Abstract
Objective: To explore the pathological mechanism and clinical treatment of cerebellar infarction through the clinical imaging changes of cerebellar infarction.
Materials and Methods: The clinical cases of cerebellar infarction were collected randomly, and the changes of imaging manifestations and clinical treatment were observed, analyzed and discussed.
Results: The delayed enhancement from the cortex is caused by the countercurrent of the contrast medium into the capillaries through the vein and unobstructed capillary network. This phenomenon of venous reflux can provide pathological basis for the choice of treatment. Dehydration can significantly improve the condition of patients with cerebellar infarction.
Conclusions: Patency of capillaries and venous reflux are important pathological mechanisms of cerebellar infarction. The duration of traditional ischemic penumbra is prolonged and the treatment time window is wider than that of cerebral infarction. Dehydration is one of the first choice for clinical treatment of cerebellar infarction, and it can even replace craniotomy decompression. (APCs).
Keywords
Cerebellar infarction, Delayed enhancement, Venous reflux, Ischemic penumbra, Dehydration.
Introduction
The cerebellum is an important organ that regulates movement, emotion, attention and language, but people do not pay as much attention to cerebellar infarction as cerebral infarction. Cerebellar infarction is rare, and the related literature shows that it accounts for about 3% of stroke (660 stroke 23426) [1]. The clinical symptoms of some cerebellar infarcts are not typical and need the help of imaging to diagnose and guide the treatment. Although it is realized that the blood supply artery of the cerebellum originates from the vertebrobasilar artery system, the blood vessels are circuitous, and there are relatively many anatomical variations, people still compare the imaging findings of cerebral infarction to judge cerebellar infarction. Imaging and pathology echo each other, so the imaging findings of cerebellar infarction can reflect the pathological changes of the infarcted area. At present, there are no specific guidelines for the treatment of cerebellar infarction. The American Heart Association (AHA) ‘s guidelines on acute ischemic stroke are often used internationally [2]., and the golden 6 hours of cerebral infarction are also used to guide the treatment of cerebellar infarction. Is it completely correct? The purpose of this study is to explore the pathological mechanism and clinical treatment principles of cerebellar infarction through the imaging changes of cerebellar infarction.
Materials and Methods
Four patients with clinical cerebellar infarction were randomly collected to observe the changes of imaging manifestations and treatment process, summarize the imaging features of cerebellar infarction, analyze the corresponding pathological mechanism of imaging changes, and explore the choice of treatment of cerebellar infarction.
Patient 1
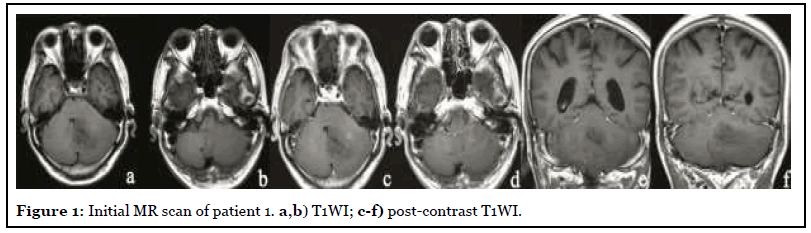
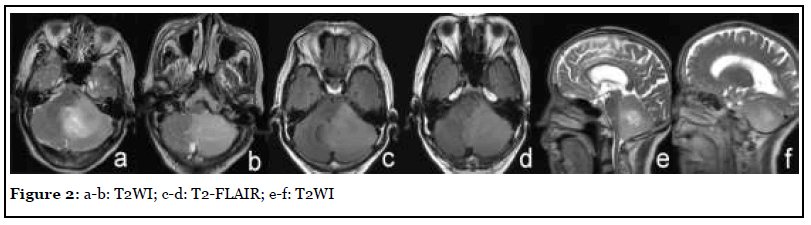
The patient was admitted with headache, vertigo and ataxia for half a year with a NIHSS score of 5. After admission, the patient underwent MR scan as shown in Figure 1. Abnormal mass in the left cerebellar hemisphere and cerebellar worm, showing long T1 and long T2 abnormal signal, high signal on T2-FLAIR, unclear boundary, obvious edema of surrounding tissue, compression near the brainstem, and the midline shifted slightly to the right. After injection of Gd-DTPA, the focus showed mild heterogeneous enhancement. Imaging diagnosis: The left cerebellar hemisphere and cerebellar worms were abnormally occupied, and followup was recommended. After three months of traditional conservative treatment, the patient had no improvement in symptoms, so he was reexamined by MRI (Figure 2). Surgical resection was performed a week later, and the final pathology was cerebellar tissue and focal cell proliferation.
