Abstract
Type 2 diabetes mellitus (T2DM) is one of the most prevalent metabolic disorders. The interference of Bisphenol A (BPA) with different cellular communication routes involved in glucose homeostasis and the onset of insulin resistance is now documented. A bibliographic collection was made using the PubMed and Scopus database, focused on BPA exposure for humans, both in daily life and in occupational settings. A comparison between exposure levels of BPA in the general population and in specific workplaces showed possible higher BPA exposure in workers. Even with some controversies about the role of BPA in the onset of T2DM, the aim of this study is shedding light on the potential risk of diabetes in workers exposed to BPA and provide helpful information for occupational physicians in directing their health checks.
Keywords
Bisphenol A, BPA, Diabetes, Workers, Workplaces, Metabolic disease
Introduction
Type 2 diabetes mellitus (T2DM) is one of the most prevalent metabolic disorders, characterized by impaired metabolism of carbohydrates, lipids and proteins [1,2]. The prevalence of diabetes worldwide has grown from about 108 million adults (4.7% of population) in 1980 [3] to 463 million (9.3%) in 2019 [4] and it is estimated that it will rise to 700 million by 2045 [4]. Diabetes leads to hyperglycemia, which results from defective insulin secretion, insulin resistance or a combination of both [5]. The etiology of this type of diabetes involves different factors, with a clear genetic background but also linked to possible environmental chemical exposure [6,7].
Bisphenol A (BPA) is the basic monomer of polycarbonate plastics and epoxy resins. It is used for food preservation in coating materials of cans and jar caps [8,9]. Other products containing BPA are dental materials and thermal paper for cash registers.
BPA has estrogenic properties [10], may affect pancreatic β-cell function and/or insulin action [11,12]. Moreover, at the hepatic level BPA can lead to glucose production, glycogen synthesis reduction and damage insulin signals [13].
Routes for BPA exposure are: food consumption, like meat products (17-602ng/g), fish (5-109 ng/g), fruits and vegetables (9-76 ng/g), beverages (1-18ng/g), infant formula (0.1-13 ng/g); dust coming from laminate flooring, paints, electronic equipment (0.8-10μg/g), thermal paper (54.0-79.0 ng/cm2), dental material, mostly in the form of BPA-glycidyl methacrylate (0.013-30 mg) and plastic (0.2- 26 ppb) [14].
The possible occupational BPA exposure can occur in industrial settings (in the petrochemical synthesis of the products; in the production of polycarbonate plastics; in the production of epoxy-based products; in the production of plastic coatings) as well as in activities in which these products are used for specific purposes (e.g., the application of epoxy resins as surface insulation, or epoxy paints) [15].
Handling thermal papers is a major occupational source of BPA exposure. Studies on cashiers [16], who routinely handle this type of receipts, have shown higher urine BPA level in workers in comparison with the general population.
Due to the risk for human health linked with BPA exposure, European legislation has adopted some preventive measures: it banned the use of this chemical in baby bottles and many limitations are present for the use of BPA in plastic for food contact [17]. Indeed, BPA levels in thermal paper has been strongly reduced by law from January 1st 2020.
The aim of this study is shedding light on the potential risk of diabetes in workers exposed to BPA and provide helpful information for the occupational physicians in directing their health checks.
Materials and Methods
A bibliographic collection was made using the PubMed and Scopus databases, with these search items: “BPA and diabetes” “BPA and workers” “BPA and occupational exposure”; surveys prior to 2015 and articles not available as full text in English, Spanish or Italian were excluded from the study.
Reviews of scientific literature were excluded as well. Epidemiological surveys on adult men and women were taken into consideration for this update.
The article selection diagram is shown in Figure 1.
