Abstract
This commentary complements data reported in Clinical Biomechanics [1] reporting reduced maximal handgrip strength in numerous patients with myalgic encephalomyelitis / chronic fatigue syndrome (ME/CFS) in proportion to their lowered maximal physical performances. The causes of muscle weakness in these patients are open to discussion. Literature data reveal a reduction of central command to skeletal muscles in some ME/CFS patients, related to encephalomyelitis. Altered muscle membrane excitability, that is “peripheral fatigue”, is also described in relation with an imbalance of the oxidant / anti-oxidant status. On the other hand, subgroups of chronically fatigued patients with clinical criteria of ME/CFS do not suffer from any muscle weakness. Thus, clinical data do not sufficiently clarify homogeneous ME/CFS pathology.
Highlights
• Altered muscle function often occurs in ME/CFS patients.
• Reduced handgrip strength is proportional to lowered physical performance
• Muscle fatigue could result from altered muscle excitability at work
• Reduced central motor command is also documented in relation of encephalomyelitis
• Subgroups of ME/CFS patients without muscle weakness are documented
Commentary
This commentary considers the occurrence of skeletal muscle weakness in patients with myalgic encephalomyelitis / chronic fatigue syndrome (ME/ CFS), already reported by Jammes et al. in the last issue of Clinical Biomechanics [1]. These authors showed that numerous patients with severe body fatigue and clinical criteria of ME/CFS had reduced values of maximal handgrip strength in proportion of their lowered maximal performance at work measured on a cycloergometer. Here, we discuss these data in terms of pathophysiological mechanisms of muscle failure.
Remember that ME/CFS is a multisystem disease characterized by an intense fatigue worsened by physical/ mental activity [2-4], often associated with postexertional malaise (PEM) [3,5]. Several body systems including the muscular and nervous systems are affected in ME/CFS. The symptoms combine fatigue, loss of memory and/or concentration, headaches, unrefreshing sleep, unexplained muscle or joint pain, sore throat and sometimes enlarged lymph nodes in neck or armpits [6]. ME/CFS pathogenesis appears to have a number of factors; different stressors (such as physical exertion, severe infections, or emotional stress or a combination of these) are continually reported in the medical history of patients with ME/CFS [4,7]. ME/CFS definition is too often solely based on clinical criteria and does not use biological markers. Primary analyses of negative biological observations, including the absence of changes in light and electron microscopy of muscles biopsies [8] and contractile muscle properties [9], have led to the former conclusion that ME/CFS is not a myopathy but that psychological/psychiatric factors appear to be of greater importance [10]. However, ME/CFS is sometimes diagnosed in elite ultra-endurance cyclists [11] and in later life of subjects who exercised frequently [12]. CFS patients also often complaint of delayed recovery after exercise, confirmed by lengthened recovery of maximal voluntary contraction after fatiguing efforts [13]. Thus, exercise-induced causes of ME/CFS pathology are highly suspected [4].
In healthy subjects, muscle fatigue primarily results from the incapacity of muscle fibres to contract and this muscle failure is called “peripheral fatigue” [14]. This may result from failure of metabolic processes due to the imbalance between oxygen demand and supply, reduced excitation-contraction coupling involving altered intracellular calcium release and mobilization, and also impaired muscle membrane excitability due to altered ionic fluxes through the sarcolemma.
Indeed, electrophysiological muscle events are closely linked with the ionic fluxes through the membranes. Recording the compound evoked muscle action potentials (M-wave) with surface electrodes is a noninvasive mean to explore peripheral muscle fatigue in exercising humans (Figure 1). An impaired excitation of the muscle fibres is suspected when the M-wave declines and becomes broader [15]. Muscle fatigue is closely linked to an excessive production of reactive oxygen species which counteract the potassium outflow [16]. In healthy subjects, muscle biopsies have demonstrated a contraction-induced loss in myoplasmic potassium (K+) concentration [17]. This potassium outflow is detectable in plasma and the kinetics of plasma K+ increase during an incremental exercise is well known in healthy subjects [18]. In sedentary subjects an increased ROS production with exercise exerts inhibitory action on the Na+-K+ pump activity [16], reducing the potassium outflow and the muscle membrane excitability. “Peripheral fatigue” affects the muscle machine itself but it may also coexist with an altered central nervous system command to muscles (“central fatigue”). In humans, non-invasive tools are used to assess the occurrence of “central” fatigue. They combine electromyographic recordings of maximal or submaximal voluntary contractions with interpolation of twitches, and analysis of post-exercise recovery of maximal contraction [14].
