Abstract
Minocycline is being tested in clinical trials for the treatment of stroke and traumatic brain injury. As an antibiotic it reduces microglia activation. Can minocycline be used to treat mild repetitive head injury? To that end, minocycline was tested in a novel, closed-head, momentum exchange model of repetitive mild head injury in female rats impacted while fully awake. Magnetic resonance imaging (MRI) revealed there was no brain damage or contusion attesting to the mild nature of the head impacts in this model. It was hypothesized that drug treatment would reduce edema and brain neuroinflammation. Female rats maintained on a reverse light-dark cycle were head impacted three times while fully awake with and without drug treatment. The impacts, separated by 24 hrs each, were delivered under red light illumination. Within 1-2 hrs of the last impact, rats were assessed for changes in water diffusion using diffusion weighted imaging. The data were registered to a 3D MRI rat atlas with 173 segmented brain areas providing site specific information on altered brain gray matter microarchitecture. Postmortem histology was performed 18 days post head injury. Head injury without minocycline treatment was characterized by multiple areas of increased fractional anisotropy, evidence of cytotoxic edema. Treatment with minocycline reversed these measures in many of the same areas and several others (e.g., hippocampus, basal ganglia, prefrontal cortex, sensory and motor cortices and thalamus). Histology for gliosis showed no evidence of neuroinflammation in the thalamus, hippocampus and cerebellum for control or experimental groups in this female model of mild head injury. These studies provide clear evidence that treatment with minocycline within hours after mild repetitive head injury significantly reduce measures of cytotoxic edema in a female rat model of mild repetitive head injury.
Keywords
Concussion, Closed head momentum exchange, Microgliosis, Diffusion weighted imaging, Cytotoxic edema
Introduction
Minocycline is a tetracycline antibiotic commonly used to treat bacterial infections (e.g., acne), but has a potent anti-inflammatory effect independent of its antibacterial action [1] The anti-inflammatory effects of minocycline have been reported in various animal models of CNS disease like Alzheimer’s [2], Parkinson’s [3], Huntington’s [4] and traumatic brain injury [5,6]. There is compelling evidence that the anti-inflammation is caused by suppressing microglia activation; reducing inducible nitric oxide synthase (iNOS), an enzyme catalyzing the production of nitric oxide; and by decreasing matrix metalloproteases associated with blood brain barrier (BBB) permeability [7]. There is ever growing evidence that failure in the BBB lies at the foundation of cerebral small vessel disease (cSVD), the underlying pathophysiological process affecting arterioles, capillaries, and venules [8,9]. Cerebral SVD is a leading cause of dementia [10-12] and thought to be a significant source of neurological disability in aging and a key pathogenic factor in Alzheimer’s [13]. Indeed, there is an ongoing clinical trial testing the efficacy of minocycline to reduce inflammation and BBB leakage in small vessel disease (MINERVA) [14].
These conditions of neuroinflammation, microglia activation, and BBB leakage have been reproduced in a model of mild repetitive head injury using anesthetized male rats [15], (i.e., “the bump on the head” incurred while playing organized sports, car accidents, falls, or in military combat [16-18]). This closed head, momentum exchange model was developed to replicate the human experience of a mild head injury without neuroradiological evidence of damage to the skull or brain [19]. While the impacts are mild they result in changes in BBB permeability [20] and sustained neuroinflammation and microglia activation in heterogenous brain areas, specifically the midbrain dopaminergic system [21]. The present study was undertaken to advance the model to include female rats, impacted while fully awake and during the dark phase when they are active, and to evaluate the treatment effect of minocycline using magnetic resonance imaging (MRI). Given the effectiveness of minocycline in various rodent models of neurodegenerative diseases, we hypothesized it would have a beneficial effect in this model of mild head injury. Minocycline treatment selectively reduced measures of water diffusion that would suggest alteration in the gray matter microarchitecture associated with cytotoxic edema.
Methods
Animals
Twenty-four Sprague Dawley female rats (250-300g) were purchased from Charles River Laboratories (Wilmington, MA, USA), housed on a reverse 12:12 light-dark cycle (lights off at 9:00 hr), maintained in ambient temperature (22–24°C) and provided with food and water ad libitum. All experiments were conducted under dim red illumination between 10:00 hrs and 18:00 hrs to avoid the transitions between the L-D dark cycles. Rats were randomly assigned to three experimental groups: 1) vehicle treated shams with no head injury (n = 8), 2) vehicle treated with head injury (n = 8), and 3) minocycline treated with head injury (n = 8). Minocycline hydrochloride (Thermo Fisher Scientific, Waltham. MA) was taken up in saline and given IP in a dose of 45 mg/kg in a volume of 1 mL/kg within 1 hr post head impact. A single dose was given after each heat impact. The dose was taken from the literature using minocycline (45-50 mg/kg) to treat TBI in rats [22-24]. All animals were cared for in accordance with the NIH Guide to the Care and Use of Laboratory Animals. Methods and procedures used in this study were pre-approved by the Northeastern University Institutional Animal Care and Use Committee protocol 21-0824R. The protocols used in this study complied with the regulations of the institution and adhere to the ARRIVE guidelines for reporting in vivo experiments in animal research [25]. Animals were monitored daily over the duration of the study for general health, food and water consumption. A 15% loss in body weight was set as a humane endpoint.
Mild head impact
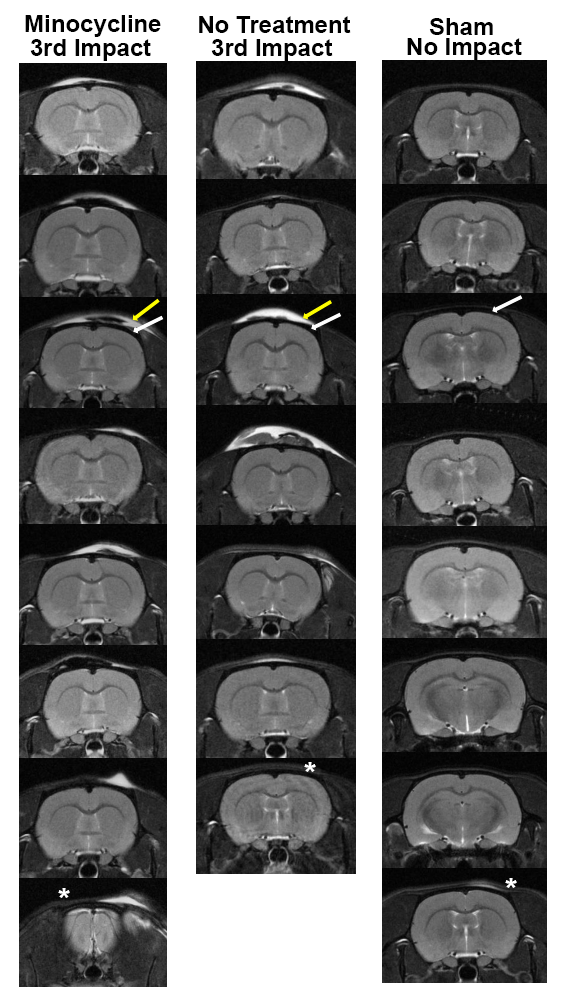
Head impacts were generated with a pneumatic pressure driven 50 g compactor described by Viano and colleagues [26] and refined by Mychasiuk et al. [27] to reliably produce the 7.4 m/s impact velocities described for mild rat head injury. Before the first impact, all rats were treated with 0.1 mg/kg slow-releasing buprenorphine via subcutaneous injection for its analgesic effect lasting through the duration of the three-day repetitive injury period. Rats were lightly anesthetized with 1-2% isoflurane for impact setup. While anesthetized, rats were secured with a bite bar and strapped to a cradle to allow for acceleration along its chassis upon impact. When fully awake (typically within 1-2 minutes), the impact piston was directed to the top of the skull, midline, in the approximate area of Bregma for closed-head momentum exchange mild head injury. All rats showed normal ambulatory behavior within seconds of being placed into their home cage after head impact. There were no mortalities. There was no evidence of skull damage or contusion (Figure 1). Rats were subjected to three mild head impacts separated by 24 hours each as previously described [15,20]. The kinetic energy at impact is 1.37 joules. We have used this model to publish on the long-term neuroradiological effects of repetitive mild head impacts in isoflurane anesthetized, male rats [15,21]. This model is comparable to CHIMERA developed for mouse mild head injury [28,29]. All rats were imaged for edema using DWI within 1-2 hours of the third head impact. Eighteen days following the last impact rats were euthanized and the brain harvested for histology.
Figure 1. Neuroradiology. Shown are radiograms of each subject in each experimental group. The edema (yellow arrow) that appears on the skin and tissue overlying the skull (dark area noted by white arrow) appears white using a T2 weighted pulse sequence.
Imaging
Imaging sessions were conducted using a Bruker Biospec 7.0 T/20-cm USR horizontal magnet (Bruker, Billerica, MA, USA) and a 2 T/m magnetic field gradient insert (ID=12 cm) capable of a 120-μs rise time. Radio frequency signals were sent and received with a quadrature volume coil built into the animal restrainer (Ekam Imaging, Boston MA, USA) [30]. The design of the restraining system included a padded head support obviating the need for ear bars, helping to reduce discomfort while minimizing motion artifact. All rats were imaged under 1–2% isoflurane while keeping a respiratory rate of 40–50 breaths/min. At the beginning of each imaging session, a high-resolution anatomical data set was collected for assessment of structural damage using the RARE pulse sequence with following parameters: 35 slice of 0.7mm thickness; field of view (FOV) 3 cm; 256×256; repetition time (TR) 3900 msec; effective echo time (TE) 48 msec; number of excitations (NEX) 3; 6 min 14 sec acquisition time.
