Abstract
Coronary artery disease is a major healthcare problem. Acute myocardial infarction is a leading cause of morbidity and mortality. Contemporary therapy is percutaneous coronary intervention, performed by balloon angioplasty and stent placement. Percutaneous coronary intervention is often complicated by microvascular obstruction (MVO), characterized by poor microvascular perfusion of myocardium distal to the site of primary occlusion. MVO is a multifactorial condition that is associated with adverse ventricular remodeling, arrythmia, greater infarct size, and poor prognosis. Given the high prevalence and worsened outcomes associated with MVO, there is a clear need for an innovative approach to restore microvascular perfusion. Our group and others have investigated sonoreperfusion therapy for the treatment of MVO. This technique involves the application of both diagnostic imaging and therapeutic ultrasound pulses to the site of MVO in the presence of transiting microbubbles. This review will discuss the pathophysiology of MVO and the clinical translation of sonoreperfusion therapy.
Keywords
Sonoreperfusion Therapy; Microvascular Obstruction; Coronary artery disease; percutaneous coronary intervention; angioplasty; stent
Microvascular Obstruction
Coronary artery disease and acute myocardial infarction are a leading cause of morbidity and mortality. Contemporary therapy is percutaneous coronary intervention (PCI), performed by balloon angioplasty and stent placement. PCI is nearly ubiquitously accompanied by some degree of microvascular obstruction (MVO), and in many cases characterized by poor microvascular perfusion of myocardium distal to the site of primary occlusion. This phenomenon, also termed “no reflow”, was first documented in 1974 [1]. MVO is a powerful independent predictor of mortality, independent of age, infarct size, or ejection fraction [2]. Ultrasound targeted microbubble cavitation, termed “sonoreperfusion therapy”, is a promising treatment option to relieve MVO and reestablish microvascular perfusion. The purpose of this review is to discuss the pathophysiology of MVO and the clinical translation of sonoreperfusion therapy.
MVO is a multifactorial condition that is associated with adverse ventricular remodeling, arrythmia, greater infarct size, and poor prognosis [3]. It occurs in up to 56% of STelevation myocardial infarction patients treated with PCI as measured by cardiac magnetic resonance imaging [2].
Bekkers et al. [4] postulated that endothelial cells and myocytes become edematous from osmotic overload in the setting of ischemia and subsequently occlude capillaries. This process promotes infiltration and activation of neutrophils and platelets, and promotes fibrin deposition. Adherence of activated neutrophils to the endothelium can drive increases in microvascular resistance. Lipowsky et al. [5] demonstrated that adherence of one white blood cell per high power field doubles microvascular resistance. Furthermore, coronary microembolization of atherosclerotic debris after PCI has been implicated in MVO pathophysiology [6]. All together, these phenomena result in increased microvascular resistance and reduced microvascular perfusion.
Sonoreperfusion Therapy
Contemporary therapy for MVO consists of medications such as nicardipine, nitroprusside, adenosine, etc. which provide microvascular vasodilation but also decrease systemic blood pressure. Often, however, patients undergoing PCI for acute myocardial infarction are hypotensive and therefore administration of these agents is contraindicated. Given the high prevalence of MVO and the accompanying worse outcomes, there is an urgent unmet need for an innovative approach to restore microvascular perfusion. The use of ultrasound and microbubble therapy, referred to as sonothrombolysis, has been used to treat occlusive large thrombi in supplying vessels, including epicardial coronary arteries and larger cerebral arteries [7-12] and offers a unique approach to treating MVO.
Our group has investigated the therapeutic potential of ultrasound targeted microbubble cavitation, termed sonoreperfusion therapy (SRP), specifically to relieve microvascular obstruction and restore microvascular perfusion. This process involves the application of both imaging and therapeutic ultrasound (US) pulses to microbubbles (MBs) transiting through the microcirculation. This causes MB oscillations which generates mechanical forces and shear stress which disrupt microthrombi to relieve MVO. Additionally, this shear stress stimulates endothelial cells and acute release of nitric oxide (NO), a potent vasodilator. NO counteracts the numerous pathophysiological sequalae that emanate from MVO, namely inflammation, platelet activation, endothelial cell permeability and edema, and vasoconstriction. MBs are composed of a perfluorocarbon gas encapsulated in a lipid shell. Low frequency, high intensity US has been shown to effectively dissolve thrombi [13], while high frequency, low intensity US has been shown to enhance thrombus dissolution when combined with tissue plasminogen activator (tPa), a clinically approved thrombolytic agent [13]. High intensity US causes inertial cavitation, non-linear microbubbles expansions and eventual collapse. Low intensity US causes stable cavitation, or cyclic microbubble vibrations. One major advantage of SRP is its potential for dual diagnostic and therapeutic functions in that contrast-enhanced US imaging can identify areas of MVO, with the same US transducer then being used to deliver therapeutic pulses.