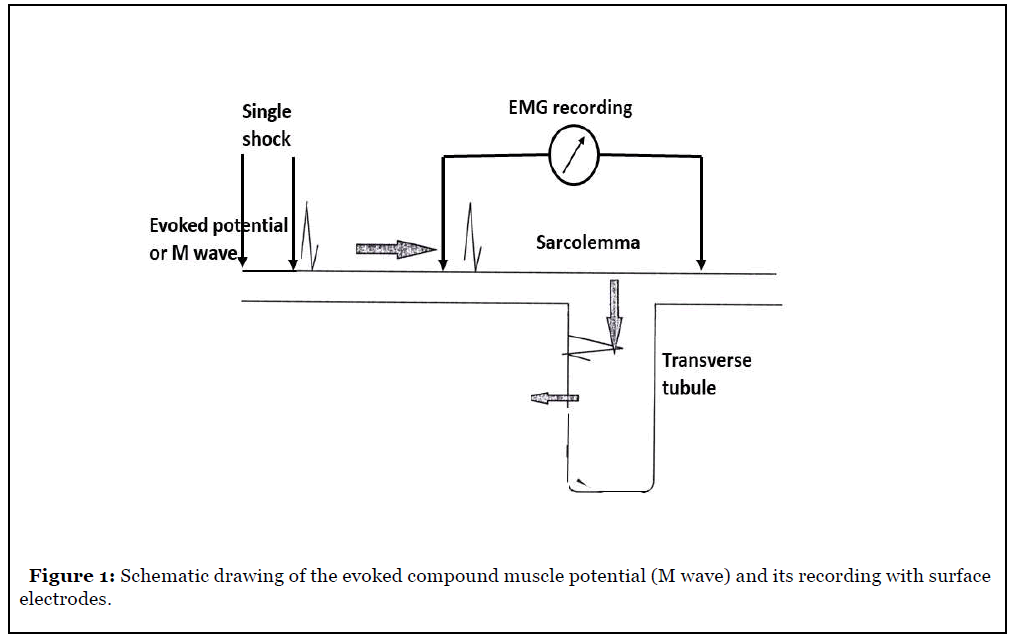
Several studies have documented the occurrence of peripheral skeletal muscle fatigue in ME/CFS patients. Experimental evidences indicate that exercising skeletal muscles are often affected because marked alterations of myopotentials in response to direct muscle stimulation (M-wave) occur [7,19-24]. The M-wave alterations show an impaired muscle membrane excitability; it begins early in the exercising muscles before culminating during the 10-min recovery period. In contrast, M-wave alterations are absent or modest during exercise in healthy subjects and in some of them the amplitude of myopotentials often increases with the incremental pedalling force [25]. In ME/CFS patients, M-wave alterations are also present in resting muscles; indeed, it was recently reported an altered excitability in resting forearm muscles during maximal cycling exercise [26]. The alteration of myopotentials was proportional to the magnitude of the imbalance between oxidant and anti-oxydant status in plasma, often measured in these patients. Several studies in patients with ME/CFS have examined the changes in resting blood oxidant–anti-oxidant status and reported lower vitamin E concentration and higher levels of oxidized LDL, thiobarbituric acid reactive substances (TBARS), and malondialdehyde (MAL) [27-30]. Fulle et al. [20] observed in ME/CFS patients a dysregulation of the Na+/ K+ ATPase pump which could result from an increased fluidity of the sarcoplasmic reticulum membrane in relation to an excessive production of reactive oxygen species. Thus, blood disorders of the oxidant status seem to result in an overall alteration of the muscle membrane excitability also present in resting muscles. The heat shock proteins (HSPs) protect the cells against the deleterious effects of reactive oxygen species (ROS) produced during exercise [31,32], reducing the generation of ROS through the activation of anti-oxidants. In patients with ME/CFS, the responses of plasma HSP27 and HSP70 to exercise are delayed and often reduced, and resting levels of plasma HSP70 are lower than in healthy volunteers [22]. The lack of HSPs response to exercise might explain the augmented oxidative stress measured in these patients. A downregulation of HSP production in some individuals could be caused by the repetition of exercise bouts at high energetic levels and/or severe infectious events. Further studies, including in high-intensity sport programs and military training, are needed to show that the repetition of exercise bouts at high levels might depress the expression of inducible HSP factors.