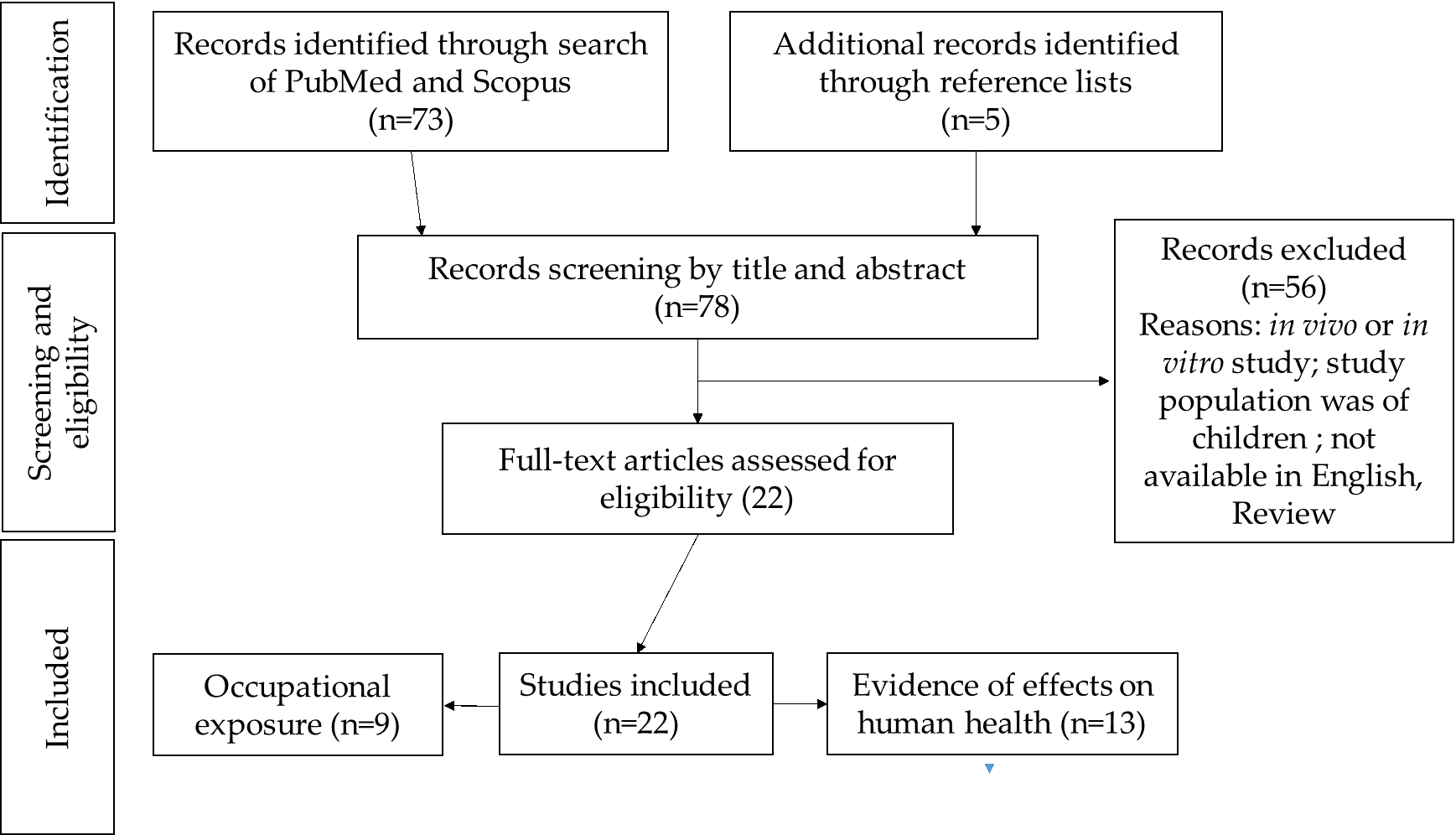
Figure 1. Selection diagram of the bibliographic research.
Results
Studies on the population
The interference of BPA with different cellular communication routes involved in the glucose homeostasis and the onset of insulin resistance has been presented in literature and this involved the BPA exposure with T2DM [18].
A brief illustration about published studies is provided in Table 1.