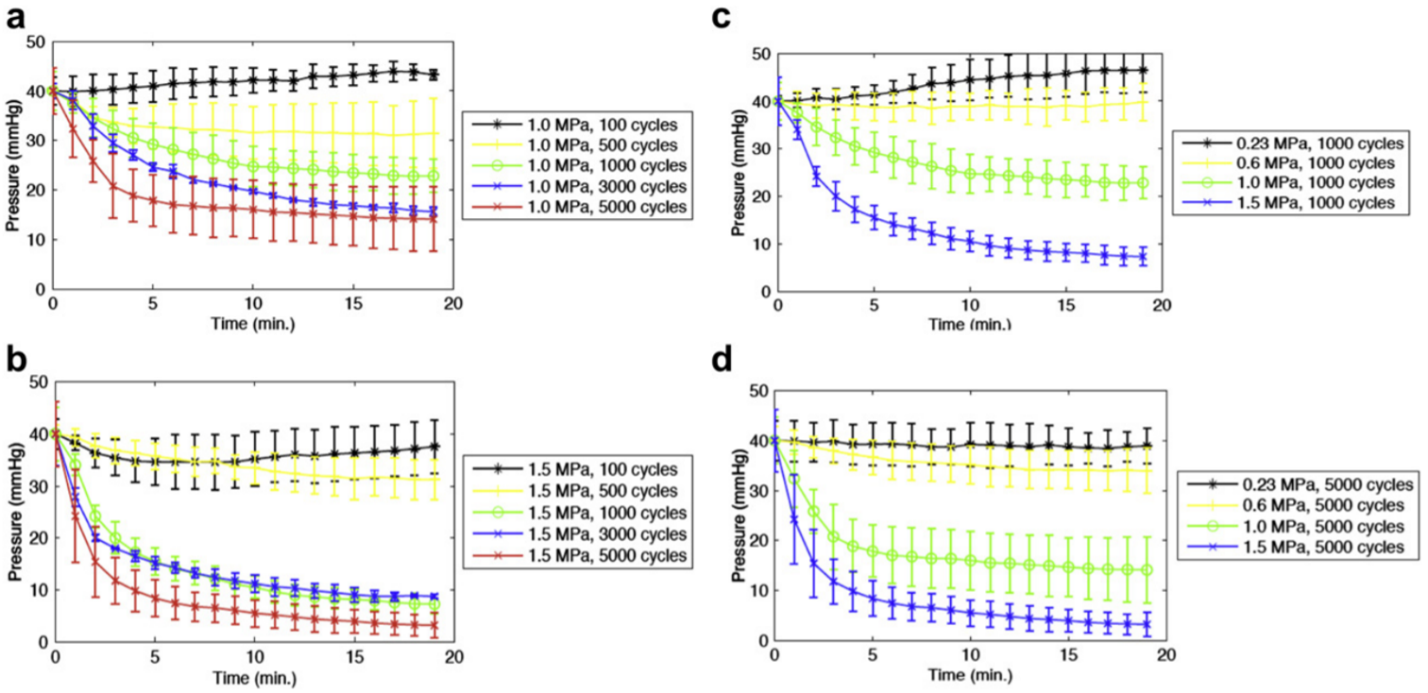
Initial in vitro studies by our group identified US parameters that optimized microthrombus dissolution. Leeman et al. [14] assessed SRP efficacy in an in vitro model of MVO composed of a silicone-rubber flow phantom model. To model MVO, microthrombi made from venous blood were deposited onto a mesh with 40 um pores, resulting in increased resistance and upstream hydrostatic pressure. MBs were diluted in phosphate buffered saline and infused while therapeutic pulsed US was delivered to the mesh to induce MB oscillations adjacent to microthrombi. Upstream pressure was measured as a surrogate for thrombus burden. Figure 1 demonstrates upstream pressure over a 20 min therapy session at various acoustic pressures and pulse lengths [14]. More rapid and complete clot dissolution occurred with increasing pressure and pulse length. The most effective acoustic regime was 1 MHz, 1.5 MPa and 5000 cyc (5 msec) pulse length every 3 sec. This in vitro system provided an effective means to evaluate the pure mechanical effects of SRP.