Numerous studies also support the existence of central fatigue in ME/CFS patients perhaps in relation to encephalomyelitis. Kent-Braun et al. [33] showed that the voluntary contraction of the tibialis muscle during maximal isometric exercise was lowered. Sacco et al. [34] reported a reduced amplitude of motor potentials evoked in the biceps brachii muscle by transcranial magnetic stimulation of the motor cortex. The same observations were made by Schillings et al. [35] and Davey et al. [36] who reported a reduction of corticospinal excitability with a deficit in motor preparatory cortical areas. Also, in ME/CFS patients, Siemionow et al. [37] reported a modification of the central motor command to muscles during isometric handgrip compared with healthy volunteers. Recently, we confirmed the occurrence of reduced maximal handgrip strength in these patients, in proportion to the decrease in peak oxygen uptake and maximal work rate [1]. These observations suggest that ME/CFS pathology may be associated with an altered central nervous system command to muscles. Indeed, the reduction of maximal muscle strength cannot result from an altered muscle membrane excitability because in ME/CFS this only occurs during and after lengthened dynamic exercise.
However, it merits to be underlined that an altered muscle function cannot be documented in all subjects in our large series of patients with clinical ME/CFS criteria [7,21-23,38]. Indeed, reduced handgrip values are absent in 62/220 patients and M-wave alteration were not found in 126/296 patients. Thus, the clinical criteria of ME/CFS are unable to distinguish subgroups of patients and do not sufficiently clarify a homogeneous pathology.
The ME/CFS diagnosis also explores the innate immunity and inflammatory response to exercise [39]. Indeed, these patients often present an immune imbalance, characterized by a depressed function of natural killer cells, reduced T cell responses to mitogens and specific antigens, IgG subclass deficiencies (IgG1, IgG3) and decreased complement levels [10]. However, no clear evidence of a link between abnormal immunity and CFS was established [40]. It merits to be underlined that the commonly used EMG / nerve conduction velocity testing does not bring any help in the ME/CFS diagnosis.
The differential for ME/CFS is idiopathic fatigue (the patient has none of the four ME/CFS symptoms), fibromyalgia (the main symptom of fibromyalgia is pain whereas the main symptom of ME/CFS is extreme tiredness that does not get better with sleep and rest), and also multiple sclerosis, polymyalgia rheumatica, post-infectious Lyme disease and the rare myasthenia gravis.
Commonly used treatments are exercise therapy and psychotherapy, dietary therapy, and also probiotic uses because the gut microbiota might play an important role in ME/CFS pathophysiology [41,42].
In conclusion, muscle weakness occurs in numerous ME/ CFS patients limiting their capacities at work. The causes of muscle weakness in ME/CFS are not fully understood and perhaps combine peripheral and central fatigue.
Because measurement of maximal exercise capacities on a cycloergometer or a treadmill is often difficult in severely fatigued patients, the simple determination of maximal handgrip strength can be useful to evaluate the limitation of physical performance.
References
2. Fukuda K, Strauss SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Journal of Chronic Fatigue Syndrome. 1995 Jan 1;1(2):67-84.
3. Carruthers BM. Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works. Journal of Clinical Pathology. 2007 Feb 1;60(2):117-9.
4. Prins JB. Meer JWM van der, Bleijenberg G. Chronic fatigue syndrome (review). Lancet. 2006;367:346-55.
5. Institute of Medicine of the National Academies. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations. National Academics Press, Washington DC, 2015.
6. De Korwin JD, Chiche L, Banovic I, Ghali A, Delliaux S, Authier FJ, et al. Le syndrome de fatigue chronique: unenouvelle maladie?. La Revue de Médecine Interne. 2016 Dec 1;37(12):811-9.