Patient 2
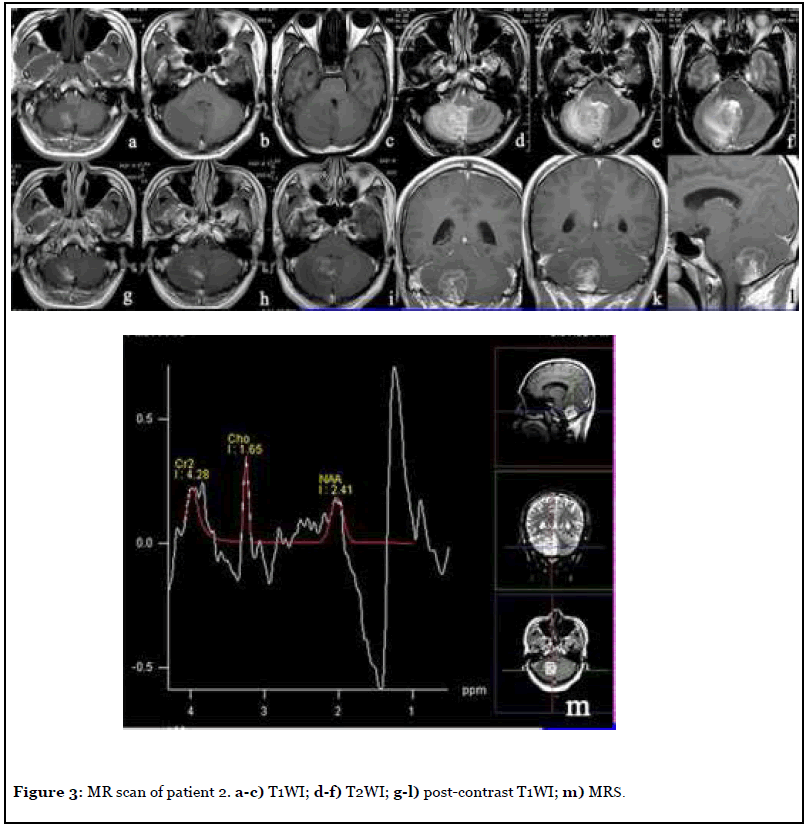
The patient was admitted to hospital with headache and dizziness for 2 years, and the NIHSS score was 4. After admission, MR scan was per formed as shown in Figure 3. The right cerebellar hemisphere showed abnormal space occupation, showing long and short T1 and long T2 abnormal signal, unclear boundary, obvious surrounding tissue edema, delayed contrast -enhanced scan showed obvious heterogeneous enhancement, NAA peak in the central area of the lesion decreased significantly and Cho peak increased slightly. Imaging diagnosis: Abnormal mass in the right cerebellar hemisphere, tumor cannot be excluded. Surgical resection was performed a week later, and the final pathology was cerebellar tissue necrosis and angiogenesis.