In order to test the effects of viscosity on SRP efficacy, Roos et al. [15] used an identical system as was used in Leeman et al. For more clinical relevance, however, the authors used whole blood as the infusate. The viscosity of whole blood is ~4 times higher than that of phosphate buffered saline and the viscosity of the surrounding medium is known to affect MB oscillatory behavior [16]. The authors demonstrated that clot dissolution with venous microthrombi increased with pulse length, with the most effective thrombolysis occurring at 1.5 MPa and 5000 cycles (5 msec) pulse length every 3 sec. Addition of tPa further enhanced SRP efficacy. This study further supported clinical translation of high acoustic pressure, long pulse length US for SRP.
Black et al. [17] used this same in vitro model of MVO to study SRP on arterial thrombi, which are more representative of microthrombi associated with post- PCI MVO. The authors showed that for a given acoustic energy, there was greater dissolution of venous vs. arterial thrombi, but that arterial thrombi were successfully dissolved. They also demonstrated that increased viscosity of the surrounding medium was associated with less microbubble cavitation, particularly in whole blood. These results further supported the utilization of high mechanical indices and long pulse lengths for sonoreperfusion and restoration of microvascular blood flow (MBF).
Multiple studies have assessed both the mechanical and biologic mechanisms of MVO in vitro and in vivo. Chen et al. [18] optically characterized MB-microthrombus interactions in the presence of a short US pulse using a specialized ultra-high-speed microscopy imaging system, capable of imaging up to 25 million frames per second [19], to visualize acoustic behavior. With inertial cavitation, large amplitude MB oscillations caused thrombus deformation and pitting of the clot surface. These results illustrated the purely mechanical mechanism of SRP, brought upon by microjetting formed by MBs. Given the observed increased thrombolytic effect using long pulse length and high mechanical indices, Chen et al. [20] further investigated MB behavior under these US conditions. The authors showed that MBs underwent stable or inertial cavitation depending on the acoustic pressure and then formed gas filled clusters that continued to oscillate, fragment, and form new clusters. For the first time, they demonstrated that MB cavitation persisted throughout the 5 msec US pulse, providing new directions for future ultrasound system design.
Further studies were also conducted to evaluate the biological mechanism of SRP. Yu et al. [21] showed that MB oscillations activate the endothelial nitric oxide synthase (eNOS) pathway and contribute to increased perfusion via nitric oxide (NO), a potent vasodilator. Intramuscular NO and perfusion were monitored in response to US targeted MB cavitation (UTMC) in a rodent hindlimb muscle. Following 2 minutes of UTMC, intramuscular NO increased at 6 min over 30 min and was higher than baseline after 13 min. Furthermore, contrast enhanced US imaging following UTMC showed increased perfusion flow rate. Importantly, enhanced flow persisted for ~4 hours. This prolonged effect raises the possibility that UTMC induces transcriptional changes and upregulation of eNOS. Other mechanisms that may also be implicated include sonoporation [22], or transient endothelial cell pore formation leading to intracellular calcium influx occurring in a similar time span as SRP. Of note, both NO and flow rate increases were blunted with LNAME, an NO inhibitor. Subsequent testing in an MVO rodent hindlimb model showed that perfusion rates did not return to baseline after SRP with LNAME pretreatment.