7. Jammes Y, Steinberg JG, Delliaux S. Chronic fatigue syndrome: acute infection and history of physical activity affect resting levels and response to exercise of plasma oxidant/antioxidant status and heat shock proteins. Journal of Internal Medicine. 2012 Jul;272(1):74-84.
8. Edwards RH, Gibson H, Clague JE, Helliwell T. Muscle histopathology and physiology in chronic fatigue syndrome. Chronic Fatigue Syndrome. 1993:102-31.
9. Gibson H, Carroll N, Clague JE, Edwards RH. Exercise performance and fatiguability in patients with chronic fatigue syndrome. Journal of Neurology, Neurosurgery & Psychiatry. 1993 Sep 1;56(9):993-8.
10. Evengård B, Schacterle RS, Komaroff AL. Chronic fatigue syndrome: new insights and old ignorance. Journal of Internal Medicine. 1999 Nov;246(5):455-69.
11. Rowbottom DG, Keast DA, Green SI, Kakulas BY, Morton AR. The case history of an elite ultra-endurance cyclist who developed chronic fatigue syndrome. Medicine and Science in Sports and Exercise. 1998 Sep;30(9):1345-8.
12. Harvey SB, Wadsworth M, Wessely S, Hotopf M.Etiology of chronic fatigue syndrome: testing popular hypotheses using a national birth cohort study. Psychosomatic Medicine. 2008 May 1;70(4):488-95.
13. Paul L, Wood L, Behan WM, Maclaren WM.Demonstration of delayed recovery from fatiguing exercise in chronic fatigue syndrome. European Journal of Neurology. 1999 Jan;6(1):63-9.
14. Enoka RM, Stuart DG. Neurobiology of muscle fatigue. Journal of Applied Physiology. 1992 May 1;72(5):1631-48.
15. Bigland-Ritchie B, Kukulka CG, Lippold OC, Woods JJ. The absence of neuromuscular transmission failure in sustained maximal voluntary contractions. The Journal of Physiology. 1982 Sep 1;330(1):265-78.
16. Juel C. Muscle fatigue and reactive oxygen species. The Journal of Physiology. 2006 Oct 1;576(Pt 1):1.
17. Sjøgaard G. Exercise-induced muscle fatigue: the significance of potassium. Acta Physiologica Scandinavica.Supplementum. 1990;140(593):1-63.
18. Marcos E, Ribas J. Kinetics of plasma potassium concentrations during exhausting exercise in trained and untrained men. European Journal of Applied Physiology and Occupational Physiology. 1995 Mar 1;71(2-3):207-14.
19. Samii A, Wassermann EM, Ikoma K, Mercuri B, George MS, O’fallon A, et al. Decreased postexercise facilitation of motor evoked potentials in patients with chronic fatigue syndrome or depression. Neurology. 1996 Dec 1;47(6):1410-4.
20. Fulle S, Belia S, Vecchiet J, Morabito C, Vecchiet L, Fano G. Modification of the functional capacity of sarcoplasmic reticulum membranes in patients suffering from chronic fatigue syndrome. Neuromuscular Disorders. 2003 Aug 1;13(6):479-84.
21. Jammes Y, Steinberg JG, Mambrini O, Bregeon F, Delliaux S. Chronic fatigue syndrome: assessment of increased oxidative stress and altered muscle excitability in response to incremental exercise. Journal of Internal Medicine. 2005 Mar;257(3):299-310.
22. Jammes Y, Steinberg JG, Delliaux S, Brégeon F.Chronic fatigue syndrome combines increased exerciseinduced oxidative stress and reduced cytokine and Hsp responses. Journal of Internal Medicine. 2009 Aug;266(2):196-206.
23. Jammes Y, Steinberg J, Guieu R, Delliaux S. Chronic fatigue syndrome with history of severe infection combined altered blood oxidant status, and reduced potassium efflux and muscle excitability at exercise. Open Journal of Internal Medicine. 2013 Sep;3(3):98-105.
24. Fenouillet E, Vigouroux A, Steinberg JG, Chagvardieff A, Retornaz F, Guieu R, et al. Association of biomarkers with health-related quality of life and history of stressors in myalgic encephalomyelitis/chronic fatigue syndrome patients. Journal of Translational Medicine. 2016 Dec;14(1):251.
