted:(1) Name of the first author, (2) year of publication, (3) country of data source, (4) study design (retrospective or prospective), (5) sample size, (6) male to female ratio, and (7) key results.
Quality assessment
The Newcastle– Ottawa Scale (NOS), comprising three sections of selection, comparability, and outcome of interest, was used to assess the quality of the included studies. NOS values varied from 0 to 9.
Results
Overview
Table 2 shows the general characteristics of included studies. Ten studies reported the association between NLR and NMOSD [16,19-27]. All of them were written in English and were designed retrospective. Seven studies were conducted in China [16,20-22, 25-27] , one in the United Kingdom [19], one in Germany and Austria [23], and one in Latin America [24].
|
First author |
Year |
Region |
Design |
Sample size |
NOS score |
|
Lin [16] |
2017 |
China |
Retrospective |
166 |
8 |
|
Benetou [19] |
2020 |
UK |
Retrospective |
6 |
8 |
|
Qingli [20] |
2021 |
China |
Retrospective |
155 |
8 |
|
Xie [21] |
2021 |
China |
Retrospective |
324 |
7 |
|
Tong [22] |
2021 |
China |
Retrospective |
111 |
6 |
|
Peternell [23] |
2021 |
Germany and Austria |
Retrospective |
270 |
6 |
|
Contentti [24] |
2021 |
Latin America |
Retrospective |
90 |
8 |
|
Pan [25] |
2021 |
China |
Retrospective |
169 |
7 |
|
Zhou [26] |
2021 |
China |
Retrospective |
428 |
8 |
|
Chen [27] |
2021 |
China |
Retrospective |
32 |
8 |
Lin et al. [16] initiated research to analyze the association between NLR and NMOSD for the first time in 2017. They studied 120 healthy controls, and 46 NMOSD patients admitted to Wenzhou Medical university’s hospitals between 2010 and 2016. NLR level in NMOSD patients was higher (1.56 ± 0.53, p<0.001) than that of healthy controls (1.56 ± 0.53, p<0.001). Increased level of NLR was detected in NMOSD patients experiencing acute attack (n=33, NLR=2.83 ± 1.00) compared to patients at the remission stage (n=13, NLR=2.22 ± 0.74, p<0.001).
Benetou et al. [19] initiated research to analyze the association between NLR and recruited 62 RDS (relapsing demyelination syndrome) children, including 39 MS, 17 myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), and six AQP4-Ab NMOSD patients compared to 94 healthy controls to assess if disease activity correlates with NLR in RDS. Full blood counts were used to calculate NLR. These were tested clinically in three stages: 1) during remission, 2) during the attack stage but prior to steroid treatment or 3) during steroid treatment. Steroid treatment artifactually increases NLR (3.75 (1.78–5.28)) compared to patients at remission (2.82 (1.4–3.21)) or the attack group (2.87 (2.14–7.42)); however, p values were not reported. Median NLR during remission (IQR) in NMOSD group (2.82 (1.4–3.21)) was higher than MS (1.42 (1–2.04), p=0.01), and MOGAD (1.43 (1.14–2.18), p=0.04). Median NLR during attack prior to steroids (IQR) in the NMOSD group (2.87 (2.14–7.42)) was higher than MS group (1.41 (1.08–1.75), p=0.001), and was like the MOGAD group (3.22 (1.92–6.01), p=not significant). NLR values in NMOSD patients during relapse prior to steroids (2.87 (2.14–7.42), p=0.001), during steroid therapy (3.75 (1.78–5.28), p= not reported), and remission (2.82 (1.4–3.21), p=0.01) were higher than healthy controls (1.27 (0.96–1.71)).