Diffusion weighted imaging – quantitative anisotropy
DWI was acquired with a spin-echo echo-planar-imaging (EPI) pulse sequence having the following parameters: TR/TE = 500/20 msec, eight EPI segments, and 10 non-collinear gradient directions with a single b-value shell at 1000 s/mm2 and one image with a B-value of 0 s/mm2 (referred to as B0) as previously described [15,31,32]. Geometrical parameters were: 48 coronal slices, each 0.313 mm thick (brain volume) and with in-plane resolution of 0.313×0.313 mm2 (matrix size 96×96; FOV 30 mm3). The imaging protocol was repeated two times for signal averaging. Each DWI acquisition took 35 min and the entire MRI protocol including the anatomy lasted about 90 min. There are numerous studies detailing the benefits of multi-shot EPI in BOLD imaging [33-37]. We avoided using single shot EPI because of its severe geometrical distortion at high field strengths (≥ 7T) and loss of effective spatial resolution as the readout period increases [33,38,39]. There is also the possibility of signal loss in single shot EPI due to accumulated magnetic susceptibility or field inhomogeneity [34].
DWI analysis was completed with MATLAB and MedINRIA (1.9.0; http://www-sop.inria.fr/asclepios/software/MedINRIA/index.php) software. Because sporadic excessive breathing during DWI acquisition can lead to significant image motion artifacts that are apparent only in the slices sampled when motion occurred, each image (for each slice and each gradient direction) was screened prior to DWI analysis. For statistical comparisons among rats, each brain volume was registered to the 3D MRI rat brain atlas allowing voxel- and region-based statistics. All image transformations and statistical analyses were carried out using the in-house EVA software (Ekam Solutions LLC, Boston MA). For each rat, the B0 image was co-registered with the MRI atlas using a 9-parameter affine transformation. Insight Toolkit (https://itk.org/) registration framework was used with Affine transformation, mutual information similarity matrix and gradient descent optimizer with following parameters: Max step length 0.3 mm, Min step length 0.0001 mm, Number of iterations 100, Scan threshold 20%. Finally, before segmentation, registration of the scans were closely inspected for quality and manually corrected if necessary. The average value for each ROI was computed using map files for indices of apparent diffusion coefficient (ADC) and fractional anisotropy (FA).
For statistical comparisons among rats, each brain volume was registered to a 3D MRI Rat Brain Atlas (© 2012 Ekam Solutions LLC, Boston, MA) allowing voxel- and region-based statistics. All image transformations and statistical analyses were carried out using the in-house MIVA software (http://ccni.wpi.edu/). For each rat, the B0 image was co-registered with the B0 template (using a 6-parameter rigid-body transformation). The co-registration parameters were then applied on the DWI ADC and FA maps. Normalization was performed on the maps because they provided the most detailed visualization of brain structures and allowed for more accurate normalization. The normalization parameters were then smoothed with a 0.3-mm Gaussian kernel. To ensure that ADC and FA values were not affected significantly by the pre-processing steps, the ‘nearest neighbor’ option was used following registration and normalization. Statistical differences in measures of DWI between experimental groups were determined using one-way ANOVA followed by Bonferroni post hoc tests (alpha set at 5%). Statistical differences in measures of DWI between experimental groups were determined using mixed effects analyses followed by Tukey’s post hoc test.
The 3D MRI rat atlas has 173 segmented, annotated brain areas. For DWI analysis (see below), 150 areas were chosen because they could be organized into well-defined neuroanatomical regions. Areas excluded were white matter tracts because they traverse several brain regions. Circumventricular organs (e.g., anterior and posterior pituitary, pineal gland, area postrema, median eminence) and small adjacent areas like the arcuate and retrochiasmatic nuclei, were also excluded because of their larger, more fenestrated blood vessels. Also excluded were areas with no clear regional organization (e.g., prerubral field). The remaining 150 brain areas were divided into 11 brain regions: cerebellum (20), cortex (19), thalamus (20), basal ganglia (10), hypothalamus (14), hippocampus (9), prefrontal cortex (9), olfactory bulb/cortex (8), amygdala (8), midbrain/pons (12), brainstem (21). The organization was based on conventional neuroanatomy and an effort to keep individual brain areas localized and contiguous within a region. The olfactory bulb/cortex is the exception as the piriform cortex extends some distance caudally along the ventral lateral cortex away from the bulbs.
Immunohistochemistry
Following perfusion and tissue collection, brains were sectioned and immunohistochemically stained for visualization of astrocyte (GFAP+) and microglia (IBA1+) populations. Sections were obtained at 50 mm increments using a cryostat (Leica Biosystems) at -20°C and refrigerated at 4°C until staining. From each sectioned brain, 2-3 representative samples were selected per region of interest (thalamus, hippocampus, substantia nigra, and cerebellum) approximating the coordinates shown in Figure 4. Free-floating immunohistochemistry was conducted in 12-well plates on a lightly rotating shaker at 4°C. Sections were triple-rinsed in PBS (5 min/rinse) and blocked in 0.2% Triton X-100 (85111; ThermoFisher Scientific, Rockford, IL, USA) and 5% Normal Goat Serum (S26; EMD Millipore, Temecula, CA, USA) in PBS for 1 hour. After blocking, sections were incubated in primary antibody solution overnight for 18 hours [1:1000 rabbit anti-GFAP (Z0334; Agilent Dako, Cedar Creek, TX, USA) or 1:200 rabbit anti-AIF1/IBA1 (SAB5701363; Sigma-Aldrich, St. Louis, MO, USA) in blocking solution]. After primary antibody incubation, sections were triple-rinsed in PBS before incubation in secondary fluorescent antibody solution [1:400 goat anti-rabbit Alexa Fluor 488 (111-545-003; Jackson ImmunoResearch, West Grove, PA, USA) in blocking solution] for 1 hour. Following secondary antibody incubation, sections were triple-rinsed in PBS and mounted onto microscope slides in distilled water, cover slipped using Fluoroshield with DAPI mounting medium (F6057; Sigma-Aldrich, St. Louis, MO, USA), and sealed with clear polish. Slides were allowed to set for at least 24 hours at 4°C before confocal microscopy.
Images were acquired using a Zeiss LSM 800 confocal microscope (Carl Zeiss Meditec AG, Jena, Germany; housed in the Institute for Chemical Imaging of Living Systems at Northeastern University) at 100X magnification. Data were collected from consistent sites within the thalamus, hippocampus, substantia nigra, and cerebellum (Figure 4). GFAP or IBA1 signal intensity was measured using FIJI ImageJ at a consistent threshold. One-way ANOVA for each region was conducted using IBM SPSS Statistics and graphed using Prism GraphPad software.
Results
Radiograms of head impact
Shown are radiograms of each subject in each experimental group. The edema (yellow arrow) that appears on the skin and tissue overlying the skull (dark area noted by white arrow) appears white using a T2 weighted pulse sequence. One rat in the no treatment impact group did not present with edema at the putative site of impact (see asterisk). In all other cases the impact site and edema at the forebrain (level of Bregma) was consistent across all rats following the 3rd impact except for the last rat in the minocycline treated group where the impact sited is more rostral near the cerebrum (see asterisk). While all sham rats had no hits, one showed some evidence of edema that could not be accounted for (see asterisk).
Diffusion weighted imaging
Table 1a shows the significant changes in measures of FA between vehicle treated sham rats and rats that were untreated and hit. In this case the increase in FA is considered a surrogate measure of cytotoxic edema. This comparison shows the extent of head injury as 50/173 brain areas presented with an increase in FA values without treatment. These areas are ranked in order of their significance. Reported are the mean (highlighted in gray) and standard deviation (SD) together with their probability values and the omega square (ω Sq) for effect size. The critical value was set at p <0.05 A false discovery rate (FDR) for multi-comparisons gave a significant level of p = 0.057. Table 1b lists 39/173 brains areas that showed a significant decrease in FA values with minocycline treatment. The FDR was p = 0.039. The location of many of these brains areas are shown in the 2D maps and summarized in the 3D reconstructions in Figure 3. When sham untreated rats were compared with head injured rats treated to minocycline there were very few significant differences (See Supplementary File S5).