| Study type | No. of subjects | Geographic area of study | BPA mean levels | Results | Ref. |
| Case/ control | 54/47 | Saudi Arabia | 3.9 vs 1.3a | Urinary level of BPA is associated with increased risk of T2DM | 19 |
| Cross sectional | 400 (247 with diabetes/153 without diabetes) | Pakistan | 2.10 vs 1.86a | BPA exposure is a risk factor of T2DM | 20 |
| Cross sectional | 8498 | USA | 3.7a | No correlation between urinary concentration of BPA and diabetes | 21 |
| Longitudinal | 775/201 with diabetes after 9 years follow up | France | 1.75ax | Positive association between BPA exposure and the incidence of T2DM | 22 |
| Case control | 70/334 | Mexico | 6.60/4.80c | Association between urinary BPA level and diabetes (OR=1.85, 95% CI 1.04-3.38) | 23 |
| Longitudinal | 2336 with follow up for 4 years | China | 0.93ab | No significant association between urinary BPA and glucose metabolic markers in men. In women higher OR values were identified (OR=1.37, 95% CI 1.10-1.72 for hyperglycaemia and OR=1.30, 95% CI 1.02-1.65 for β-cell dysfunction) |
24 |
| Case control | 251/251 | China | 0.599 vs 0.726bc | No significant differences in BPA concentration between cases and controls | 25 |
| Cross sectional | 350 (only women) | USA | 1.3ab | No association between BPA and glucose levels | 26 |
| Cross sectional | 296 | Korea | 1.38c | Positive association between urinary BPA levels and insuline resistance. | 27 |
| Cross sectional | 240 | Thailand | 1.1d | No association between BPA and T2DM, but a positive association with impaired glucose tolerance | 28 |
| Cross sectional | 4320 | Canada | 1.21ab | Urinary BPA levels were positively associated with adverse glucose homeostasis in men | 29 |
| Cross sectional | 2581 | Thailandia | 0.34d | Serum BPA was positively associated with T2DM | 30 |
| Longitudinal | 3510/232 with diabetes after 5 years follow up | China | 1.3 vs 1.6e | No significant difference in serum BPA concentration between patients and controls | 31 |
| a µg/L, unadjusted urinary BPA; bmedian; cµg/g, urinary BPA adjusted for creatinine; dµg/L, serum BPA; e µmoL/L, serum BPA; xurinary BPA glucuronide, 50th percentile | |||||
The workplace: epidemiological and exposure data
While in the literature, in the last decade, numerous researches were published about BPA exposure and metabolic disease, mainly to characterize the possible risks for the general population; in workplaces the attention has been focused on the level of exposure more than to the possible target organ/system. Studies regarding adverse effects were mainly focused on the ability of BPA to act as an endocrine disruptor, particularly for male or female reproductive functions; while, at the moment, only one article has focused on metabolic disease [32].
The possibility of dermal exposure related to contact with thermal papers (e.g., for a cashier) is an aspect of interest that should require additional considerations, with respect to the possible exposure routes [33]. Indeed, the Risk Assessment Committee of the European Chemical Agency released a scientific opinion alerting that occupational BPA exposure for dermal contact via thermal paper might not be adequately controlled so the European Commission restricted the BPA level in this type of paper from January 2020 [34].
A description of what emerged in the workplace is provided in Table 2.
| Study type | Focus | No. of subjects | Population type | Geographic area of study | BPA mean levels |
Results | Ref. |
| Cross sectional | Exposure/ hormones |
592 | Male workers in industry | China | 685.9cd | Association between urinary BPA and prolactina, estradiol, elevated sex hormon binding globulin level. | 35 |
| Cross sectional | Exposure/ thyroid hormones | 90 | Workers of plastic industry | Egypt | 15.6ad | No correlation between BPA levels and thyroid hormones concentration | 36 |
| Cross sectional | Biological monitoring | 29 | Workers in waste incinerator | Spain | 0.58e/ 0.86a |
All workers had shown BPA exposure | 37 |
| Case/ control |
Exposure/ hormones |
106/250 | Female workers in epoxy resin production vs not exposed | China | 22.2 bc / 0.9bc |
Urinary BPA levels were positive associated to serum prolactin and progesterone concentration. |
38 |
| Case-control | Exposure/ hormones |
110/113 | Petrolchemical factory workers and not | Korea | 0.628e/ 0.457e |
Serum BADGE* produced hormonal alterations but no difference between cases and controls. | 39 |
| Case/ control |
Exposure/ hormones |
281/278 | Workers exposed to BPA vs not exposed | China | 18.75e/ 3.37e |
Increased serum BPA level was associated with decreased mean serum androstenedione level and increased serum SHBG level. | 40 |
| Case/ control |
Exposure/ hormones |
62/62 | PCOS women, working as market seller vs healthly women | Iran | 0.48e/ 0.16e |
In cases, BPA level was higher than controls, together with higher levels of triglyceride, cholesteriol, TSH and LH:FSH ratio. | 41 |
| Case/ control |
Biological monitoring | 90/44 | Cashiers exposed vs not exposed | France | 6.76c/ 2.89c |
Cashiers handling daily thermal paper receipts had significant higher urinary BPA concentration | 33 |
| Longitudinal | Exposure/ metabolic syndrome (MS) | 1227/200 with MS after a follow up of 4 years |