Aquaporin 4
Qingli et al. [20] retrospectively studied a total of 55 NMOSD patients admitted to Perking University third hospital between 2015 and 2020 and 100 healthy controls. NLR was calculated using the routine blood test in less than one week of the first manifestation of new clinical symptoms. They followed up 44 patients and performed their routine blood test 6 months after the last treatment. NLR in NMOSD patients at the relapse stage was significantly elevated (3.52 ± 1.85) compared to that of remission stage (2.28 ± 1.27, p=0.001) and healthy control group (1.98 ± 1.17, p=0.001). There was not any difference between patients in the remission group and the healthy group in terms of NLR (p=0.158). They also compared NLR in AQP4(+) patients to AQP4(-) patients, which was almost equal (3.51 ± 1.81 and 3.52 ± 1.98 respectively, p=0.98). ROC curve of NLR for the diagnosis of inflammatory changes in NMOSD patients during relapse compared to healthy controls showed that the AUC was 0.806 (P ≤ 0.001, 95% CI: 0.735 to 0.876). The optimal cut-off point, the sensitivity, and the specificity were 0.727, 72.7%, and 75%. The optimal cut-off point ROC curve for NMOSD patients during relapse in comparison with those in the remission stage indicated that the best cut-off point was 2.3045 with an AUC of 0.728 (P ≤ 0.001, 95% CI: 0.629 to 0.828), the sensitivity of 72.7%, and the specificity of 63.6%.
Prognosis
In another article, Xie et al. [21] reported a retrospective cohort study to evaluate the association of NLR with the prognosis of first attack NMOSD. Three hundred twenty-four first episode NMOSD patients were studied in the research. Poor recovery was defined as a follow-up Expanded Disability Status Scale (EDSS) score of ≥ 4. According to NLR value, 162 patients were categorized as the high-NLR group (median NLR = 4.28, IQR = 3.01, 6.72) and 162 patients as the low-NLR group (median NLR = 1.63, IQR = 1.28, 2.05). Based on the univariate analysis, NLR was correlated with both poor recovery (OR=1.32, 95% CI: 1.20–1.46, P<0.001) and relapse (OR=1.28, 95% CI: 1.16–1.41, P<0.001). The multifactorial analysis was also performed and indicated that an increase in NLR is associated with a higher likelihood of relapse (OR= 1.33, 95% CI=1.11, 1.59, p= 0.002) and poor recovery (OR=1.23, 95% CI=1.06, 1.43, p= 0.007). The ROC curve for the prognostic value of NLR in NMOSD relapse and poor recovery was plotted. The optimal cut-off values of NLR in NMOSD as a predictor of relapse was 2.38 with the sensitivity of 81.8%, specificity of 64.7%, and AUC of 0.781 (95% CI=0.731 to 0.832, p=0.001). In addition, the best cut-off point for prediction of poor recovery was 2.63 with a sensitivity of 76.3%, specificity of 68%, and AUC of 0.746 (95% CI=0.688 to 0.804, p=0.001).
Tong et al. [22] analyzed 111 NMOSD patients admitted to one of the hospitals of Zhengzhou University from January 2016 to January 2018. NLR in NMOSD patients was correlated with the disability degree. Patients with an increase in the NLR level showed a higher level of disability in the following three years (R2 = 0.053, p= 0.015). Only the abstract of this article was available.
Individual sites
Peternell et al. [23] conducted research in Austria and Germany. They studied 270 pediatric patients (62 MOGAD, 10 AQP4 ab positive NMOSD, 56 MS, 128 other neurological disorders (OND), and 14 healthy controls (HC)). The highest NLR at the clinical attack was for NMOSD patients. An elevated level of NLR was observed during acute treatment. NLR level was still high in AQP4-ab-positive NMOSD patients between clinical attack and remission. Only the abstract of this article was available.
Contentti et al. [24] studied 90 AQP4-IgG-positive NMOSD patients in Latin America (Argentina, Ecuador, and Mexico) retrospectively. They compared NMOSD patients’ NLR to that of 369 healthy controls. In comparison to healthy controls, NLR was higher in NMOSD patients (2.9 ± 1.6 vs. 1.8 ± 0.6; p<0.001). Patients’ data were also collected at 12 and 24 months after the attack to evaluate the prognosis. NLR was still higher in NMOSD patients at these stages (12 months: 2.8 ± 1.3; p<0.001 and 24 months: 3.1 ± 1.6; p<0.001) than controls. However, when considering multiple observations, there was no association between NLR and physical disability, new/enlarging MRI lesions, or relapses at one and two years.
It has been established that the complement C3 and C4 levels in NMOSD patients were lower compared to those in healthy controls [28]. So, Pan et al. [25] designed a study to evaluate the association between NLR and C4. 169 AQP4-ab-positive NMOSD patients admitted to one of the hospitals of Zhengzhou University from December 2013 to March 2021 were retrospectively assessed. They categorized patients into two groups: 54 with low-complement C4 patients and 115 with normal-complement C4 patients. The NLR levels were not different between C4 normal group (median= 2.61, IQR = 1.78–4.50) and C4 low-level group (median NLR= 2.92, IQR= 1.66–4.69, p=0.832).