Preclinical and Clinical Translation of Sonoreperfusion Therapy
Promising early data regarding SRP efficacy in treating MVO [23,24] encouraged investigators to pursue further in vivo studies and human trials. To specifically address isolated MVO, Pacella et al. [25] developed a rodent hindlimb model of MVO to demonstrate SRP efficacy. In this model, an imaging transducer was placed on the biceps femoris muscle, perpendicular to a single element 1 MHz treatment transducer. Contrast enhanced US imaging was performed at baseline, after MVO, and after each of two successive US therapy sessions consisting of 5000 cycles, 0.33 Hz, PRF 5, 1.5 MPa for 10 min during continuous infusion of lipid encapsulated MBs. Control rats were injected with microthrombi and imaged at corresponding timepoints but did not receive therapeutic US. There was a 90% reduction in perfusion after MVO in both groups. Treatment rats demonstrated return of MBF to baseline, while control rats had persistently blunted MBF. Overall, this study demonstrated the clear effectiveness of a high mechanical index and long pulse length in restoring MBF after MVO.
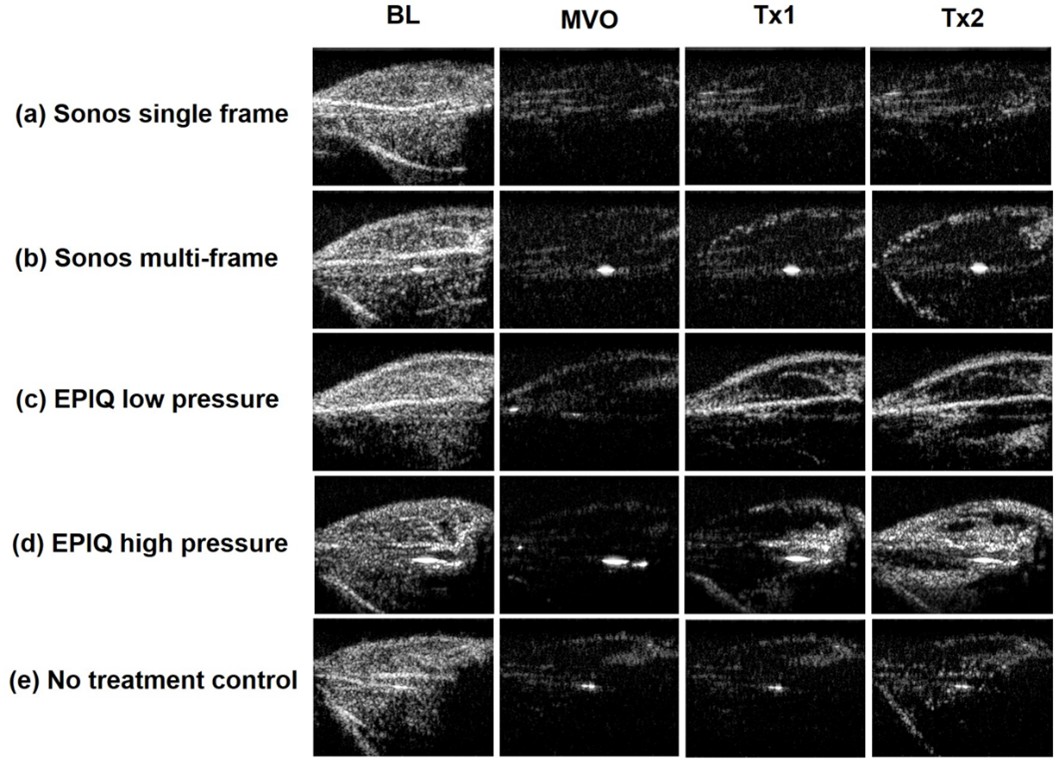
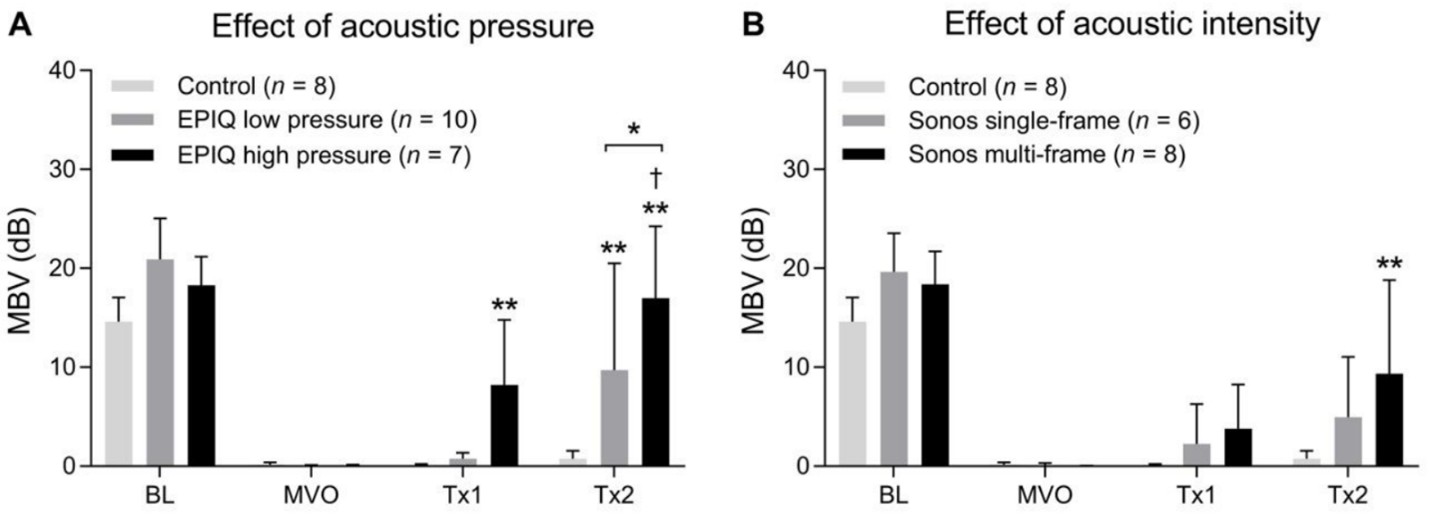
Istvanic et al. [26] investigated the therapeutic potential of two clinically available US systems, the Philips Sonos 7500 vs the Philips EPIQ. The same rodent hindlimb model as previously described [25] was used with changes in the therapeutic US delivery system. The EPIQ was modified to deliver long pulse lengths (1 msec) as was shown to be most effective in prior in vitro studies [14,15,17] and in the rodent hindlimb moded [25]. The therapeutic efficacy of SRP utilizing various acoustic pressures and acoustic intensities was also assessed. The US parameters for each of the four experimental groups were