|
|
Sham |
|
Hit |
|
|
||
|
Brain Area |
Ave |
SD |
|
Ave |
SD |
P-val |
Ω Sq |
|
paraventricular n. |
0.27 |
0.05 |
< |
0.40 |
0.06 |
0.003 |
0.579 |
|
lateral preoptic n. |
0.31 |
0.04 |
< |
0.41 |
0.05 |
0.004 |
0.529 |
|
anterior hypothalamic n. |
0.28 |
0.03 |
< |
0.37 |
0.06 |
0.005 |
0.527 |
|
ventral medial striatum |
0.31 |
0.05 |
< |
0.41 |
0.06 |
0.005 |
0.505 |
|
lateral hypothalamus |
0.37 |
0.04 |
< |
0.45 |
0.04 |
0.005 |
0.502 |
|
ventral lateral striatum |
0.30 |
0.04 |
< |
0.37 |
0.04 |
0.006 |
0.478 |
|
extended amygdala |
0.35 |
0.06 |
< |
0.46 |
0.07 |
0.006 |
0.477 |
|
ventral posteriolateral thalamic n. |
0.40 |
0.02 |
< |
0.47 |
0.05 |
0.007 |
0.458 |
|
ventromedial thalamic n. |
0.35 |
0.05 |
< |
0.42 |
0.04 |
0.008 |
0.454 |
|
tenia tecta ctx |
0.32 |
0.06 |
< |
0.43 |
0.05 |
0.008 |
0.453 |
|
dorsal lateral striatum |
0.34 |
0.04 |
< |
0.40 |
0.06 |
0.009 |
0.433 |
|
ventral orbital ctx |
0.30 |
0.04 |
< |
0.39 |
0.06 |
0.009 |
0.430 |
|
prerubral field |
0.35 |
0.05 |
< |
0.42 |
0.05 |
0.010 |
0.412 |
|
ventral medial n. |
0.30 |
0.05 |
< |
0.38 |
0.05 |
0.010 |
0.410 |
|
posterior hypothalamic n. |
0.32 |
0.04 |
< |
0.41 |
0.06 |
0.011 |
0.409 |
|
prelimbic ctx |
0.32 |
0.04 |
< |
0.41 |
0.06 |
0.011 |
0.409 |
|
reuniens n. |
0.32 |
0.04 |
< |
0.40 |
0.05 |
0.011 |
0.409 |
|
substantia nigra compacta |
0.41 |
0.04 |
< |
0.49 |
0.05 |
0.012 |
0.388 |
|
anterior olfactory n. |
0.37 |
0.05 |
< |
0.47 |
0.08 |
0.013 |
0.385 |
|
parafascicular thalamic n. |
0.32 |
0.03 |
< |
0.38 |
0.06 |
0.013 |
0.382 |
|
supramammillary n. |
0.35 |
0.05 |
< |
0.44 |
0.05 |
0.014 |
0.370 |
|
endopiriform n. |
0.37 |
0.05 |
< |
0.44 |
0.04 |
0.014 |
0.369 |
|
medial preoptic n. |
0.32 |
0.03 |
< |
0.40 |
0.05 |
0.014 |
0.367 |
|
rostral piriform ctx |
0.35 |
0.05 |
< |
0.42 |
0.04 |
0.015 |
0.364 |
|
infralimbic ctx |
0.31 |
0.06 |
< |
0.40 |
0.07 |
0.015 |
0.362 |
|
caudal piriform ctx |
0.36 |
0.04 |
< |
0.41 |
0.03 |
0.016 |
0.350 |
|
accumbens core |
0.34 |
0.06 |
< |
0.43 |
0.04 |
0.017 |
0.348 |
|
lateral amygdaloid n. |
0.35 |
0.04 |
< |
0.43 |
0.06 |
0.017 |
0.343 |
|
granular cell layer |
0.38 |
0.06 |
< |
0.47 |
0.08 |
0.017 |
0.342 |
|
claustrum |
0.35 |
0.03 |
< |
0.43 |
0.07 |
0.019 |
0.329 |
|
diagonal band of Broca |
0.36 |
0.04 |
< |
0.42 |
0.05 |
0.020 |
0.327 |
|
glomerular layer |
0.43 |
0.03 |
< |
0.50 |
0.05 |
0.020 |
0.327 |
|
dorsal medial n. |
0.29 |
0.05 |
< |
0.38 |
0.07 |
0.020 |
0.322 |
|
ventral pallidum |
0.33 |
0.05 |
< |
0.40 |
0.05 |
0.023 |
0.304 |
|
zona incerta |
0.41 |
0.03 |
< |
0.48 |
0.06 |
0.024 |
0.301 |
|
entorhinal ctx |
0.34 |
0.03 |
< |
0.40 |
0.06 |
0.027 |
0.284 |
|
accumbens shell |
0.35 |
0.07 |
< |
0.43 |
0.06 |
0.027 |
0.283 |
|
bed n. stria terminalis |
0.37 |
0.03 |
< |
0.44 |
0.06 |
0.027 |
0.283 |
|
central medial thalamic n. |
0.29 |
0.05 |
< |
0.37 |
0.07 |
0.027 |
0.283 |
|
suprachiasmatic n. |
0.33 |
0.07 |
< |
0.40 |
0.06 |
0.031 |
0.269 |
|
ventral tegmental n. |
0.42 |
0.05 |
< |
0.47 |
0.04 |
0.036 |
0.248 |
|
ventral posteromedial thalamic n. |
0.34 |
0.04 |
< |
0.41 |
0.05 |
0.037 |
0.246 |
|
magnocellular preoptic n. |
0.37 |
0.04 |
< |
0.44 |
0.07 |
0.042 |
0.228 |
|
reticular n. |
0.48 |
0.03 |
< |
0.52 |
0.05 |
0.047 |
0.214 |
|
premammillary n. |
0.33 |
0.06 |
< |
0.40 |
0.06 |
0.048 |
0.212 |
|
primary somatosensory ctx jaw |
0.33 |
0.05 |
< |
0.39 |
0.05 |
0.048 |
0.212 |
|
secondary somatosensory ctx |
0.29 |
0.04 |
< |
0.35 |
0.06 |
0.048 |
0.212 |
|
insular ctx |
0.35 |
0.05 |
< |
0.40 |
0.04 |
0.049 |
0.211 |
|
medial dorsal thalamic n. |
0.32 |
0.05 |
< |
0.38 |
0.06 |
0.049 |
0.211 |
|
medial orbital ctx |
0.32 |
0.05 |
< |
0.41 |
0.09 |
0.049 |
0.211 |
|
|
Vehicle |
|
Minocycline |
|
|
||
|
Brain Area |
Ave |
SD |
|
Ave |
SD |
P-val |
Ω Sq |
|
accumbens core |
0.43 |
0.04 |
> |
0.37 |
0.03 |
0.004 |
0.561 |
|
endopiriform n. |
0.44 |
0.04 |
> |
0.38 |
0.02 |
0.005 |
0.513 |
|
glomerular layer |
0.50 |
0.05 |
> |
0.44 |
0.02 |
0.008 |
0.440 |
|
prerubral field |
0.42 |
0.05 |
> |
0.35 |
0.03 |
0.011 |
0.409 |
|
reuniens n. |
0.40 |
0.05 |
> |
0.32 |
0.03 |
0.011 |
0.407 |
|
medial preoptic n. |
0.40 |
0.05 |
> |
0.32 |
0.04 |
0.012 |
0.387 |
|
paraventricular n. |
0.40 |
0.06 |
> |
0.31 |
0.05 |
0.015 |
0.365 |
|
ventral lateral striatum |
0.37 |
0.04 |
> |
0.31 |
0.02 |
0.017 |
0.347 |
|
rostral piriform ctx |
0.42 |
0.04 |
> |
0.37 |
0.03 |
0.019 |
0.330 |
|
tenia tecta ctx |
0.43 |
0.05 |
> |
0.35 |
0.04 |
0.020 |
0.327 |
|
ventromedial thalamic n. |
0.42 |
0.04 |
> |
0.37 |
0.04 |
0.020 |
0.325 |
|
9th cerebellar lobule |
0.49 |
0.08 |
> |
0.39 |
0.06 |
0.020 |
0.322 |
|
central gray |
0.43 |
0.08 |
> |
0.35 |
0.04 |
0.023 |
0.304 |
|
dorsal lateral striatum |
0.40 |
0.06 |
> |
0.35 |
0.03 |
0.023 |
0.304 |
|
dorsal paragigantocellularis n. |
0.44 |
0.09 |
> |
0.34 |
0.05 |
0.023 |
0.304 |
|
ventral medial striatum |
0.41 |
0.06 |
> |
0.35 |
0.04 |
0.026 |
0.289 |
|
ventral medial n. |
0.38 |
0.05 |
> |
0.31 |
0.06 |
0.027 |
0.283 |
|
lateral hypothalamus |
0.45 |
0.04 |
> |
0.39 |
0.04 |
0.028 |
0.282 |
|
supramammillary n. |
0.44 |
0.05 |
> |
0.37 |
0.06 |
0.031 |
0.268 |
|
infralimbic ctx |
0.40 |
0.07 |
> |
0.34 |
0.02 |
0.036 |
0.248 |
|
insular ctx |
0.40 |
0.04 |
> |
0.36 |
0.02 |
0.036 |
0.247 |
|
sub coeruleus n. |
0.46 |
0.08 |
> |
0.39 |
0.05 |
0.036 |
0.247 |
|
accumbens shell |
0.43 |
0.06 |
> |
0.37 |
0.04 |
0.037 |
0.246 |
|
pontine reticular n. caudal |
0.44 |
0.10 |
> |
0.35 |
0.07 |
0.037 |
0.245 |
|
reticulotegmental n. |
0.47 |
0.10 |
> |
0.35 |
0.11 |
0.037 |
0.245 |
|
ventral orbital ctx |
0.39 |
0.06 |
> |
0.33 |
0.02 |
0.039 |
0.239 |
|
parafascicular thalamic n. |
0.38 |
0.06 |
> |
0.32 |
0.03 |
0.040 |
0.234 |
|
interpeduncular n. |
0.46 |
0.08 |
> |
0.39 |
0.07 |
0.042 |
0.227 |
|
premammillary n. |
0.40 |
0.06 |
> |
0.31 |
0.07 |
0.042 |
0.227 |
|
principal sensory n. trigeminal |
0.48 |
0.06 |
> |
0.41 |
0.04 |
0.048 |
0.213 |
|
anterior hypothalamic n. |
0.37 |
0.06 |
> |
0.31 |
0.04 |
0.048 |
0.212 |
|
reticular n. midbrain |
0.41 |
0.08 |
> |
0.35 |
0.04 |
0.048 |
0.212 |
|
posterior hypothalamic n. |
0.41 |
0.06 |
> |
0.34 |
0.06 |
0.048 |
0.211 |
|
dorsal medial n. |
0.38 |
0.07 |
> |
0.30 |
0.07 |
0.049 |
0.210 |
Table 2a shows the significant changes in measures of ADC between vehicle treated shams and rats that were untreated and hit. In this case an increase in ADC would be interpreted as an increase in vasogenic edema. This comparison shows that only 7/173 brain areas presented with a change in ADC values. The critical value was set at p <0.05. An FDR for multi-comparisons gave a significant level of p = 0.008. Table 2b lists 48/173 brains areas that showed a significant decrease in ADC values with minocycline treatment. All DWI measures for each experimental condition and their tables for all 173 brain areas are provided in Supplementary Data Files S1-S4.