Male workers | China | 3.89bc | Exposure to BPA may increase the risk of MS, urinary BPA levels are positively associated to higher incidence, particularly for smokers |
32 |
| a µg/L; bgeometric mean; cµg/g, urinary BPA adjusted for creatinine; dmedian; eµg/L, serum BPA; ; eµg/L, serum; BPAdiglycidyl ether- BADGE (BPA precursor in vivo) | |||||||
Discussion
Looking at the overall recent data on the general population, some controversy persists about the association of BPA exposure and incidence of T2DM. Indeed four of the seven cross sectional studies suggest a possible positive association with a useful sample dimension (4,320, 2,581, 400 and 296 subjects) [20,27,29,30], while the other articles express doubts about this conclusion. However, different types of bias should be considered: in the National Health and Nutrition Examination Survey NHANES study, on 8,498 subjects [21], cases of diabetes were self- reported and the authors do not distinguish between type 1 and type 2 diabetes; Bellavia et al. [26] used a specific sample, only pregnant women, and this poses problems for an extrapolation of data regarding a different population; the survey carried out by Chailurkit et al. [27] on 240 subjects, analysed serum BPA using an ELISA method, a comparison of accuracy between ELISA and HPLC/ MS/MS methods shows very remarkable differences that should be taken into consideration. Furthermore cross-sectional study characteristics severely restrict the possibility to extrapolate elements of causality, this type of study only leads to formulate hypotesis.
Nevertheless, the case/control studies do not clarify the doubt. Two papers confirm the positive association , even if in one case [19] the sample dimension is too low (54 cases and 47 controls) for a robust result, the work of Murphy et al. [23] seems to be more consistent in this respect, but the third paper [25] with a useful number of subject does not confirm the starting hypotesis.
Finally, the longitudinal surveys, which by an epidemiological standpoint represent the golden study, confirm the correlation between BPA and T2DM, with a follow up of 9 years [22], while a shorter follow up (4/5 years) showed a partial confirmation, only for the female population [24] or no evidence [31].
Some methodological limitations make the conclusions of lesser impact; for example an influencing factor can be the choice to characterize the magnitude of exposure to BPA with urinary BPA concentration, on a spot urine sample, without proceeding with a standardization regarding the grams of urinary creatinine; this implies a very limited interpretation of the datum [42].
The studies carried out in the workplaces showed a detectable BPA exposure in every situation, sometimes at considerable higher level than the general population [35,36,38]. Attention was primarily given to the detectable concentration of BPA and then to the possible interaction between BPA and hormones. Evidences emerged about a positive correlation between BPA and sexual hormones, while data about thyroid hormones seems to be less clear.
The only published prospective study [32] about occupational exposure to BPA and metabolic problems confirms the positive correlation between this chemical and the metabolic syndrome (a cluster of cardio-metabolic risk factors like obesity and insulin resistance that pose a major threat for future diabetes and cardiovascular disease).
Conclusion
The onset of diabetes sees in the genetic predisposition and environmental factors clear elements of promotion, nevertheless other factors play a role [43], because the former may not fully clarify the increase in this type of pathology over the last century [44].
Exploratory studies on volunteers confirmed the association between BPA and glucose metabolism. 8 men and 3 women were orally administered a BPA dose (50 μg/Kg body weight, considered safe by US regulators) and an alteration of glucose-stimulated insulin response was found [45]. Moreover, a second study enrolled 11 subjects, in a double-blinded survey with placebo and orally administered BPA: evidence emerged of immediate effects on glucose, insulin and C-peptide concentration in the subgroup exposed to BPA [46]. A meta-analysis [47] carried out to clarify the role of BPA in T2DM, involved data from about 41,320 subjects: biological levels of BPA, both in urine and in serum, were collected and showed a positive correlation with T2DM risk (OR 1.28, 95% CI 1.14- 1.44).
Starting from these literature evidences, even if some uncertainties persist, it is appropriate to consider BPA as possibly involved in the interference of glucose metabolism and therefore in the onset of T2DM.
The present short review of recent studies shows, although with some controversies, an association between BPA and T2DM, even if some methodological problems should be considered. For example, the use of standardized measurement units (i.e., for the micrograms of urinary creatinine in the case of urine). Often the use of simple concentration as micrograms/liter does not permit a correct evaluation of exposure and a comparability of data.
Overall, the improvement of prospective studies to highlight the predictability of data and their statistical force is desirable [48].