Patient 3
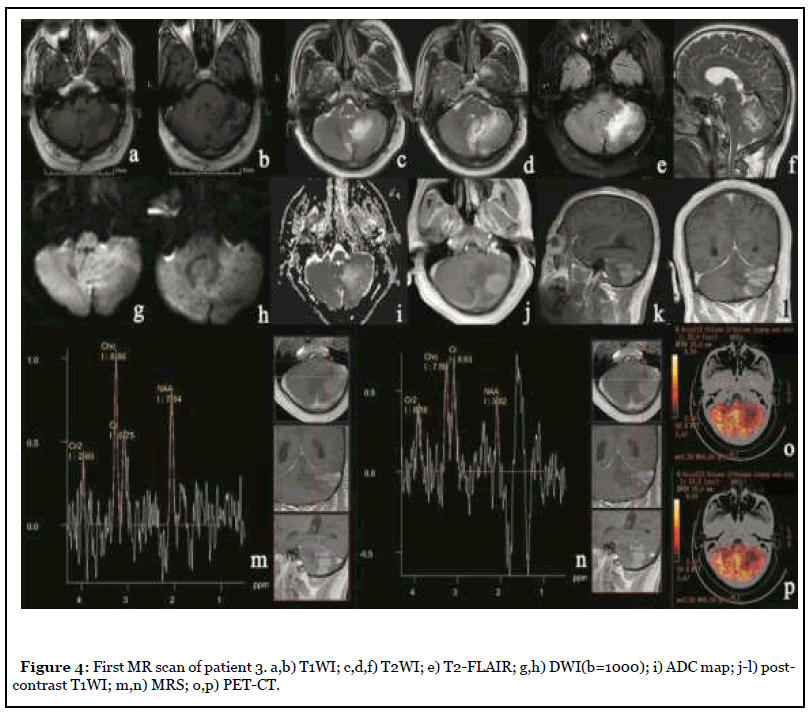
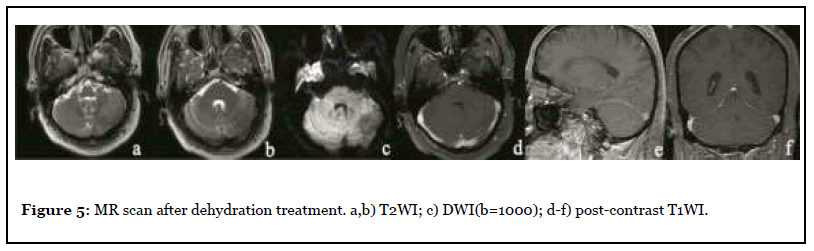
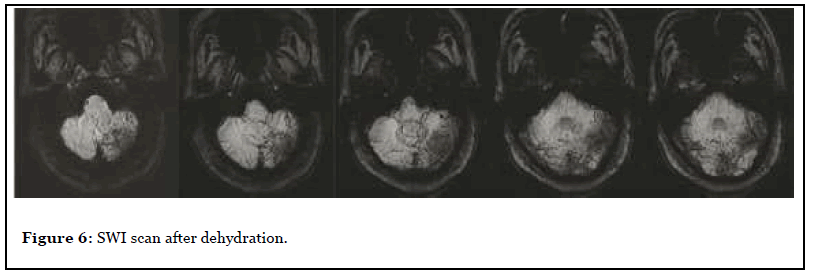
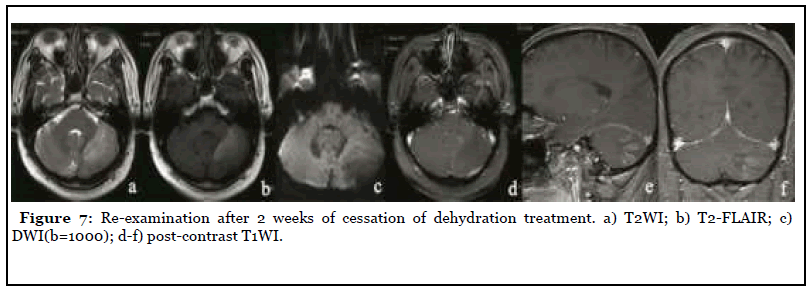
The patient was admitted to the hospital with dizziness and weakness of the left limb for 3 months, with a NIHSS score of 7. After admission, the patient underwent MR scan as shown in Figure 4. Abnormal space occupying in the left cerebellar hemisphere, showing abnormal signal of long and short T1 and long T2, high signal on T2-FLAIR, unclear boundary, obvious peripheral edema, slight pressure near the brainstem, midline shift to the right, delayed contrast-enhanced scan showed obvious heterogeneous enhancement, and NAA peak in the central area of the lesion decreased significantly while Cho peak increased. Imaging diagnosis: Abnormal mass in the left cerebellar hemisphere, tumor cannot be excluded, further examination is recommended. Three days later, PET-CT was performed as shown in Figure 4, and the diagnostic opinion was that the metabolic activity of the left cerebellar hemisphere was significantly lower than that of the contralateral cerebellar hemisphere, suggesting that malignant lesions were more likely. Clinicians recommend dehydration treatment first, and then re-examining the MR three days later, as shown in Figure 5: The lesion and