|
|
Vehicle |
|
Hit |
|
|
||
|
Brain Area |
Ave |
SD |
|
Ave |
SD |
P-val |
Ω Sq |
|
medial amygdaloid n. |
2.78 |
0.30 |
< |
2.44 |
0.14 |
0.021 |
0.320 |
|
primary somatosensory ctx jaw |
1.69 |
0.05 |
< |
1.84 |
0.14 |
0.024 |
0.302 |
|
glomerular layer |
2.24 |
0.12 |
> |
2.06 |
0.16 |
0.032 |
0.263 |
|
primary somatosensory ctx upper lip |
1.74 |
0.06 |
< |
1.93 |
0.21 |
0.049 |
0.210 |
|
anterior amygdaloid n. |
2.38 |
0.29 |
> |
2.12 |
0.17 |
0.049 |
0.209 |
|
medial orbital ctx |
2.22 |
0.33 |
> |
1.88 |
0.20 |
0.049 |
0.209 |
|
olfactory tubercles |
2.63 |
0.36 |
> |
2.25 |
0.29 |
0.049 |
0.209 |
|
|
Vehicle |
|
Minocycline |
|
|
||
|
Brain Area |
Ave |
SD |
|
Ave |
SD |
P-val |
Ω Sq |
|
lateral dorsal thalamic n. |
2.00 |
0.17 |
> |
1.75 |
0.08 |
0.001 |
0.690 |
|
central medial thalamic n. |
1.86 |
0.18 |
> |
1.65 |
0.08 |
0.003 |
0.579 |
|
anterior pretectal n. |
2.19 |
0.27 |
> |
1.74 |
0.14 |
0.004 |
0.550 |
|
primary somatosensory ctx jaw |
1.84 |
0.14 |
> |
1.59 |
0.10 |
0.004 |
0.530 |
|
ventrolateral thalamic n. |
1.89 |
0.19 |
> |
1.65 |
0.09 |
0.004 |
0.528 |
|
primary somatosensory ctx upper lip |
1.93 |
0.21 |
> |
1.65 |
0.08 |
0.005 |
0.504 |
|
posterior thalamic n. |
1.90 |
0.23 |
> |
1.64 |
0.09 |
0.005 |
0.502 |
|
ventral anterior thalamic n. |
1.85 |
0.17 |
> |
1.64 |
0.08 |
0.005 |
0.499 |
|
ventral posteriolateral thalamic n. |
1.91 |
0.20 |
> |
1.69 |
0.08 |
0.008 |
0.453 |
|
2nd cerebellar lobule |
2.24 |
0.33 |
> |
1.83 |
0.17 |
0.008 |
0.452 |
|
primary somatosensory ctx barrel field |
1.95 |
0.25 |
> |
1.69 |
0.09 |
0.008 |
0.451 |
|
anterior thalamic nuclei |
1.96 |
0.16 |
> |
1.73 |
0.12 |
0.009 |
0.432 |
|
ventral posteromedial thalamic n. |
1.88 |
0.22 |
> |
1.67 |
0.08 |
0.009 |
0.431 |
|
CA3 dorsal |
2.06 |
0.25 |
> |
1.81 |
0.09 |
0.009 |
0.429 |
|
secondary somatosensory ctx |
1.92 |
0.23 |
> |
1.65 |
0.07 |
0.009 |
0.429 |
|
primary somatosensory ctx forelimb |
1.90 |
0.20 |
> |
1.66 |
0.10 |
0.011 |
0.409 |
|
medial dorsal thalamic n. |
1.97 |
0.18 |
> |
1.71 |
0.14 |
0.011 |
0.406 |
|
CA1 dorsal |
2.10 |
0.29 |
> |
1.81 |
0.13 |
0.011 |
0.405 |
|
insular ctx |
1.86 |
0.13 |
> |
1.71 |
0.10 |
0.013 |
0.385 |
|
prelimbic ctx |
1.76 |
0.11 |
> |
1.60 |
0.11 |
0.013 |
0.385 |
|
primary somatosensory ctx shoulder |
2.04 |
0.23 |
> |
1.80 |
0.10 |
0.015 |
0.362 |
|
lateral orbital ctx |
1.74 |
0.04 |
> |
1.61 |
0.12 |
0.017 |
0.348 |
|
dorsal lateral striatum |
1.82 |
0.17 |
> |
1.65 |
0.09 |
0.017 |
0.342 |
|
1st cerebellar lobule |
2.33 |
0.45 |
> |
1.87 |
0.24 |
0.018 |
0.341 |
|
parafascicular thalamic n. |
1.95 |
0.24 |
> |
1.71 |
0.10 |
0.021 |
0.321 |
|
auditory ctx |
1.94 |
0.28 |
> |
1.69 |
0.08 |
0.024 |
0.302 |
|
7th cerebellar lobule |
1.97 |
0.45 |
> |
1.61 |
0.14 |
0.028 |
0.281 |
|
periaqueductal gray thalamus |
2.22 |
0.33 |
> |
1.92 |
0.13 |
0.028 |
0.281 |
|
ventral orbital ctx |
1.71 |
0.03 |
> |
1.61 |
0.14 |
0.036 |
0.247 |
|
primary motor ctx |
1.88 |
0.15 |
> |
1.73 |
0.15 |
0.037 |
0.246 |
|
white matter |
2.10 |
0.23 |
> |
1.94 |
0.10 |
0.037 |
0.246 |
|
pedunculopontine tegmental n. |
2.06 |
0.34 |
> |
1.79 |
0.12 |
0.037 |
0.245 |
|
parabrachial n. |
2.21 |
0.36 |
> |
1.88 |
0.14 |
0.037 |
0.244 |
|
dorsal medial striatum |
1.86 |
0.16 |
> |
1.71 |
0.10 |
0.037 |
0.244 |
|
lemniscal n. |
2.54 |
0.39 |
> |
2.21 |
0.31 |
0.037 |
0.244 |
|
medial cerebellar n. fastigial |
2.29 |
0.31 |
> |
1.93 |
0.27 |
0.037 |
0.244 |
|
ventral medial striatum |
1.77 |
0.15 |
> |
1.64 |
0.08 |
0.042 |
0.230 |
|
reticular n. midbrain |
2.07 |
0.30 |
> |
1.82 |
0.15 |
0.042 |
0.227 |
|
anterior cingulate n. |
2.02 |
0.18 |
> |
1.83 |
0.15 |
0.043 |
0.227 |
|
primary somatosensory ctx hindlimb |
2.02 |
0.23 |
> |
1.80 |
0.11 |
0.048 |
0.212 |
|
3rd cerebellar lobule |
2.23 |
0.39 |
> |
1.87 |
0.21 |
0.049 |
0.210 |
|
4th cerebellar lobule |
2.26 |
0.40 |
> |
1.91 |
0.12 |
0.049 |
0.210 |
|
claustrum |
1.76 |
0.11 |
> |
1.64 |
0.10 |
0.049 |
0.210 |
|
precuniform n. |
1.97 |
0.35 |
> |
1.69 |
0.11 |
0.049 |
0.210 |
|
superior colliculus |
2.41 |
0.32 |
> |
2.08 |
0.20 |
0.049 |
0.210 |
|
9th cerebellar lobule |
1.97 |
0.39 |
> |
1.65 |
0.17 |
0.049 |
0.209 |
|
globus pallidus |
1.82 |
0.19 |
> |
1.67 |
0.09 |
0.049 |
0.209 |
|
reticular n. |
1.96 |
0.21 |
> |
1.77 |
0.08 |
0.049 |
0.209 |
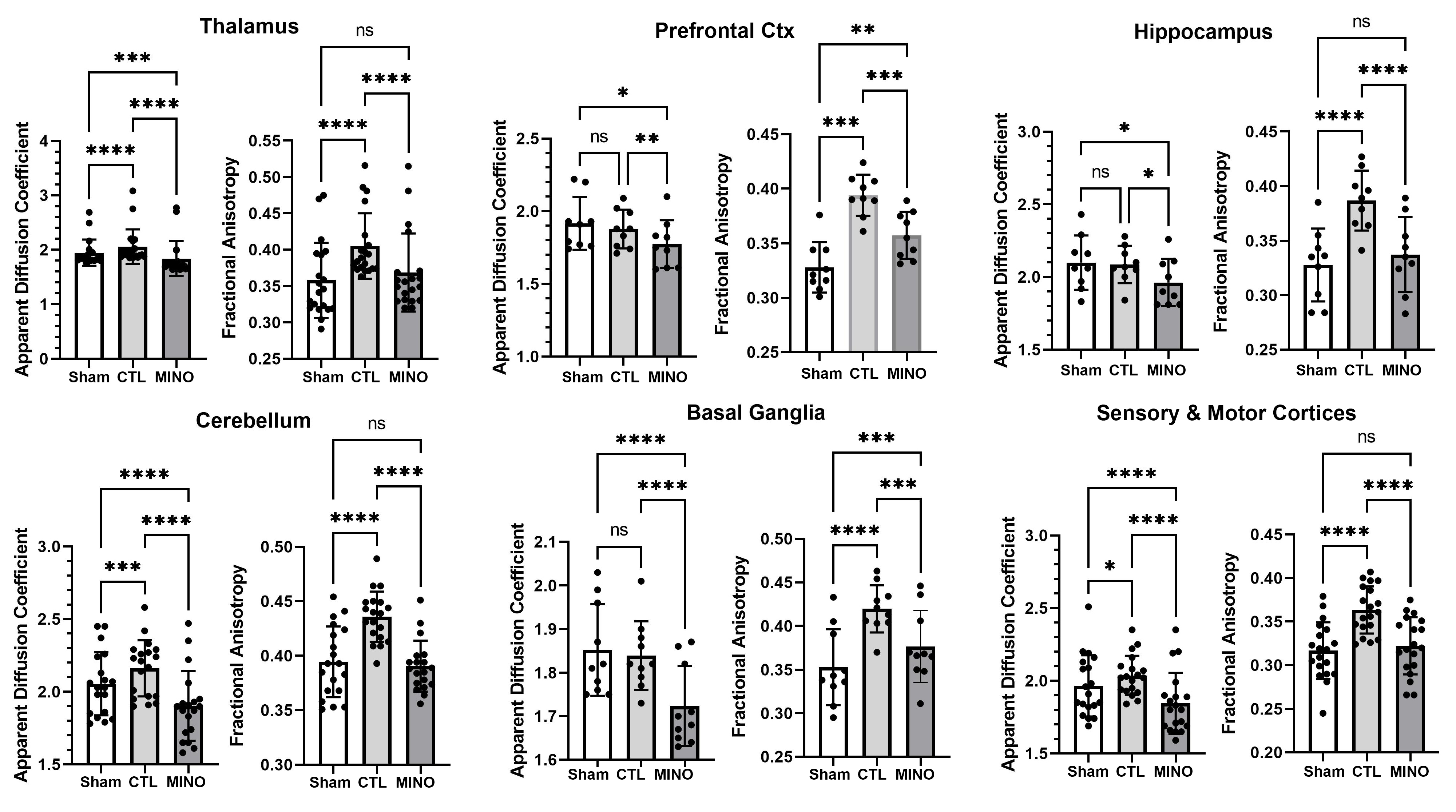