Zhou et al. [26] published research including 259 newly-diagnosed NMOSD patients within January 2013 to January 2020 and 169 healthy controls. Elevation in the level of NLR was observed in NMOSD group (2.54, IQR = 1.72–4.27 p <0.001) than control group (1.73, IQR = 1.36–2.24; p<0.001). NLR in 85 patients with low initial EDSS (≤3) was 1.91 (IQR = 1.50–2.71), which was lower than that of other 174 patients with high initial EDSS (3.06 IQR = 1.90-2-5.29; p<0.001). In addition, multivariate analysis showed that severity of neurological impairment at disease onset was associated with NLR in these patients (OR=1.146, 95%CI=1.003–1.308, p= 0.044). ROC of the prognostic value of NLR showed that the cut-off value of 2.754 with the sensitivity of 57.5%, specificity of 76.5%, and AUC of 0.687 (95% CI 0.618 to 0.755) could predict the severity of neurological impairment at disease onset.
Chen et al. [27] retrospectively studied NMOSD, MS, and MOGAD patients to investigate and compare their immunological features. They categorized patients into two groups according to the admission date: the training set, including 26 NMOSD patients, 14 MS patients, ten MOGAD patients, and the validation set, including six NMOSD patients, four MS patients, three MOGAD patients. Patients with NMOSD had elevated levels of NLR compared to MS and MOGAD patients in both training (Median= 2.54, 1.59, and 2.15, respectively) and validation set (Median=4.42, 1.76, and 2.78, respectively). However, p values were not reported.
Discussion
The main result of the current systematic review study was that NLR increased among patients with NMOSD compared to healthy controls and those with other types of neuro-autoimmune disorders, including MS and MOGAD. This is important because these diseases are often included in the differential diagnosis list of NMOSD. The results showed that NLR during steroid treatment increased artificially. In addition, NLR levels in the attack stage were higher than remission stage. NLR was positively associated with the EDSS score, a scoring system for disability in NMOSD, and could predict the relapse of NMOSD.
A significant part of many chronic diseases is played by inflammation and immunity. The neutrophil-lymphocyte ratio (NLR), measured as a simple ratio of neutrophil and lymphocyte counts in peripheral blood, is a biomarker that indicates the balance of two elements of the immune system: adaptive immunity and acute and chronic inflammation (as demonstrated by the neutrophil count). NLR has been widely studied and shown to relate to mortality and predict course of illness in patients suffering from a range of medical disorders such as cerebral hemorrhage, infectious diseases, sepsis, ischemic stroke, and major cardiac events. Besides that, higher NLR has been linked to a poor prognosis in cancer patients. These negative associations could be due to the roles of poor immune function and severe inflammation in the progression of these illnesses. Furthermore, it has been suggested that increased NLR is an independent and complementary indicator of the degree of neurological dysfunction brought on by multiple sclerosis. To inform future clinical care using the NLR's predictive capacity, we compiled the information that is currently available on the function of the NLR in predicting NMOSD.
NMOSD is an uncommon and disabling condition marked by inflammatory episodes affecting the spinal cord and optic nerves [29]. Because autoantibodies cause it, NMOSD is primarily recognized as a condition of humoral immunity [29,30]. The existence of AQP4-ab, an abundant water channel mostly expressed in astrocytic foot processes, is a classic example of an inappropriate humoral reaction and a serological hallmark of NMOSD, differentiating it from relapsing-remitting multiple sclerosis (RRMS) [31]. The principal pathophysiology of NMOSD is autoimmune astrocytopathy [32]. Neutrophils and lymphocytes are essential components of the immune response [33]. NLR may be a more accurate indicator of inflammatory response than WBC [34,35]. Autoimmune disorders such as MS [15], Behçet's disease [9], primary Sjogren's syndrome [10], systemic lupus erythematosus (SLE) [8], inflammatory bowel diseases [36], as well as NMOSD have been correlated to NLR.