surrounding edema basically disappeared, and there was no obvious abnormal enhancement after injection of Gd-DTPA. The SWI was re-examined the next day as shown in Figure 6. Patchy low-signal foci and multiple small veins were seen in the left infarcted area. Two weeks after the cessation of dehydration treatment, MR examination showed that abnormal space-occupying signal appeared again in the left cerebellar hemisphere, and the enhanced scan showed obvious heterogeneous enhancement (Figure 7). Clinical treatment continued according to cerebellar infarction.
Patient 4
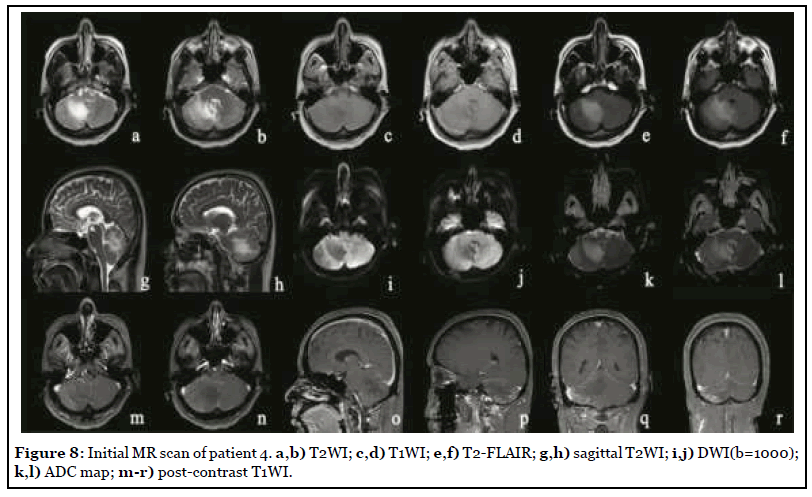
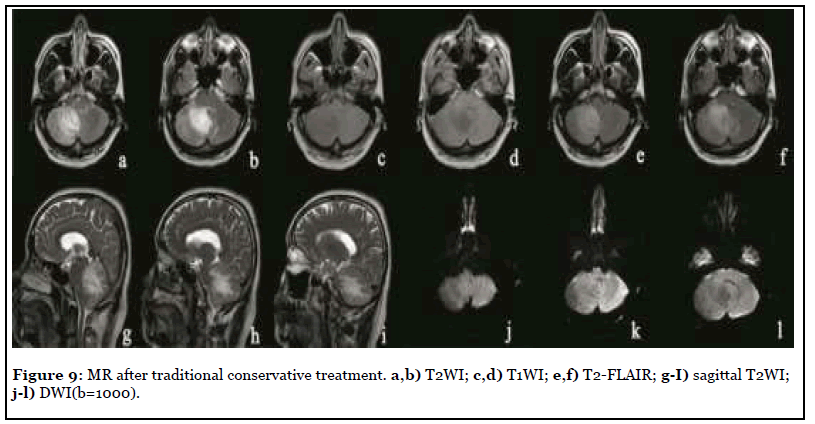
The patient was admitted to hospital with dizziness, vomiting and weakness of the right limb with a NIHSS score of 8. MR scan was performed after admission, as shown in Figure 8. The right cerebellar hemisphere showed abnormal space occupation, showing long T1 and long T2 abnormal signal, high signal on T2-FLAIR, iso-or low signal on DWI (b=1000), high signal on ADC map, unclear boundary, obvious peripheral edema, and the delayed contrast-enhanced scan showed obvious heterogeneous enhancement. After two weeks of traditional conservative treatment, the symptoms were not relieved, and the reexamination of MR was shown in Figure 9. The scope of focus and edema was enlarged.