Shown in Figure 2 are scatter plot/bar graphs (mean ± SD) for only brain regions that showed minocycline associated differences in FA and ADC. The dots represent each brain area contributing to that brain region. For example, the hippocampus is composed of nine areas: dorsal and ventral subiculum, dorsal and ventral dentate, dorsal and ventral CA3 and CA1 and a single CA2. For a list of brain areas in each region see Supplementary File S6. With the data organized into brain regions there is a significant increase in ADC values associated with vasogenic edema when comparing hit with no treatment (CTL) to sham no hit rats (SHAM) for the thalamus (p<0.0001), cerebellum (p<0.001) and sensorimotor cortices (p<0.05). FA values were significantly greater in CTL than SHAM for all brain regions indicative of cytotoxic edema with repeated head injury. What was consistent across all groups, with the exception of the olfactory system, was a decrease in measures of FA and ADC with minocycline treatment (MINO) in head injured rats compared untreated head impacted controls, suggesting a decrease in vasogenic and cytotoxic edema.
Figure 2. Regional changes in water diffusivity using DWI. Shown are bar graphs (mean ± SD) and dot plots for different brain regions for two indices of anisotropy, apparent diffusion coefficient (ADC) and fractional anisotropy (FA), for each experimental condition. The dots represent the number of brain areas in that particular brain region. For example, the hippocampus, comprised of the dorsal and ventral dentate, dorsal and ventral subiculum, dorsal and ventral CA1, dorsal and ventral CA3, and CA2, has nine dots. Each dot is the average of the values for that particular brain area in shams without head injury (n=8), rats hit three times with no treatment (n=7) and rats hit three times and treated with minocycline (n=8). The list of brain areas that comprise prefrontal ctx, basal ganglia, cerebellum, thalamus, and sensory and motor cortices can be found in Supplementary Data S1: *<0.05; ***<0.001***; ****<0.0001.
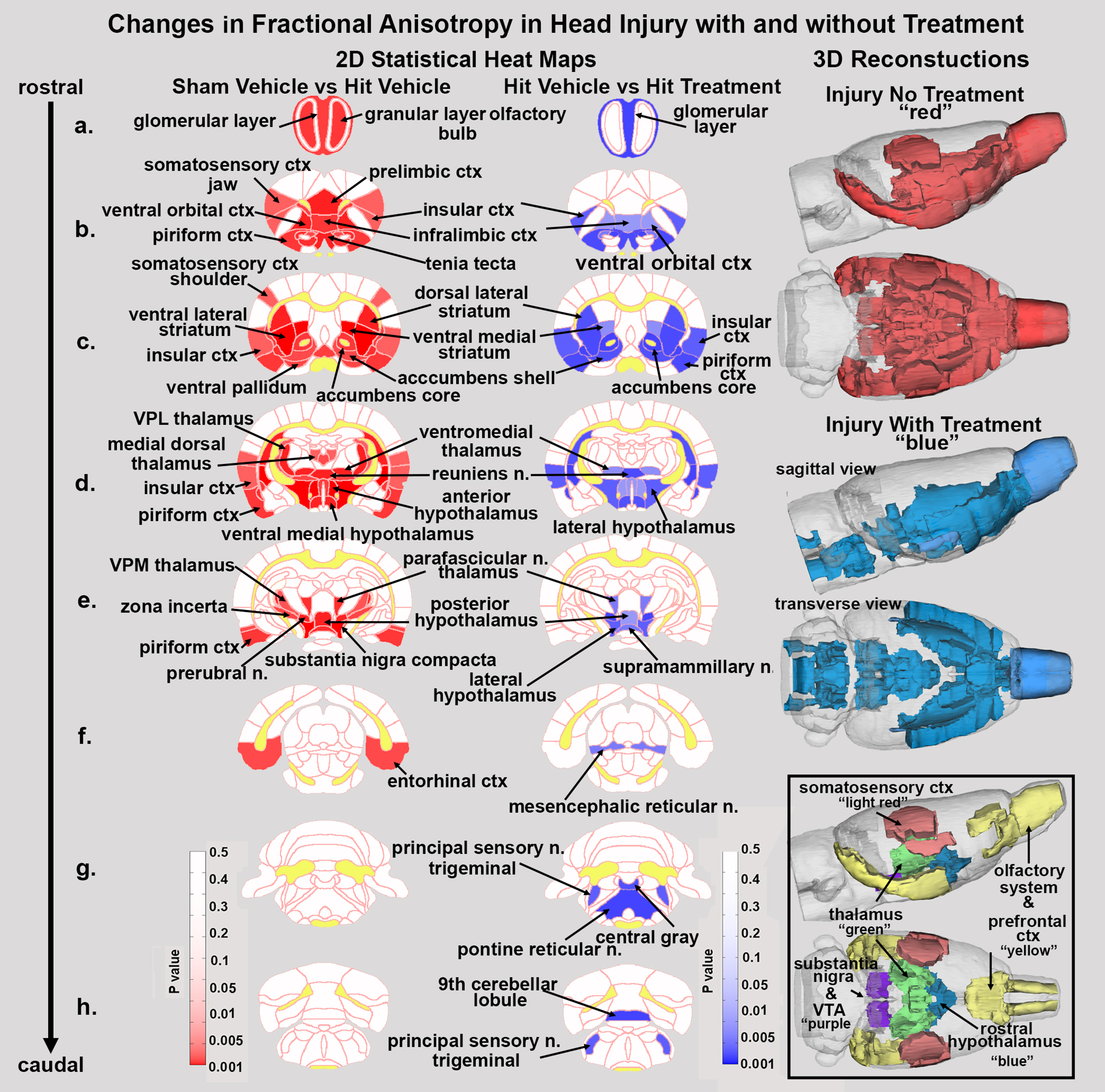
Figure 3 shows the anatomical localization of the brain areas listed in Tables 1a and 1b for FA values, presented as 2D activation maps. The coronal sections are labeled (a) through (h) and arranged from rostral (top) to caudal (bottom). The red denotes brain areas where FA values were significantly increased with head injury but no treatment. The blue denotes brain areas where FA values were significantly decreased in head injured rats treated with minocycline. Areas in yellow denote the location of white matter tracts. In brain section (a), the olfactory bulb with three different layers shows injury to the glomerular and granular layers. Treatment with minocycline significantly reduces FA values or the putative cytotoxic edema in the glomerular layer shown in blue. Brain section (b) highlights the injury in the forebrain prefrontal ctx (e.g., prelimbic, infralimbic and ventral orbital cortices) shown in red. Treatment with minocycline reversed the FA values in all of these injured areas with the exception of the prelimbic ctx and somatosensory ctx. Brain section (c) highlights injury to the dopaminergic, forebrain basal ganglia (e.g., dorsal lateral striatum, medal and lateral ventral striatum, accumbens core and shell, and ventral pallidum) shown in red. Treatment with minocycline reversed FA values in all of these injured sites with the exception of somatosensory ctx and ventral pallidum. Indeed, as you progress through each of the brain sections the areas of putative cytotoxic injury defined by an increase in FA shown in red also appear as blue with minocycline treatment, but not all injured areas are recovered with treatment. This distinction is shown in the 3D reconstructions to the right. The red reconstruction shows the injured whole brain (i.e., significantly elevated levels of FA suggestive of cytotoxic edema) and the blue reconstruction represents injured areas sensitive to minocycline treatment (i.e., significantly reduced measures of FA). The reconstructions are not identical. It should be noted that areas in brainstem sections (g & h) show significant decreases in FA values (e.g., sensory n. trigeminal, central gray, pontine reticular n. and 9th cerebellar lobule) (blue) that are not identified as having putative cytotoxic edema (no red). However, there are several areas of the hit, untreated brain that are not “recovered” with minocycline treatment (i.e., red with no matching blue). These areas are shown in the boxed insert. Of note are the thalamus and the substantia nigra and VTA.