as follows: (1) Sonos 7500 single-frame: 1.3 MHz, 1.3 MPa (MI=1.1), 27 μs pulse length (total per line), time triggered, 1 frame every 3 sec; spatial peak temporal average acoustic intensity (ISPTA) 0.5 mW/cm2; (2) Sonos 7500 multi-frame: 1.3 MHz, 1.3 MPa (MI=1.1), 27 μs pulse length (total per line), time triggered, 23 frames every 3 sec; ISPTA 11.6 mW/cm2; (3) EPIQ low pressure: 1.6 MHz, 0.5 MPa (MI=0.40), 1 ms pulse length, time triggered, 8.3 frames every 3 sec; ISPTA 23.0 mW/cm2; and (4) EPIQ high pressure: 1.6 MHz, 1.44 MPa (MI=1.1), 1 ms pulse length, time triggered, 1 frame every 3 sec, ISPTA 23.0 mW/cm2. The EPIQ high pressure condition was most effective and was the only treatment to restore MBF to baseline. This result is consistent with prior in vitro and in vivo findings that long pulse, high pressure US is associated with the most effective SRP. The EPIQ low pressure and Sonos multi-frame conditions resulted in significantly improved MBF after treatment but below baseline levels. The Sonos 7500 single-frame and control conditions showed no improvement in MBF. Figure 2 shows contrast enhanced US perfusion still-frame images at four timepoints for each experimental group [26]. Figure 3 shows microvascular blood volume at four timepoints for each experimental group [26]. Importantly, this study demonstrated that a clinically available US system is readily capable of delivering therapeutic long pulses. Though previous studies have demonstrated SRP efficacy using high mechanical indices from diagnostic US transducers in STEMI patients, as detailed below, they did not assess SRP efficacy using a modified clinical system capable of delivering long pulses. Moreover, they did not address isolated, pure MVO, as would be seen post PCI; they focused on restoring epicardial coronary artery patency.