Results
The delayed enhancement of cerebellar infarction from the cortex was caused by the contrast medium countercurrent flowing into the capillaries through the vein and unobstructed capillary network. This phenomenon of venous backflow can improve the state of hypoxia in the infarcted area and provide pathological basis for the choice of treatment. Dehydration can significantly improve the condition of patients with cerebellar infarction.
Discussion
Imaging features and differential diagnosis
Thirteen patients with cerebellar infarction first reported by Momose et al. [3]; all had obvious space-occupying effect and brain stem compression. Obvious spaceoccupying effects often lead to obstructive hydrocephalus and herniation of the cerebellar tonsil, which are lifethreatening [4,5]. In our study, four cases of cerebellar infarction also had obvious space occupying effect, accompanied by obvious edema. The infarction was located in the blood supply area of (PICA) of posterior inferior cerebellar artery and (SCA) of superior cerebellar artery. The gray matter and white matter were involved, and no obvious obstructive hydrocephalus and cerebellar tonsillar hernia were caused. Its imaging features are difficult to distinguish from gliomas, especially lowgrade gliomas. It should be pointed out that cerebellar infarction is distributed along the blood supply area of the artery [6]. Therefore, despite the obvious spaceoccupying effect and edema, cerebellar infarction does not cross the midline, while gliomas are not limited by the blood supply area and can cross the midline. Cerebral infarction rarely has obvious space-occupying effect, and peripheral edema is not obvious.
Value of special sequence
Magnetic resonance spectroscopy (MRS) has been widely used in recent years and plays an important role in the diagnosis and differential diagnosis of nervous system diseases [7]. The MRS of patients 2 and 3 showed a significant decrease in NAA peak and an increase in Cho peak, while tumors, including low-grade gliomas that were difficult to distinguish from cerebellar infarction, also showed the same MRS [8]. This is because cerebellar infarction and low-grade gliomas are dominated by neuronal destruction, so NAA, which can reflect the number of neurons [9], is significantly decreased in both. Choline as an indicator of the metabolic activity of cell membrane [10], whether cell proliferation or cell lysis, will cause the increase of Cho peak [11]. Therefore, MRS plays a limited role in differentiating cerebellar infarction from glioma. PET-CT of patient 3 showed slightly low density cerebellar infarction with moderate uptake of FDG, while low-grade gliomas also showed moderate uptake of FDG [12], indicating that the differential role of PET-CT is limited.