Figure 3. Fractional anisotropy heat maps. Shown are the anatomical localization of the brain areas listed in Tables 1a and b for FA values, presented as 2D activation maps. The coronal sections are labeled (a) through (h) and arranged from rostral (top) to caudal (bottom). The red denotes brain areas where FA values were significantly increased with head injury but no treatment. The blue denotes brain areas where FA values were significantly decreased in head injured rats treated with minocycline. Areas in yellow denote the location of white matter tracts. There are several areas of the hit, untreated brain that are not represented in the minocycline treated maps (i.e., red with no matching blue). These areas are shown in the boxed insert.
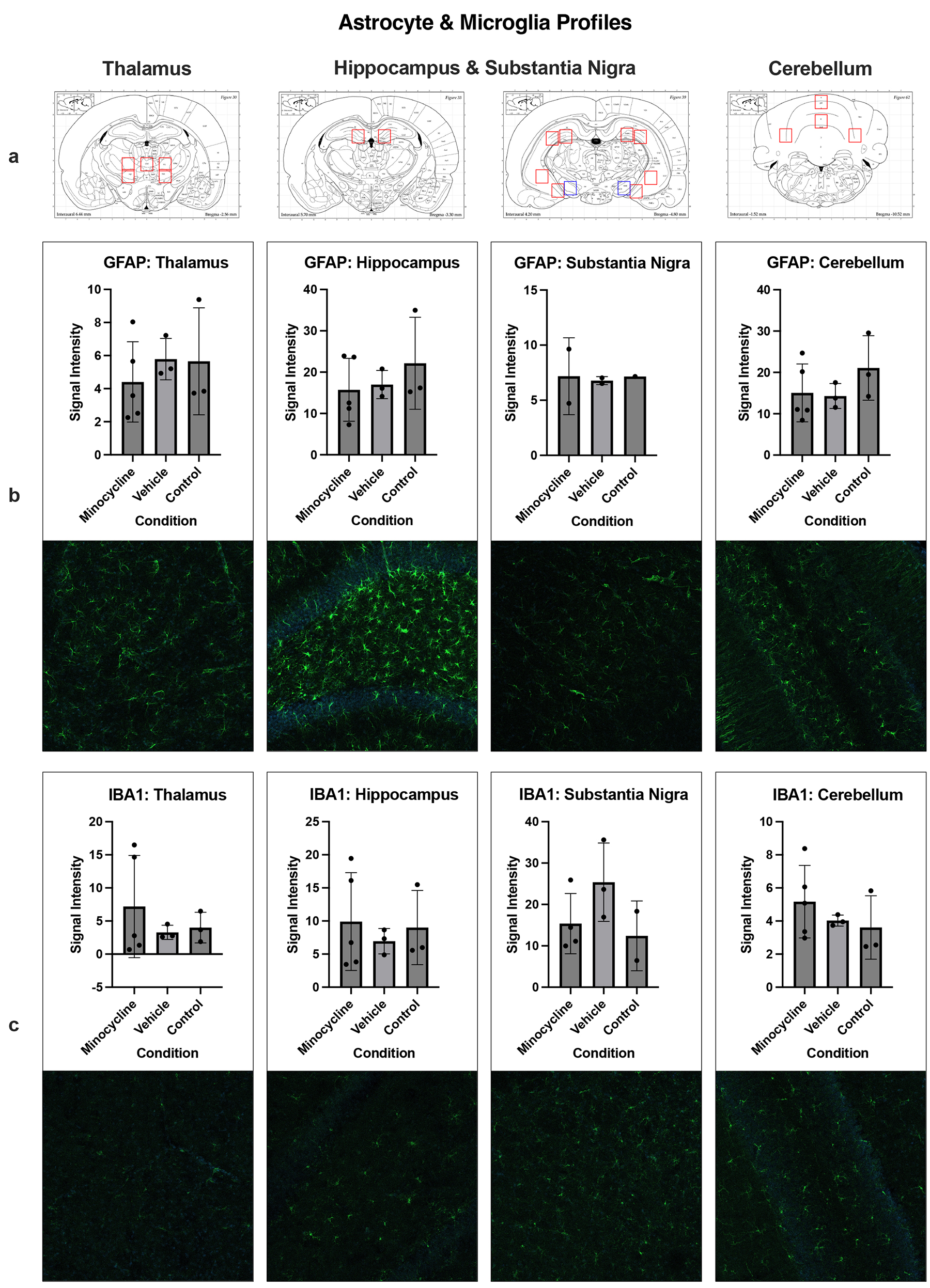
Immunohistochemistry
Shown in Figure 4 are representative micrographs of immunostaining for changes in astrocyte and microglia activation from the three experimental groups. No significant main effects of experimental condition were observed in any region. A post hoc power analysis conducted using G*Power software indicated an achieved power of 1-b = 0.153 to detect a large effect, an important limitation of this analysis.
Figure 4. Histology. a) Data collection sites within the thalamus, hippocampus, and cerebellum are outlined in red, with the substantia nigra outlined in blue. b) & c) Signal intensity values are graphed for GFAP+ astrocytes (b) and IBA1+ microglia (c) in each region above corresponding sample images. Individual data points represent the average signal intensity of the region per subject, shaded bars signify each group mean, and error bars indicate the range of ± 1 SD from the group mean.
Discussion
This study was undertaken to assess the efficacy of minocycline to treat mild repetitive head injury. Minocycline is reported to reduce microgliosis in mice and rats following significant brain damage caused by traumatic head impact [5,6,23,40-42]. All of these studies focused on the consequences of brain damage (e.g., lesion volume) and the subsequent loss of cognitive and motor behavior. The present study contributes to this body of literature in two ways. First, by using a model of mild repetitive head injury that better reflects the human experience. Head impacts were delivered during the dark period of the circadian cycle when rats are active, and then when rats were fully awake without the confound of anesthesia. There was no neuroradiological evidence of skull damage or brain contusion or noticeable deficits in motor behavior after each of three impacts. All of these findings attest to the mild nature of the head injury. Changes in diffusion weighted imaging, specifically increases in fractional anisotropy, were used as a surrogate measure of cytotoxic edema. These data are discussed below in the context of the many preclinical rodent studies on TBI with minocycline treatment and their translation to the human experience and clinical condition.
There have been numerous studies using minocycline to treat TBI in mice and rats [23,24,40-46]. All have used controlled cortical impact or weight drop protocols on anesthetized animals with open or closed skulls producing frank brain damage. The doses range from 20-90 mg/kg with various dosing regimens. Minocycline treatment under these conditions reduces neuroinflammation and microglia activation [40,41,43,44,47]. Alterations in emotion [43], motor [41] and olfactory function [45] are corrected with minocycline. Although, a study by Vonder Haar and coworkers using a dose regimen meant to mimic clinical practice reported only modest results with respect to restoration of behavioral functions [46]. Similarly, Pechacek et al. reported deficits in motor impulsivity and attention following head injury were unaffected by minocycline treatment [23], raising questions about the efficacy of minocycline for the treatment of psychiatric disorders following head injury.
The dosing regimen used by Taylor et al. in male and female adult Sprague Dawley rats was very similar to that used in the present study. Following an open skull CCI injury, rats were treated with 50 mg/kg of minocycline once daily for three consecutive days. The protracted hyperthermia caused by the TBI was reduced with minocycline treatment [24]. Although it should be noted these were moderate to severe head injuries. Kovesdi et al. used mild blast injury in anesthetized adult male Sprague Dawley rats maintained on a reverse L-D cycle and reported enhanced neuroinflammation and deficits in cognition and emotion [22]. Daily IP injections of 50 mg/kg of minocycline over four days reduced the biomarkers of inflammation and mitigated the behavioral deficits.