Investigating the roles of neutrophils and lymphocytes is essential to attain a comprehensive explanation for NLR as a biomarker in NMOSD. Although neutrophils have long been seen in Central nervous system (CNS) lesions and Cerebrospinal fluid (CSF) of NMOSD cases, their involvement in NMOSD pathophysiology has not yet been known entirely [37-39]. Neutrophils have a crucial role in developing NMOSD lesions [37, 40]. According to Saadoun et al.’s study, the severity of NMOSD lesions was decreased in neutropenic mice and elevated in mice with neutrophilia [40]. A study by Jacob et al. [41] reported a patient whose first NMOSD episode was aggravated by unintended administration of granulocyte colony-stimulating factor, indicating the pathogenetic role of neutrophils in NMOSD. In relatively early NMOSD model lesions, Gong et al. [42] confirmed that neutrophils are the principal inflammatory cells throughout the acute phase (24h).
Neutrophils, macrophages, and eosinophils are the most recruited inflammatory cells in the NMOSD lesions, as well as a few T lymphocytes [40,43]. These inflammatory cells show temporal dynamic changes during the NMOSD pathogenesis. In the Saadoun et al. study, the first cells attracted into NMOSD lesions were neutrophils, which happened within a few hours after NMO-IgG and human complement were injected intracerebrally [40]. Perivascular complement activation occurred within 12 hours, with early myelin loss and AQP4 loss [40,44]. By 24 hours, neutrophils had entered the lesion and concentrated around small vessels, where AQP4 is abundantly expressed [40,44]. Few neutrophils existed in the lesions during the chronic stage (7 days), and macrophages take over and penetrate the white matter extensively [40,44]. At seven days, perivascular eosinophils could also be seen. However, the involvement of eosinophils and macrophages in NMOSD lesions is unknown [40].
Levels of Interleukin (IL)-17 and IL-8 are increased in Cerebrospinal fluid (CSF) of NMO patients, which could answer why there are more neutrophils in NMO [45]. intrathecal activation of the IL-17/IL-8 axis causes considerable neutrophil infiltration in optic–spinal MS (OSMS) (OSMS is known as the usual manifestation of NMO in native inhabitants of tropical and subtropical countries [46]). IL-17 predominantly leads to elevated neutrophil recruitment by IL-8 release. IL-8 is a CXC chemokine for neutrophils and boosts the activity of myeloperoxidase and elastase, and therefore contributes to the breakdown of the blood-brain barrier (BBB) and the creation of extensive spinal cord lesions [47].
In vitro investigations have shown anti-AQP4 Ab bound astrocytes to cause complement-dependent granulocyte migration through the BBB [48]. The degree of damage to the CNS in mouse models of NMOSD was related to neutrophil infiltration, and inhibiting neutrophil protease lowered anti-AQP4 Ab-induced brain tissue damage [40,49]. Piatek et al. [50] investigated how neutrophils contributed to NMOSD pathogenesis, finding evidence of their direct participation in astrocyte function dysregulation by accelerating the generation of reactive astrocytes with proinflammatory potential deficient glutamate metabolism.
B cells and T cells have been shown to play significant roles in the development of NMOSD in numerous investigations. Anti-AQP4 antibodies are often generated by CD19, CD38, and CD27 phenotypic B cell subsets [51]. In addition, astrocytes activate the T cells in the CNS, playing a role in BBB disruption [52]. Th17-related cytokines, such as IL-21, IL-17, and IL-23, are significantly increased in the sera of NMOSD patients [53]. The Th17-mediated responses and elevated release of Th17-related cytokines have been demonstrated to be positively related to the extent of neurological dysfunction in NMOSD patients [54].
The contribution of B lymphocytes and plasma cells in NMOSD can be perceived by the pathogenic effect of AQP4-ab [55,56]. Levels of CD38, CD27, CD19, and CD180 B cells are increased in NMOSD patients' peripheral blood circulation. These cells possess plasma blast-like phenotypes and produce AQP4-ab in response to IL-6 stimulation [57]. B cells in the CSF of NMOSD patients show signs of somatic B cell hypermutation, suggesting antigen recognition in the CNS. Also, higher levels of CXC13, IL-6, proliferation-inducing ligand (APRIL), B cell-activating factor (BAFF), and IL-6 have been observed in the CSF of NMOSD patients, all of which are thought to have a role in the recruitment and maintenance of AQP4-ab-producing cells [58,59]. Eosinophils entering the CNS (see below) have been shown to help plasma cells survive and produce AQP4-ab by producing IL-5, IL-6, and APRIL [56].