Pathological mechanism
Delayed enhancement was found in all 4 cases on the first MR scan. From the study of cerebral infarction, the T1 high signal intensity of cerebral parenchyma after intravenous thrombolysis is one of the high-risk factors of cerebral hemorrhagic transformation [13], but the cerebral parenchyma enhancement before thrombolysis is rare and has no significant correlation with thrombolytic therapy [14]. Some people think that the ischemia-reperfusion after thrombolysis leads to the destruction of the bloodbrain barrier and the extravasation of contrast medium [15], and its pathophysiological mechanism is that the stimulation of reperfusion promotes the secretion of potent vasodilator and causes the injury of vascular endothelium [16]. But our results are different. It is generally believed that the destruction of BBB occurs as early as 6 hours after infarction, but delayed enhancement occurs a few days later [17,18], and occurs around the lesion area. We believe that the delayed enhancement in 4 patients with cerebellar infarction shows that some of the capillary network is unobstructed and the contrast medium can enter the capillaries, but the arterial end is blocked, so the contrast medium comes from the venous end, and through the venous and capillary network backflow into the capillaries to produce enhancement. The enhancement in all the 4 patients began from the cortex, and thin strip enhancement could be seen locally. The thin strip enhancement was the manifestation of venule dilatation and filling after venous reflux. These venules could be observed from the patient’s SWI sequence. Patient 3 reexamined the SWI sequence after dehydration treatment, and we found that there were a large number of branched venules in the infarcted area. High-field MR has proved that there are a large number of venules in the periphery of the cerebellum, while the flaky low-density focus is caused by the overall T2* effect of deoxyhemoglobin in the capillaries [19]. After cerebellar infarction, the perfusion pressure in the infarcted area decreases, causing venous system backflow and venule filling. The extremely low blood oxygen concentration in the infarcted area caused the venous blood oxygen saturation to decrease again, which enhanced the T2* effect. Moreover, the outer margin of the cerebellum is adjacent to the sigmoid sinus and transverse sinus. There is a sufficient anatomical basis for venous backflow after cerebellar infarction to prevent or even weaken the progression of infarction. The disappearance of delayed enhancement in patient 3 after dehydration treatment does not mean that there is no venous backflow, but the problem of scanning phase, because patient 3 reappears delayed enhancement 2 weeks after the cessation of dehydration treatment, indicating that the capillaries in the enhanced area are always unobstructed. The “microcirculation” formed by venous reflux always exists, and complete coagulation has not occurred in the capillaries in the enhanced area.
Ischemic penumbra
Previous studies have suggested that infarction in the basal ganglia for more than 3 hours and cortical infarction for more than 6 hours is irreversible, and thrombolytic therapy is not recommended because it increases the risk of bleeding [20]. In this paper, the onset time of the 4 cases is very long, but there is no tendency to form a softening focus, of which patient 3 has been proved to have no complete infarction. As mentioned earlier, venous refluxing after cerebellar infarction plays a key role in the progression of cerebellar infarction. According to the traditional ischemic penumbra, only areas with low perfusion around the infarction core can be rescued [21], but venous refluxing and the establishment of collateral circulation after cerebellar infarction can change the pathophysiological state of the infarction core area, and the volume of the cerebellar hemisphere is significantly smaller than that of the cerebral hemisphere. Cerebellar infarction, especially cerebellar infarction caused by PICA, often involves the entire cerebellar hemisphere [6], the ischemic penumbra is difficult to quantify. Therefore, the traditional understanding of ischemic penumbra is difficult to apply in cerebellar infarction.
Treatment principle
Obstructive hydrocephalus and cerebellar tonsillar hernia caused by space-occupying effect of cerebellar infarction are the most common. Traditionally, suboccipital craniectomy and cerebrospinal fluid drainage by ventriculostomy are the first choice for the treatment of acute cerebellar infarction [22]. The treatment experience of patient 3 tells us that mannitol osmotic therapy is one of the first choices for acute cerebellar infarction. Dehydration can reduce intracranial pressure, reduce the pressure of interstitial fluid outside the lumen on the blood vessel wall, promote the smooth flow of microcirculation, improve the effect of thrombolytic therapy, and maintain the near-death state of brain tissue in the focus of infarction waiting for the establishment of collateral circulation. In addition, mannitol can be used in combination with hormones, which can reduce the cellular inflammatory response caused by hypoxia, increase the tolerance of tissues and cells in the infarcted area, and enhance the effect of mannitol.