25. Jammes Y, Zattara-Hartmann MC, Caquelard F, Arnaud S, Tomei C. Electromyographic changes in vastus lateralis during dynamic exercise. Muscle & Nerve. 1997;20(2):247-9.
26. Jammes Y, Adjriou N, Kipson N, Criado C, Charpin C, Rebaudet S, et al. Altered muscle membrane potential and redox status differentiates two subgroups of patients with chronic fatigue syndrome. Journal of Translational Medicine. 2020b Dec;18:1-8.
27. Fulle S, Mecocci P, Fanó G, Vecchiet I, Vecchini A, Racciotti D, et al. Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radical Biology and Medicine. 2000 Dec 15;29(12):1252-9.
28. y Keenoy BM, Moorkens G, Vertommen J, De Leeuw I. Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sciences. 2001 Mar 16;68(17):2037-49.
29. Vecchiet J, Cipollone F, Falasca K, Mezzetti A, Pizzigallo E, Bucciarelli T, De Laurentis S, Affaitati G, De Cesare D, Giamberardino MA. Relationship between musculoskeletal symptoms and blood markers of oxidative stress in patients with chronic fatigue syndrome. Neuroscience Letters. 2003 Jan 2;335(3):151-4.
30. Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJ. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radical Biology and Medicine. 2005 Sep 1;39(5):584-9.
31. Noble EG. Heat shock proteins and their induction with exercise. InExercise and stress response 2002 Mar 28 (pp. 49-84). CRC Press.
32. Whitham M, Fortes MB. Heat shock protein 72: release and biological significance during exercise. Frontiers in Bioscience. 2008 Jan 1;13(4):1328-39.
33. Kent-Braun JA, Sharma KR, Weiner MW, Massie B, Miller RG. Central basis of muscle fatigue in chronic fatigue syndrome. Neurology. 1993 Jan 1;43(1 Part 1):125- 131.
34. Sacco P, Hope PA, Thickbroom GW, Byrnes ML, Mastaglia FL. Corticomotor excitability and perception of effort during sustained exercise in the chronic fatigue syndrome. Clinical Neurophysiology. 1999 Nov 1;110(11):1883-91.
35. Schillings ML, Kalkman JS, Van der Werf SP, Van Engelen BG, Bleijenberg G, Zwarts MJ. Diminished central activation during maximal voluntary contraction in chronic fatigue syndrome. Clinical Neurophysiology. 2004 Nov 1;115(11):2518-24.
36. Davey NJ, Puri BK, Catley M, Main J, Nowicky AV, Zaman R. Deficit in motor performance correlates with changed corticospinal excitability in patients with chronic fatigue syndrome. International Journal of Clinical Practice. 2003 May;57(4):262-4.
37. Siemionow V, Fang Y, Calabrese L, Sahgal V, Yue GH. Altered central nervous system signal during motor performance in chronic fatigue syndrome. Clinical Neurophysiology. 2004 Oct 1;115(10):2372-81.
38. Jammes Y, Retornaz F. Understanding neuromuscular disorders in chronic fatigue syndrome. F1000Research. 2019;8.
39. Morris G, Maes M, Berk M, Puri BK. Myalgic encephalomyelitis or chronic fatigue syndrome: how could the illness develop?. Metabolic Brain Disease. 2019 Apr 15;34(2):385-415.
40. Bassi N, Amital D, Amital H, Doria A, Shoenfeld Y. Chronic fatigue syndrome: characteristics and possible causes for its pathogenesis. The Israel Medical Association Journal. 2008 Jan 1;10(1):79.
41. Du Preez S, Corbitt M, Cabanas H, Eaton N, Staines D, Marshall-Gradisnik S. A systematic review of enteric dysbiosis in chronic fatigue syndrome/myalgic encephalomyelitis. Systematic Reviews. 2018 Dec 1;7(1):241.
42. Vink M, Vink-Niese F. Work Rehabilitation and Medical Retirement for Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome Patients. A Review and Appraisal of Diagnostic Strategies. Diagnostics. 2019 Dec;9(4):124.