However, the role of B cells in the pathophysiology of NMOSD may go beyond the synthesis of AQP4-ab. B cells may also serve as antigen-presenting cells to form follicular effector T cells that play a role in isotype switching and differentiation [60,61], leading to a potentially pathogenic B and T cell interaction positive feedback loop. Furthermore, bystander activation may contribute to the secretion of B cell cytokines such as IL-6, GM-CSF, and TNF-α, which augment NMOSD activity. Pro-inflammatory memory B cells secrete IL-6 in NMOSD. This cytokine may elevate the disease activity by increasing the survival of plasmablasts, inducing AQP4-ab synthesis, damaging BBB integrity, and stimulating differentiation of pathogenic Th17 cells [30]. Finally, reducing regulatory B cell populations may impede function or lower IL-10 levels, exacerbating the disease [62]. However, this mechanism requires further investigation.
In NMO patients' serum, AQP4-ab are IgG1, a subtype of mature IgG that needs T cell assistance, implying that AQP4-specific CD4+ T cells are involved in developing this adaptive humoral response [63]. T cells are rare in NMOSD lesions, suggesting that they are not involved in the creation of lesions directly [64]. T cells may alter tolerance and contribute to AQP4-ab synthesis in the periphery [64,65]. In response to intact AQP4 and AQP4 peptides, T cells from NMO patients' peripheral blood proliferate, and in NMOSD patients, T cells respond robustly to p61–80 [63].
AQP4 antigenic activation polarizes the immune reaction toward a Th17 repertoire and the release of Th17-associated cytokines, including IL-21 and IL-6 [54]. Th17 cells may impair BBB integrity by secreting IL-17, increasing endothelial activation, and inducing neutrophils trans-endothelial migration [66]. Meanwhile, higher IL-6 levels in the CSF of NMOSD patients may promote the survival of AQP4-specific Th17 cells while inhibiting FOXP3+ regulatory T cells [67]. These findings point to the potential significance of AQP4-specific T cells in NMO pathogenesis as mediators of adaptive humoral and cellular immune responses [1].
Therefore, in the NMOSD immunological response, neutrophils and lymphocytes play critical roles. As the forerunners of the immune response, neutrophils secrete a considerable number of inflammatory mediators. Activated lymphocytes, on the other hand, release superoxide radicals and proteases. All these variables contribute to tissue damage [16,33]. NLR is preferable to other single leucocytes index as a composite index of these two leucocytes and a reliable indicator of inflammatory state.
Limitations
Our systematic review has two significant limitations. First, most of the included research was retrospective. Future prospective research should be conducted to confirm these results. Second, due to high heterogeneity and inconsistent findings in the literature, as well as the absence of a sufficient number of studies to be acceptable for a meta-analysis, we were unable to conduct a meta-analysis. Because there were some controversies among the results of included studies, more research on this topic needs to be undertaken before the association between NLR and NMOSD is more clearly understood.
Conclusion
The results of our study support an association between NLR values and the development of NMOSD. NLR represents a unique inflammatory marker whose elevation in NMOSD implicates immune system imbalance in the pathogenesis of the disease. Further, our findings support NLR to be a promising biomarker that can be readily integrated into clinical settings to aid in predicting and preventing NMOSD-induced disability. Ultimately, with the development of new biomarkers and therapeutic modalities, we can better prevent and treat NMOSD to decrease long-term morbidity and mortality.
Data Availability
All data generated or analyzed during this study are included in this article.
Conflicts of Interest
None.
Funding Statement
There was no funding support.
References
2. Jacob A, McKeon A, Nakashima I, Sato DK, Elsone L, Fujihara K, et al. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. Journal of Neurology, Neurosurgery & Psychiatry. 2013;84(8):922.
3. Wu Y, Zhong L, Geng J. Neuromyelitis optica spectrum disorder: Pathogenesis, treatment, and experimental models. Multiple Sclerosis and Related Disorders. 2019;27:412-8.
4. Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clinical and Experimental Immunology. 2014;176(2):149-64.
5. Hor JY, Asgari N, Nakashima I, Broadley SA, Leite MI, Kissani N, et al. Epidemiology of Neuromyelitis Optica Spectrum Disorder and Its Prevalence and Incidence Worldwide. Frontiers in Neurology. 2020;11:501.
6. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. The Lancet. 2004;364(9451):2106-12.