Roos et al. [27] attempted to apply long pulse, high mechanical index US in STEMI patients in a phase 2 clinical trial. However, the study was aborted after 6 patients were enrolled. 3 patients with acute myocardial infarction developed coronary vasoconstriction of the culprit artery that was deemed unresponsive to nitroglycerin by the investigators. To further investigate this isolated and newly described event, coronary artery diameters were measured in five pigs. Diameters distal to the injury site decreased after (1) balloon injury, (2) thrombus injection, and (3) administration of US. The authors concluded that long pulse US may cause coronary vasoconstriction. Mathias et al. [28] applied a similar US pulse regime in 10 patients and reported no occurrence of coronary vasoconstriction.
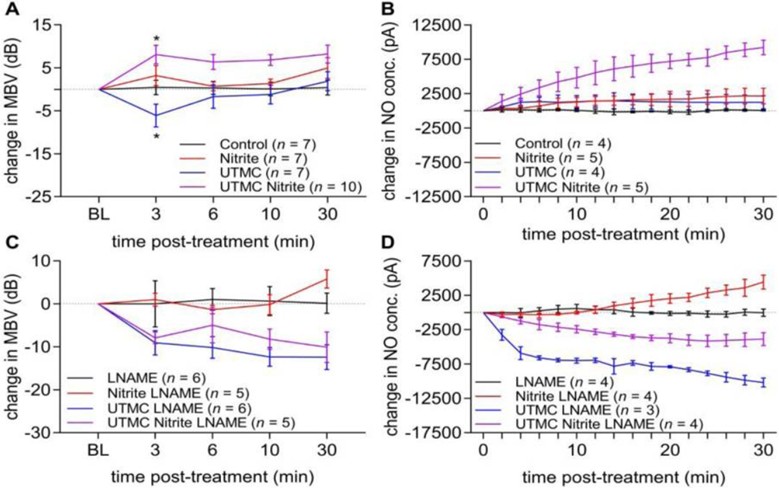
Yu et al. [29] assessed UTMC cavitation in rodent hindlimb microvascular perfusion using nitrite, an NO donor. Several preclinical and clinical studies have demonstrated the cardioprotective effect of nitrite [30,31]. Nitrite has increased bioactivity in the hypoxic and acidotic conditions characteristic of MVO. UTMC and nitrite co-therapy increased MBF and NO levels in rodent microvasculature. High mechanical index, long pulse length (5 msec) US was used in this study. Importantly, UTMC with nitrite yielded no evidence of vasoconstriction as was observed in Roos et al. [27]. Figure 4 shows changes in microvascular blood volume and NO concentration after UTMC therapy [29].
Recent human trials have also been conducted to promote clinical translation of SRP. Mathias et al. [28] analyzed the application of high mechanical index impulses from a diagnostic US transducer in STEMI patients. Results showed a smaller proportion of MVO at 1 month and overall improvement in LVEF. Of note, the US parameters used in the study are standard features of diagnostic US systems. Up to 20 usec pulse lengths were used. It is important to note that this study did not assess pure adjunctive SRP therapy immediately following PCI. SRP was administered at variable times and durations before and after PCI, with variable US regimes and contrast doses, making it impossible to identify optimal therapeutic approach. Additionally, more than twice as many patients in the treatment group had complete restoration of coronary blood flow prior to stenting, making it impossible to determine whether post-PCI adjunctive SRP conferred any additional benefit.
Mathias et al. [32] subsequently conducted a randomized control trial using high mechanical index SRP plus PCI vs PCI only in STEMI patients. <5 usec pulse length was used. In the high mechanical index plus PCI group, ST segment resolution occurred in 32% before PCI (4% in PCI only), angiographic recanalization was evident in 48% (20% in PCI only), infarct size was reduced, and LVEF increased immediately after PCI and remained higher at 6 months. Finally, the need for ICD was only 5% compared to 18% in PCI only group. Of note, this work also did not assess the efficacy of SRP as a pure adjunct following PCI, as a significant portion of patients received ultrasound therapy prior to PCI. This study was also done at a center with a very high volume of STEMI patients (Sao Paolo, Brazil) where patients often wait for cath lab availability and subsequently sometimes experience delays in door to balloon time. Importantly, our group is involved in an ongoing multicenter trial in the United States to test the effect of SRP on post-PCI MVO specifically.