From all of these preclinical studies on TBI in rodents the ones most relevant to the findings in this study are those involved in measuring edema and the integrity of the BBB. Enhanced neuroinflammation, elevated proinflammatory cytokines and disruption in the BBB is common with most head injuries [48]. Homsi et al. provided the first evidence that a specific treatment regimen of minocycline could reduce brain edema in mice following head injury [47]. More recently, Lu and coworkers reported a 45 mg/kg dose of minocycline given within 30 min of head injury reduced edema and preserved BBB integrity in mice [42]. In another example using a different method other than head impact to cause brain injury, mice exposed to the neurotoxin 1,2-dicholorethane show many of the same pathological sequalae of head injury characterized by an increase in proinflammatory cytokines, gliosis, disruption in BBB integrity and edema. Treating mice with 45 mg/kg minocycline one hr before 1,2-DCE exposure reduces edema [49].
Edema makes a significant contribution to the neuropathology of head injury [50,51]. Vasogenic edema is caused by injury to the BBB and the immediate translocation of fluid to the extracellular space of the brain parenchyma. An increase in ADC, a quantitative measure of water mobility, is used as a surrogate marker for this change in extracellular volume [51]. The increase in ADC is usually accompanied by a decrease in FA. If the head injury is moderate or severe, cytogenic edema occurs characterized by cellular swelling due to loss of homeostatic regulation of osmolarity across the plasma membrane. This phase of brain edema usually presents with a decrease in ADC and increase in FA [52]. This increase in brain water contributes to parenchymal swelling and increase in intracranial pressure. Changes in BBB permeability and subsequent cerebral edema are dynamic with acute and chronic phases. For example, Logsdon and colleagues showed two mild blast injuries that cause an immediate increase in BBB over much of the brain, resolving with 24 h only to return 72 hrs later [53]. The vulnerable areas were the prefrontal cortex, hippocampus, thalamus, and medulla. In brain injury with contusion, Ren and Lu used DWI at multiple times over 72 hrs to follow the dynamic changes in edema in rats [54]. The initial vasogenic edema at 1 hr evolved into a combination of vasogenic and cytotoxic edema by 12 hrs that resolved by 24 hrs but reappeared after 48 that was prominently cytotoxic edema by 72 hrs.
In a previous study we reported a single mild impact devoid of any neuroradiological evidence of brain damage causes a short-lived increase in vasogenic edema in the thalamus, basal ganglia and cerebellum as evidenced by an increase in ADC [19]. The increase in extracellular fluid volume peaked at 6 hrs but returned to baseline by 24 hrs. In the present study female rats were subjected to three mild head impacts and imaged for changes in ADC and FA within a few hours of the last insult. We anticipated the severity of the vasogenic edema in these animals would be greater than that of a single impact characterized by an increase in ADC and decrease in FA. Indeed, the level of putative injury based on measures of DWI was widespread as shown in Figure 2. To our surprise, the expected increase in ADC and decrease in FA was not realized; instead, all affected brain regions (e.g., thalamus, prefrontal ctx, hippocampus, cerebellum, basal ganglia and sensorimotor cortices) presented with little change in ADC but robust increases in FA. In all cases, treatment with minocycline reversed the increase in FA measures. As shown in Figure 3, many of the same brain areas identified as sustaining a putative cytotoxic injury were treated with minocycline. Areas less responsive to minocycline were the midbrain dopaminergic system and the thalamus, raising questions about the sensitivity and vulnerability of these areas to head injury. Cai et al. reported two mild head impacts in anesthetized male rats interrupted perivascular clearance and aquaporin 4 expression in the substantia nigra [21]. Interestingly, patients with mild-to-moderate TBI with persistent symptoms of diminished cognitive function present with higher FA values and lower ADC values in the midbrain [55].
Data interpretation and limitations
The major limitation in this study was the absence of males to assess sex differences in this model of repetitive mild head injury. In previous studies using anesthetized male Sprague Dawley rats we interrogated the brain injury with several different imaging modalities including a quantitative ultra short time to echo-contrast enhanced procedure to assess blood brain barrier disruption at the level of the microvasculature using the contrast agent ferumoxytol and resting state functional connectivity. This project was designed to evaluate head injury in awake animals with and without minocycline treatment using DWI alone, a modality we have run in all of our previous studies and an MRI procedure readily performed in the clinic. While the changes in DWI would suggest brain injury caused by edema, there were no significant changes in astrocyte or microglia activation that would confirm the presence of neuroinflammation. As noted, the histological analysis was limited to 3-4 subjects and may have been underpowered. It is also very possible that use of only females and attending to the circadian time of injury i.e. the dark phase of the light-dark cycle may have significant effects on disease progression in this model of mild repetitive head injury.
Summary
A recent review by Cox et al. questioned the validity of preclinical models in guiding the development of new therapeutics for the treatment of head injury [56], a view shared by others to account for the many failed clinical trials for TBI [57,58]. To that end we chose to eschew the standard models of TBI that routinely cause brain damage leading to measures of cognitive and motor dysfunction. Instead, we have focused on mild head injury common in organized sports, soldiers in combat and everyday accidents in the young and old. Critical to this model is the absence of any damage as confirmed by neuroradiology. The only evidence of injury is the “bump on the head” from the edema on the skin overlying the skull as shown in Figure 1. This model, as reported in other studies on mild head injury [27,59-62], does not effectively alter behavior, discounting this measure as an endpoint when interpreting disease progression and drug efficacy using rodents. To make our model more relevant to human experience, rats were head impacted while fully awake, eliminating the confound of anesthesia, and during the dark phase of their L-D cycle when they are most active. Magnetic resonance imaging for changes in indices of anisotropy using DWI and BBB permeability using blood contrast enhanced techniques are readily performed in the clinic, aiding in the translation of data from rats to humans by using the same techniques. Previous studies from our lab using imaging with mild impacts directed to the forehead have identified the thalamus, cerebellum, hippocampus, basal ganglia, and midbrain dopaminergic system as vulnerable areas [15,19-21]. The data in this study is consistent with those findings, but using a model further refined to reflect the human experience of repetitive head injury. Moreover, we provide evidence using DWI that the alterations in gray matter microarchitecture affected by edema can be treated with minocycline given after head impact.
Conflict of Interest
CFF has a financial interest in Animal Imaging Research, a company that makes the radiofrequency electronics and holders for awake animal imaging. The CFF and PK have a partnership interest in Ekam Solutions the company that develops 3D MRI atlases for animal research.
Authors' Contributions
All of the authors have contributed substantially to the manuscript. All authors have read and agreed to the published version of the manuscript.
Concept, drafting and interpretation – Hightower, Ferris, Kulkarni,
Execution and analysis – Hightower, Prom, Brengel.
Funding
This work was supported by Elam Imaging, Inc.
Acknowledgements
We owe a dept of gratitude to Dr. Andrew Schafer whose scholarship supported the training and research of Ms. Rosemarie Hightower, an undergraduate student enrolled in the College of Science, Northeastern University.
Data Availability
All data is available on request.
References
2. Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology. 2007 Nov;32(11):2393-404.
3. Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001 Dec 4;98(25):14669-74.
4. Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med. 2000 Jul;6(7):797-801.
5. Bergold PJ, Furhang R, Lawless S. Treating Traumatic Brain Injury with Minocycline. Neurotherapeutics. 2023 Oct;20(6):1546-64.
6. Sanchez Mejia RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001 Jun;48(6):1393-9; discussion 1399-401.
7. Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005 Aug;11(4):308-22.
8. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689-701.
9. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al;STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013 Aug;12(8):822-38.
10. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013 Nov 20;80(4):844-66.
11. Jiang Y, Wang Y, Yuan Z, Xu K, Zhang K, Zhu Z, et al. Total Cerebral Small Vessel Disease Burden Is Related to Worse Performance on the Mini-Mental State Examination and Incident Dementia: A Prospective 5-Year Follow-Up. J Alzheimers Dis. 2019;69(1):253-62.
12. Wardlaw JM, Makin SJ, Hernández MC, Armitage PA, Heye AK, Chappell FM, et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimer's & Dementia. 2017 Jun 1;13(6):634-43.
13. Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. 2011 Feb;134(Pt 2):335-44.
14. Brown RB, Tozer DJ, Loubière L, Hong YT, Fryer TD, Williams GB, et al. MINocyclinE to Reduce inflammation and blood brain barrier leakage in small Vessel diseAse (MINERVA) trial study protocol. Eur Stroke J. 2022 Sep;7(3):323-30.
15. Kulkarni P, Morrison TR, Cai X, Iriah S, Simon N, Sabrick J, Neuroth L, Ferris CF. Neuroradiological Changes Following Single or Repetitive Mild TBI. Front Syst Neurosci. 2019 Aug 2;13:34.
16. Howlett JR, Nelson LD, Stein MB. Mental Health Consequences of Traumatic Brain Injury. Biol Psychiatry. 2022 Mar 1;91(5):413-420.
17. Langer L, Levy C, Bayley M. Increasing Incidence of Concussion: True Epidemic or Better Recognition? J Head Trauma Rehabil. 2020 Jan/Feb;35(1):E60-6.
18. Lefevre-Dognin C, Cogné M, Perdrieau V, Granger A, Heslot C, Azouvi P. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie. 2021 May;67(3):218-21.