Conclusions
The opening and patency of the capillary network and venous refluxing are the important pathological mechanisms of cerebellar infarction, which change the pathophysiological state of the infarcted area, prolong the duration of the traditional ischemic penumbra and widen the time window for treatment. The golden 6 hours of cerebral infarction is difficult to apply. Mannitol osmotic therapy combined with hormone is one of the first choice for the treatment of acute cerebellar infarction, timely dehydration can even replace craniotomy decompression.
References
2. Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke, 2007,38: 1655–711.
3. K. Jack Momose, and James R. Lehrich. Acute Cerebellar Infarction Presenting as a Posterior Fossa Mass. Radiology,1973,109:343-352.
4. Heros RC. Cerebellar hemorrhage and infarction. Stroke,1982,13:106–109.
5. Kase CS. Cerebellar infarction. Heart Dis Stroke,1994,3:38–45.
6. Peter J. Cormier, Eugene R. Long and Eric J. MR Imaging of Posterior Fossa Infarctions: Vascular Territories and Clinical Correlates.
7. Oz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology, 2014,70(3):658-79. Doi: 10.1148/radiol.13130531.
8. Bruhn H, Frahm J, Gyngell ML, et al. Noninvasive differentiation of tumors with use of localized H-1 MR spectroscopy in vivo:initial experience in patients with cerebral tumors. Radiology ,1989,172(2):541–548.
9. Moffett JR, Namboodiri MA, Cangro CB,Neale JH. Immunohistochemical localization of N-acetylaspartate in rat brain. Neuroreport,1991,2(3):131–134.
10. Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med, 2003,49(2):223–232.
11. Licata SC, Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and the irrelevance to addiction. Ann N Y Acad Sci, 2010,1187:148–171.
12. Della Puppa A, Zustovich F, Gardiman M, Manara R, Cecchin D, Scienza R. Haemorrhagic presentation of low-grade glioma in adults. Acta Neurochir (Wien), 2007 Nov,149(11):1151-5
13. Andreas Kastrup, Klaus Gro¨schel, Thomas M. Ringer. Early Disruption of the Blood– Brain Barrier After Thrombolytic Therapy Predicts Hemorrhage in Patients with Acute Stroke. Stroke,2008,39:2385-2387. DOI: 10.1161/STROKEAHA.107.505420.
14. Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood– brain barrier disruption in human focal brain ischemia. Ann Neurol, 2004,56:468–477.
15. Hua-Shan Liu, Hsiao-Wen Chung, Ming-Chung Chou, et al. Effects of Microvascular Permeability Changes on Contrast-Enhanced T1 and Pharmacokinetic MR Imagings after Ischemia. Stroke,2013,44:1872-1877.
16. Lee SK, Kim DI, Kim SY, Kim DJ, Lee JE, Kim JH. Reperfusion cellular injury in an animal model of transient ischemia. AJNR Am J Neuroradiol,2004,25:1342–1347.
17. Merten CL, Knitelius HO, Assheuer J, Bergmann-Kurz B, Hedde JP, Bewermeyer H. MRI of acute cerebral infarcts, increased contrast enhancement with continuous infusion of gadolinium. Neuroradiology, 1999,41:242–248.
18. Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood– brain barrier disruption in human focal brain ischemia. Ann Neurol, 2004,56:468–477.
19. Taylor DS, Coryell CD. The magnetic susceptibility of the iron in ferrohenIoglobin. J Am Chem Soc,1938, 60:1177–1181.
20. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med ,1995,333:1581–1587.
21. Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia: the ischemic penumbra. Stroke,1981,12:723–725.
22. Sang-Beom Jeon, Younsuck Koh, H. Alex Choi, Kiwon Leec. Critical Care for Patients with Massive Ischemic Stroke. Journal of Stroke ,2014,16(3):146-160.