7. Sato DK, Callegaro D, Lana-Peixoto MA, Waters PJ, de Haidar Jorge FM, Takahashi T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474-81.
8. Wu Y, Chen Y, Yang X, Chen L, Yang Y. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. International Immunopharmacology. 2016;36:94-9.
9. Rifaioglu EN, Bülbül Şen B, Ekiz Ö, Cigdem Dogramaci A. Neutrophil to lymphocyte ratio in Behçet's disease as a marker of disease activity. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23(4):65-7.
10. Hu ZD, Sun Y, Guo J, Huang YL, Qin BD, Gao Q, et al. Red blood cell distribution width and neutrophil/lymphocyte ratio are positively correlated with disease activity in primary Sjögren's syndrome. Clinical Biochemistry. 2014;47(18):287-90.
11. Tunç A. Early predictors of functional disability in Guillain-Barré Syndrome. Acta Neurologica Belgica. 2019;119(4):555-9.
12. Tamhane UU, Aneja S, Montgomery D, Rogers E-K, Eagle KA, Gurm HS. Association Between Admission Neutrophil to Lymphocyte Ratio and Outcomes in Patients With Acute Coronary Syndrome. The American Journal of Cardiology. 2008;102(6):653-7.
13. Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the Neutrophil-to-Lymphocyte Ratio in Predicting Short- and Long-Term Mortality in Breast Cancer Patients. Annals of Surgical Oncology. 2012;19(1):217-24.
14. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-Lymphocyte Ratio Predicts the Outcome of Acute Intracerebral Hemorrhage. Stroke. 2016;47(6):1654-7.
15. Demirci S, Demirci S, Kutluhan S, Koyuncuoglu HR, Yurekli VA. The clinical significance of the neutrophil-to-lymphocyte ratio in multiple sclerosis. International Journal of Neuroscience. 2016;126(8):700-6.
16. Lin J, Xue B, Li J, Xu H, Huang X, Yao Z, et al. Neutrophil to lymphocyte ratio may be a helpful marker to evaluate disease activity in NMOSD. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2017;38(10):1859-63.
17. Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. International Archives of Medicine. 2012;5(1):2.
18. Nazeri M, Bazrafshan H, Foroughi AA. Serum inflammatory markers in patients with multiple sclerosis and their association with clinical manifestations and MRI findings. Acta Neurologica Belgica. 2021:1-7.
19. Benetou C, Berti F, Hemingway C, Hacohen Y, Lim M. Neutrophil-to-lymphocyte ratio correlates with disease activity in myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD) in children. Multiple Sclerosis and Related Disorders. 2020;45:102345.
20. Qingli SUN, Dongsheng FAN. Research Square. 2021.
21. Xie H, Zhao Y, Pan C, Zhang J, Zhou Y, Li Y, et al. Association of neutrophil-to-lymphocyte ratio (NLR) with the prognosis of first attack neuromyelitis optica spectrum disorder (NMOSD): a retrospective cohort study. BMC Neurology. 2021;21(1):389.
22. Tong Y, Wang L, Liu K, Liu W, Li S, Song B, et al. Serum Immunoglobulin G Level and Neutrophils to Lymphocytes Ratio Associated with the Prognosis of Neuromyelitis Optica Spectrum Disorder. Current Neurovascular Research. 2021;18(2):227-36.
23. Peternell A, Breu M, Preisel M, Schimmel M, Eisenkölbl A, Zobel J, et al. Blood Parameter Analysis in Pediatric MOG-Antibody-Associated Disorders. Neuropediatrics. 2021;52(S 01):P1. 25.
24. Carnero Contentti E, Delgado-García G, Criniti J, López PA, Pettinicchi JP, Cristiano E, et al. An Abnormally High Neutrophil-to-Lymphocyte Ratio Is Not an Independent Outcome Predictor in AQP4-IgG-Positive NMOSD. Frontiers in Immunology. 2021;12(80).
25. Pan C, Zhao Y, Xie H, Zhou Y, Duan R, Li Y, et al. Effect of Low Complement C4 on Clinical Characteristics of Patients with First-Episode Neuromyelitis Optica Spectrum Disorder. Neuropsychiatric Disease and Treatment. 2021;17:2859-66.
26. Zhou Y, Xie H, Zhao Y, Zhang J, Li Y, Duan R, et al. Neutrophil-to-Lymphocyte Ratio on Admission is an Independent Risk Factor for the Severity of Neurological Impairment at Disease Onset in Patients with a First Episode of Neuromyelitis Optica Spectrum Disorder. Neuropsychiatric Disease and Treatment. 2021;17:1493-503.