Conclusions & Future Directions
In conclusion, high mechanical index and long pulse US is effective at restoring MBF. Further studies in porcine models using nitrite co-administration are underway and will allow for additional characterization of SRP efficacy. In addition to continuing clinical translation of SRP, further investigation of adjunctive agents is warranted. Prior in vitro studies have demonstrated increased thrombolytic efficacy of tPa when coupled with sonothrombolysis [14,27,33-35]. This approach can promote targeted drug delivery of tPa to sites of vascular obstruction and thus limit systemic exposure to tPa. Clinical trials in acute ischemic stroke patients treated with sonothrombolysis and adjunctive tPa have demonstrated safety and increased recanalization rates [36,37]. These trials support further investigation of adjunctive low-dose tPa during SRP in porcine models of MVO. Overall, SRP presents an innovative and promising method to treat MVO.
References
2. Kranenburg MV, Magro M, Thiele H, Waha SD, Eitel I, Cocheet A, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014 Sep;7(9):930-9.
3. Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical implications of the ‘no reflow’ phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223-8.
4. Bekkers SM, Yazdani SK, Virmani R, Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010 Apr 20;55(16):1649-60.
5. Lipowsky HH, Usami S, Chien S. In vivo measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc Res. 1980 May;19(3):297- 319.
6. Nakano M, Otsuka F, Finn AV, Virmani R. Microvascular obstruction is caused by atherothrombosis in ACS patients undergoing PCI. Circ Cardiovasc Imaging. 2011 Nov; 4(6): 597-600.
7. Birnbaum Y, Luo H, Nagai T, Fishbein MC, Peterson TM, Li SP, et al. Noninvasive in vivo clot dissolution without a thrombolytic drug: Recanalization of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130-134.
8. Brown AT, Flores R, Hamilton E, Roberson PK, Borrelli MJ, Culp WC. Microbubbles improve sonothrombolysis in vitro and decrease hemorrhage in vivo in a rabbit stroke model. Investigative Radiology. 2011;46:202-207.
9. Culp WC, Flores R, Brown AT, Lowery JD, Roberson PK, Hennings LJ, et al. Successful microbubble sonothrombolysis without tissue-type plasminogen activator in a rabbit model of acute ischemic stroke. Stroke. 2011;42:2280-2285.
10. Kutty S, Wu J, Hammel JM, Xie F, Gao S, Drvol LK, et al. Microbubble mediated thrombus dissolution with diagnostic ultrasound for the treatment of chronic venous thrombi. PLoS One. 2012;7:e51453.
11. Nishioka T, Luo H, Fishbein MC, Cercek B, Forrester JS, Kim CJ, et al. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: An in vitro and in vivo study. J Am Coll Cardiol. 1997;30:561-568.
12. Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. J Ultrasound Med. 2001;20:1313-1325. quiz 1326.
13. Slikkerveer J, Juffermans LJM, van Royen N, Appelman Y, Porter TR, Kamp O. Therapeutic application of contrast ultrasound in ST elevation myocardial infarction: Role in coronary thrombosis and microvascular obstruction. Eur Heart J Acute Cardiovasc Care. 2019 Feb; 8(1):45-53.
14. Leeman JE, Kim JS, Yu FTH, Chen X, Kim K, Wang J, et al. Effect of acoustic conditions on microbubblemediated micro-vascular sonothrombolysis. Ultrasound Med Biol. 2012;38:1589-1598.
15. Roos ST, Yu FTH, Kamp O, Chen X, Villanueva FS, Pacella JJ. Sonoreperfusion therapy kinetics in whole blood using ultrasound, microbubbles, and tissue plasminogen activator. Ultrasound Med Biol. 2016;42(12):3001- 3009.15.
16. de Jong N, Bouakaz A, Frinking P. Basic acoustic properties of microbubbles. Echocardiography. 2002 Apr;19(3):229-40.
17. Black JJ, Yu FTH, Schnatz RG, Chen X, Pacella JJ. Effect of thrombus composition and viscosity on sonoreperfusion efficacy in a model of microvascular obstruction. Ultrasound Med Biol. 2016;42(9):2220-31.
18. Chen X, Leeman JE, Wang J, Pacella JJ, Villanueva FS. New insights into mechanisms of sonothrombolysis using ultra high speed imaging. Ultrasound Med Biol. 2014;40:258-262.