19. Kulkarni P, Bhosle MR, Lu SF, Simon NS, Iriah S, Brownstein MJ, et al. Evidence of early vasogenic edema following minor head impact that can be reduced with a vasopressin V1a receptor antagonist. Brain Res Bull. 2020 Dec;165:218-27.
20. Leaston J, Qiao J, Harding IC, Kulkarni P, Gharagouzloo C, Ebong E, et al. Quantitative Imaging of Blood-Brain Barrier Permeability Following Repetitive Mild Head Impacts. Front Neurol. 2021 Sep 30;12:729464.
21. Cai X, Harding IC, Sadaka AH, Colarusso B, Kulkarni P, Ebong E, et al. Mild repetitive head impacts alter perivascular flow in the midbrain dopaminergic system in awake rats. Brain Commun. 2021 Nov 3;3(4):fcab265.
22. Kovesdi E, Kamnaksh A, Wingo D, Ahmed F, Grunberg NE, Long JB, et al. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front Neurol. 2012 Jul 16;3:111.
23. Pechacek KM, Reck AM, Frankot MA, Vonder Haar C. Minocycline fails to treat chronic traumatic brain injury-induced impulsivity and attention deficits. Exp Neurol. 2022 Feb;348:113924.
24. Taylor AN, Tio DL, Paydar A, Sutton RL. Sex Differences in Thermal, Stress, and Inflammatory Responses to Minocycline Administration in Rats with Traumatic Brain Injury. J Neurotrauma. 2018 Feb 15;35(4):630-8.
25. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother. 2010 Jul;1(2):94-9.
26. Viano DC, Hamberger A, Bolouri H, Säljö A. Concussion in professional football: animal model of brain injury--part 15. Neurosurgery. 2009 Jun;64(6):1162-73; discussion 1173.
27. Mychasiuk R, Hehar H, Candy S, Ma I, Esser MJ. The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. J Neurosci Methods. 2016 Jan 15;257:168-78.
28. Namjoshi DR, Cheng WH, Bashir A, Wilkinson A, Stukas S, Martens KM, et al. Defining the biomechanical and biological threshold of murine mild traumatic brain injury using CHIMERA (Closed Head Impact Model of Engineered Rotational Acceleration). Exp Neurol. 2017 Jun;292:80-91.
29. Namjoshi DR, Cheng WH, McInnes KA, Martens KM, Carr M, Wilkinson A, et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): a novel, surgery-free model of traumatic brain injury. Mol Neurodegener. 2014 Dec 1;9:55.
30. Ferris CF. Applications in Awake Animal Magnetic Resonance Imaging. Front Neurosci. 2022 Apr 5;16:854377.
31. Cai X, Qiao J, Knox T, Iriah S, Kulkarni P, Madularu D, et al. In search of early neuroradiological biomarkers for Parkinson's Disease: Alterations in resting state functional connectivity and gray matter microarchitecture in PINK1 -/- rats. Brain Res. 2019 Mar 1;1706:58-67.
32. Ferris CF, Nodine S, Pottala T, Cai X, Knox TM, Fofana FH, et al. Alterations in brain neurocircuitry following treatment with the chemotherapeutic agent paclitaxel in rats. Neurobiol Pain. 2019 May 27;6:100034.
33. Hoogenraad FG, Pouwels PJ, Hofman MB, Rombouts SA, Lavini C, Leach MO, et al. High-resolution segmented EPI in a motor task fMRI study. Magn Reson Imaging. 2000 May;18(4):405-9.
34. Kang D, Sung YW, Kang CK. Fast Imaging Technique for fMRI: Consecutive Multishot Echo Planar Imaging Accelerated with GRAPPA Technique. Biomed Res Int. 2015;2015:394213.
35. Menon RS, Thomas CG, Gati JS. Investigation of BOLD contrast in fMRI using multi-shot EPI. NMR Biomed. 1997 Jun-Aug;10(4-5):179-82.
36. Poser BA, Norris DG. Investigating the benefits of multi-echo EPI for fMRI at 7 T. Neuroimage. 2009 May 1;45(4):1162-72.
37. Swisher JD, Sexton JA, Gatenby JC, Gore JC, Tong F. Multishot versus single-shot pulse sequences in very high field fMRI: a comparison using retinotopic mapping. PLoS One. 2012;7(4):e34626.
38. Farzaneh F, Riederer SJ, Pelc NJ. Analysis of T2 limitations and off-resonance effects on spatial resolution and artifacts in echo-planar imaging. Magn Reson Med. 1990 Apr;14(1):123-39.
39. Jesmanowicz A, Bandettini PA, Hyde JS. Single-shot half k-space high-resolution gradient-recalled EPI for fMRI at 3 Tesla. Magn Reson Med. 1998 Nov;40(5):754-62.
40. Bye N, Habgood MD, Callaway JK, Malakooti N, Potter A, Kossmann T, et al. Transient neuroprotection by minocycline following traumatic brain injury is associated with attenuated microglial activation but no changes in cell apoptosis or neutrophil infiltration. Exp Neurol. 2007 Mar;204(1):220-33.
41. Homsi S, Piaggio T, Croci N, Noble F, Plotkine M, Marchand-Leroux C, et al. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J Neurotrauma. 2010 May;27(5):911-21.
42. Lu Q, Xiong J, Yuan Y, Ruan Z, Zhang Y, Chai B, et al. Minocycline improves the functional recovery after traumatic brain injury via inhibition of aquaporin-4. Int J Biol Sci. 2022 Jan 1;18(1):441-58.
43. Celorrio M, Shumilov K, Payne C, Vadivelu S, Friess SH. Acute minocycline administration reduces brain injury and improves long-term functional outcomes after delayed hypoxemia following traumatic brain injury. Acta Neuropathol Commun. 2022 Jan 28;10(1):10.
44. Ng SY, Semple BD, Morganti-Kossmann MC, Bye N. Attenuation of microglial activation with minocycline is not associated with changes in neurogenesis after focal traumatic brain injury in adult mice. J Neurotrauma. 2012 May 1;29(7):1410-25.
45. Siopi E, Calabria S, Plotkine M, Marchand-Leroux C, Jafarian-Tehrani M. Minocycline restores olfactory bulb volume and olfactory behavior after traumatic brain injury in mice. J Neurotrauma. 2012 Jan 20;29(2):354-61.
46. Vonder Haar C, Anderson GD, Elmore BE, Moore LH, Wright AM, Kantor ED, et al. Comparison of the effect of minocycline and simvastatin on functional recovery and gene expression in a rat traumatic brain injury model. J Neurotrauma. 2014 May 15;31(10):961-75.
47. Homsi S, Federico F, Croci N, Palmier B, Plotkine M, Marchand-Leroux C, et al. Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 2009 Sep 29;1291:122-32.
48. Diaz-Arrastia R, Kochanek PM, Bergold P, Kenney K, Marx CE, Grimes CJ, et al. Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J Neurotrauma. 2014 Jan 15;31(2):135-58.
49. Yang J, Wang T, Jin X, Wang G, Zhao F, Jin Y. Roles of Crosstalk between Astrocytes and Microglia in Triggering Neuroinflammation and Brain Edema Formation in 1,2-Dichloroethane-Intoxicated Mice. Cells. 2021 Oct 3;10(10):2647.
50. Katz DI, Cohen SI, Alexander MP. Mild traumatic brain injury. Handb Clin Neurol. 2015;127:131-56.
51. Toth A. Magnetic Resonance Imaging Application in the Area of Mild and Acute Traumatic Brain Injury: Implications for Diagnostic Markers? In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015.
52. Jha RM, Kochanek PM, Simard JM. Pathophysiology and treatment of cerebral edema in traumatic brain injury. Neuropharmacology. 2019 Feb;145(Pt B):230-46.
53. Logsdon AF, Meabon JS, Cline MM, Bullock KM, Raskind MA, Peskind ER, et al. Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci Rep. 2018 Jul 27;8(1):11344.
54. Ren H, Lu H. Dynamic features of brain edema in rat models of traumatic brain injury. Neuroreport. 2019 Jun 12;30(9):605-11.
55. Hartikainen KM, Waljas M, Isoviita T, Dastidar P, Liimatainen S, Solbakk AK, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J Clin Exp Neuropsychol. 2010 Aug;32(7):767-74.
56. Cox CS Jr, Juranek J, Bedi S. Clinical trials in traumatic brain injury: cellular therapy and outcome measures. Transfusion. 2019 Feb;59(S1):858-68.
57. Schwamm LH. Progesterone for traumatic brain injury--resisting the sirens' song. N Engl J Med. 2014 Dec 25;371(26):2522-3.
58. Stein DG. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015;29(11):1259-72.
59. Christensen J, Wright DK, Yamakawa GR, Shultz SR, Mychasiuk R. Repetitive Mild Traumatic Brain Injury Alters Glymphatic Clearance Rates in Limbic Structures of Adolescent Female Rats. Sci Rep. 2020 Apr 10;10(1):6254.
60. Kane MJ, Angoa-Pérez M, Briggs DI, Viano DC, Kreipke CW, Kuhn DM. A mouse model of human repetitive mild traumatic brain injury. J Neurosci Methods. 2012 Jan 15;203(1):41-9.
61. Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, et al. 'Hit & Run' model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013 Jun;33(6):834-45.
62. Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang XL, et al. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018 Feb 1;141(2):422-58.