27. Chen B, Gui MC, Ji SQ, Xie Y, Tian DS, Bu BT. Distinct Immunological Features of Inflammatory Demyelinating Diseases of the Central Nervous System. Neuroimmunomodulation. 2021:1-11.
28. Pache F, Ringelstein M, Aktas O, Kleiter I, Jarius S, Siebert N, et al. C3 and C4 complement levels in AQP4-IgG-positive NMOSD and in MOGAD. Journal of Neuroimmunology. 2021;360:577699.
29. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-89.
30. Fujihara K, Bennett JL, de Seze J, Haramura M, Kleiter I, Weinshenker BG, et al. Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurology(R) Neuroimmunology & Neuroinflammation. 2020;7(5).
31. Paul F, Jarius S, Aktas O, Bluthner M, Bauer O, Appelhans H, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Medicine. 2007;4(4):e133.
32. Takai Y, Misu T, Takahashi T, Nakashima I, Fujihara K. [NMO spectrum disorders and anti AQP4 antibody]. Brain and nerve = Shinkei kenkyu no shinpo. 2013;65(4):333-43.
33. Kedziora-Kornatowska KZ. Production of superoxide and nitric oxide by granulocytes in non-insulin-dependent diabetic patients with and without diabetic nephropathy. IUBMB life. 1999;48(3):359-62.
34. Gibson PH, Croal BL, Cuthbertson BH, Small GR, Ifezulike AI, Gibson G, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. American Heart Journal. 2007;154(5):995-1002.
35. Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. The American Journal of Cardiology. 2008;101(6):747-52.
36. Celikbilek M, Dogan S, Ozbakır O, Zararsız G, Kücük H, Gürsoy S, et al. Neutrophil-lymphocyte ratio as a predictor of disease severity in ulcerative colitis. Journal of Clinical Laboratory Analysis. 2013;27(1):72-6.
37. Hertwig L, Pache F, Romero-Suarez S, Stürner KH, Borisow N, Behrens J, et al. Distinct functionality of neutrophils in multiple sclerosis and neuromyelitis optica. Multiple Sclerosis (Houndmills, Basingstoke, England). 2016;22(2):160-73.
38. Lewkowicz N, Mycko MP, Przygodzka P, Ćwiklińska H, Cichalewska M, Matysiak M, et al. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunology. 2016;9(2):364-78.
39. Yang T, Wang S, Zheng Q, Wang L, Li Q, Wei M, et al. Increased plasma levels of epithelial neutrophil-activating peptide 78/CXCL5 during the remission of Neuromyelitis optica. BMC Neurology. 2016;16:96.
40. Saadoun S, Waters P, MacDonald C, Bell BA, Vincent A, Verkman AS, et al. Neutrophil protease inhibition reduces neuromyelitis optica-immunoglobulin G-induced damage in mouse brain. Annals of Neurology. 2012;71(3):323-33.
41. Jacob A, Saadoun S, Kitley J, Leite M, Palace J, Schon F, et al. Detrimental role of granulocyte-colony stimulating factor in neuromyelitis optica: clinical case and histological evidence. Multiple Sclerosis (Houndmills, Basingstoke, England). 2012;18(12):1801-3.
42. Gong Y, Zhang YL, Wang Z, Song HH, Liu YC, Lv AW, et al. Tanshinone IIA alleviates brain damage in a mouse model of neuromyelitis optica spectrum disorder by inducing neutrophil apoptosis. Journal of Neuroinflammation. 2020;17(1):198.
43. Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194-205.
44. Saadoun S, Waters P, Macdonald C, Bridges LR, Bell BA, Vincent A, et al. T cell deficiency does not reduce lesions in mice produced by intracerebral injection of NMO-IgG and complement. Journal of Neuroimmunology. 2011;235(1-2):27-32.
45. Argyriou AA, Makris N. Neuromyelitis optica: a distinct demyelinating disease of the central nervous system. Acta Neurologica Scandinavica. 2008;118(4):209-17.
46. Cree BA, Khan O, Bourdette D, Goodin DS, Cohen JA, Marrie RA, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. 2004;63(11):2039-45.