19. Chen X, Wang J, Versluis M, de Jong N, Villanueva FS. Ultra-fast bright field and fluorescence imaging of the dynamics of micrometer-sized objects. Rev Sci Instrum. 2013;84:063701.
20. Chen X, Wang J, Pacella JJ, Villanueva FS. Dynamic behavior of microbubbles during long ultrasound toneburst excitation: mechanistic insights into ultrasoundmicrobubble mediated therapeutics using high-speed imaging and cavitation detection. Ultrasound Med Biol. 2016 Feb;42(2):528-538.
21. Yu FH, Chen X, Straub AC, Pacella JJ. The role of nitric oxide during sonoreperfusion of microvascular obstruction. Theranostics. 2017;7(14):3527-3538.
22. Helfield B, Chen X, Watkins SC, Villanueva FS. Transendothelial perforations and the sphere of influence of single site sonoporation. Ultrasound Med Biol. 2020 Jul;46(7):1686-1697.
23. Xie F, Gao S, Wu J, Lof J, Radio S, Vignon F, et al. Diagnostic ultrasound induced inertial cavitation to noninvasively restore coronary and microvascular flow in acute myocardial infarction. PLoS One. 2013;8:1-7.
24. Porter TR, Mathias W Jr. Cardiovascular Sonothrombolysis. Curr Card Rep. 2019 Jul 25;21(8):86.
25. Pacella JJ, Brands J, Schnatz FG, Black JJ, Chen X, Villanueva FS. Treatment of microvascular microembolization using microbubbles and long-tone-burst ultrasound: an in vivo study. Ultrasound Med Biol. 2015;41(2):456-464.
26. Istvanic F, Yu GZ, Yu FH, Powers J, Chen X, Pacella JJ. Sonoreperfusion therapy for microvascular obstruction: A step toward clinical translation. Ultrasound Med Biol. 2020 Mar;46(3):712-720.
27. Roos ST, Juffermans LM, van Royen N, van Rossum AC, Xie F, Appelman Y, et al. Unexpected high incidence of coronary vasoconstriction in the reduction of microvascular injury using sonolysis (ROMIUS) trial. Ultrasound Med Biol. 2016 Aug;42(8):1919-28.
28. Mathias W Jr, Tsutsui JM, Tavares BG, Xie F, Aguiar MO, Garcia DR, et al. Diagnostic ultrasound impulses improve microvascular flow in patients with STEMI receiving intravenous microbubbles. J Am Coll Cardiol. 2016;67(21):2506-2515.
29. Yu GZ, Istvanic F, Chen X, Nouraie M, Shiva S, Straub AC, et al. Ultrasound-targeted microbubble cavitation with sodium nitrite synergistically enhances nitric oxide production and microvascular perfusion. Ultrasound Med Biol. 2020 Mar;46(3):667-678.
30. Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232-40.
31. Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, et al. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986-94.
32. Mathias W Jr, Tsutsui JM, Tavares BG, Fava AM, Aguiar MOD, et al. Sonothrombolysis in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2019;pii:S0735-1097(19)33870-7.
33. Goyal A, Yu FTH, Tenwalde MG, Chen X, Althouse A, Villanueva FS, Pacella JJ. Inertial cavitation ultrasound with microbubbles improves reperfusion efficacy when combined with tissue plasminogen activator in an in vitro model of microvascular obstruction. Ultrasound Med Biol. 2017;43(7):1391-1400.
34. Kooiman K, Roovers S, Langeveld SAG, Kleven RT, Dewitte H, O’Reilly MA, et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med Biol. 2020 Jun;46(6):1296-1325.
35. Petit B, Bohren Y, Gaud E, Bussat P, Arditi M, Yan F, et al. Sonothrombolysis: the contribution of stable and inertial cavitation to clot lysis. Ultrasound Med Biol. 2015 May;41(5):1402-10.
36. Bor-Seng-Shu E, Nogueira RDC, Figueiredo EG, Evaristo EF, Conforto AB, Teixeira MJ. Sonothrombolysis for acute ischemic stroke: a systematic review of randomized controlled trials. Neurosurg Focus. 2012 Jan;32(1):E5.
37. Ricci S, Dinia L, Sette MD, Anzola GP, Mazzoli T, Cenciarelli S, et al. Sonothrombolysis for Acute Ischemic Stroke. Stroke. 2013;44:e6-e7.