47. Ishizu T, Osoegawa M, Mei FJ, Kikuchi H, Tanaka M, Takakura Y, et al. Intrathecal activation of the IL-17/IL-8 axis in opticospinal multiple sclerosis. Brain. 2005;128(Pt 5):988-1002.
48. Vincent T, Saikali P, Cayrol R, Roth AD, Bar-Or A, Prat A, et al. Functional consequences of neuromyelitis optica-IgG astrocyte interactions on blood-brain barrier permeability and granulocyte recruitment. Journal of Immunology (Baltimore, Md : 1950). 2008;181(8):5730-7.
49. Zhang H, Bennett JL, Verkman AS. Ex vivo spinal cord slice model of neuromyelitis optica reveals novel immunopathogenic mechanisms. Annals of Neurology. 2011;70(6):943-54.
50. Piatek P, Domowicz M, Lewkowicz N, Przygodzka P, Matysiak M, Dzitko K, et al. C5a-Preactivated Neutrophils Are Critical for Autoimmune-Induced Astrocyte Dysregulation in Neuromyelitis Optica Spectrum Disorder. Frontiers in Immunology. 2018;9(1694).
51. Etemadifar M, Salari M, Mirmosayyeb O, Serati M, Nikkhah R, Askari M, et al. Efficacy and safety of rituximab in neuromyelitis optica: Review of evidence. Journal of research in medical sciences : the official journal of Isfahan University of Medical Sciences. 2017;22:18.
52. Graber DJ, Levy M, Kerr D, Wade WF. Neuromyelitis optica pathogenesis and aquaporin 4. Journal of Neuroinflammation. 2008;5:22.
53. Li M, Yan Y. Experimental models of neuromyelitis optica: current status, challenges and future directions. Neuroscience Bulletin. 2015;31(6):735-44.
54. Linhares UC, Schiavoni PB, Barros PO, Kasahara TM, Teixeira B, Ferreira TB, et al. The ex vivo production of IL-6 and IL-21 by CD4+ T cells is directly associated with neurological disability in neuromyelitis optica patients. Journal of Clinical Immunology. 2013;33(1):179-89.
55. Graf J, Mares J, Barnett M, Aktas O, Albrecht P, Zamvil SS, et al. Targeting B cells to modify MS, NMOSD, and MOGAD: Part 2. Neurology(R) Neuroimmunology & Neuroinflammation. 2021;8(1).
56. Bennett JL, O'Connor KC, Bar-Or A, Zamvil SS, Hemmer B, Tedder TF, et al. B lymphocytes in neuromyelitis optica. Neurology(R) Neuroimmunology & Neuroinflammation. 2015;2(3):e104.
57. Chihara N, Aranami T, Oki S, Matsuoka T, Nakamura M, Kishida H, et al. Plasmablasts as migratory IgG-producing cells in the pathogenesis of neuromyelitis optica. PLoS One. 2013;8(12):e83036.
58. Kaneko K, Sato DK, Nakashima I, Ogawa R, Akaishi T, Takai Y, et al. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: a cross-sectional study and potential therapeutic implications. Journal of Neurology, Neurosurgery, and Psychiatry. 2018;89(9):927-36.
59. Vaknin-Dembinsky A, Brill L, Orpaz N, Abramsky O, Karussis D. Preferential increase of B-cell activating factor in the cerebrospinal fluid of neuromyelitis optica in a white population. Multiple Sclerosis (Houndmills, Basingstoke, England). 2010;16(12):1453-7.
60. Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33(2):241-53.
61. Molnarfi N, Schulze-Topphoff U, Weber MS, Patarroyo JC, Prod'homme T, Varrin-Doyer M, et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. The Journal of Experimental Medicine. 2013;210(13):2921-37.
62. Quan C, Yu H, Qiao J, Xiao B, Zhao G, Wu Z, et al. Impaired regulatory function and enhanced intrathecal activation of B cells in neuromyelitis optica: distinct from multiple sclerosis. Multiple Sclerosis (Houndmills, Basingstoke, England). 2013;19(3):289-98.
63. Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, Nelson PA, Stroud RM, Cree BA, et al. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Annals of Neurology. 2012;72(1):53-64.
64. Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. The Lancet Neurology. 2012;11(6):535-44.
65. Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Annals of Neurology. 2009;66(5):630-43.
66. Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nature Medicine. 2007;13(10):1173-5.
67. Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(47):18460-5.