A comparison among biological BPA levels in workers and in the general population has led to a concern: often in workplaces there is a higher exposure than that of the general population. Considering that even at low doses some evidence emerged of interference between BPA and glucose metabolism, the possibility of onset of diabetes in workers, with higher and documented exposure levels, could be greater. Even if the interest of researchers in workplaces was directed to a different target, data from literature, based on the general adult population, call for the definition of new epidemiological investigations to assess the role of BPA in diabetes, to further direct the activities of occupational doctors.
The role of occupational exposure to BPA in the etiology of diabetes is a possibility that the occupational doctor must consider in his or her working activity, in order to protect the workers’ health.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This research received no external funding.
Author Contributions
Conceptualization, methodology, writing—original draft preparation L. Caporossi; resources, B. Papaleo; data curation L. Caporossi; editing, supervision, M. De Rosa and B. Papaleo. All authors have read and agreed to the published version of the manuscript.
References
2. Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked?. Journal of Biomedical Science. 2016 Dec;23(1):1-8.
3. World Health Organization. Global Report on Diabetes.
4. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Research and Clinical Practice. 2019 Nov 1;157:107843.
5. Kahn SE. The importance of β -cell failure in the development and progression of type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism. 2001 Sep 1;86(9):4047-58.
6. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology. 2018 Feb;14(2):88.
7. Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012 Sep 1;153(9):4097-110.
8. Oldring PK, Castle L, O’mahony C, Dixon J. Estimates of dietary exposure to bisphenol A (BPA) from light metal packaging using food consumption and packaging usage data: a refined deterministic approach and a fully probabilistic (FACET) approach. Food Additives & Contaminants: Part A. 2014 Mar 4;31(3):466-89.
9. Cao XL, Corriveau J, Popovic S. Bisphenol A in canned food products from Canadian markets. Journal of Food Protection. 2010 Jun;73(6):1085-9.
10. Dodds EC, Lawson W. Synthetic strogenic agents without the phenanthrene nucleus. Nature. 1936 Jun;137(3476):996-.
11. Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nature Reviews Endocrinology. 2011 Jun;7(6):346-53.
12. Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reproductive Toxicology. 2017 Mar 1;68:3-3.
13. Menale C, Mita DG, Diano N, Diano S. Adverse effects of bisphenol A exposure on glucose metabolism regulation. The Open Biotechnology Journal. 2016 Mar 31;10(1).
14. Akash MS, Sabir S, Rehman K. Bisphenol A-induced metabolic disorders: From exposure to mechanism of action. Environmental Toxicology and Pharmacology. 2020 Jul 1;77:103373.
15. Candura F, Candura SM, Colli G. Elementi di tecnologia industriale a uso dei cultori di medicina del lavoro. La tribuna; 2002; pp.11.3-11.99.
16. Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environmental Health Perspectives. 2011 Jan;119(1):131- 7.
17. Almeida S, Raposo A, Almeida-González M, Carrascosa C. Bisphenol A: Food exposure and impact on human health. Comprehensive Reviews in Food Science and Food Safety. 2018 Nov;17(6):1503-17.
18. Rancière F, Lyons JG, Loh VH, Botton J, Galloway T, Wang T, et al. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environmental Health. 2015 Dec 1;14(1):46.
19. Li AJ, Xue J, Lin S, Al-Malki AL, Al-Ghamdi MA, Kumosani TA, et al. Urinary concentrations of environmental phenols and their association with type 2 diabetes in a population in Jeddah, Saudi Arabia. Environmental Research. 2018 Oct 1;166:544-52.
20. Haq ME, Akash MS, Sabir S, Mahmood MH, Rehman K. Human exposure to bisphenol A through dietary sources and development of diabetes mellitus: a cross-sectional study in Pakistani population. Environmental Science and Pollution Research International. 2020 May 2.
21. Ward JB, Casagrande SS, Cowie CC. Urinary phenols and parabens and diabetes among US adults, NHANES 2005-2014. Nutrition, Metabolism and Cardiovascular Diseases. 2020 Jan 25.
22. Rancière F, Botton J, Slama R, Lacroix MZ, Debrauwer L, Charles MA, et al. Exposure to Bisphenol A and Bisphenol S and Incident Type 2 Diabetes: A Case– Cohort Study in the French Cohort DESIR. Environmental Health Perspectives. 2019 Oct 30;127(10):107013.
23. Murphy L, Mérida-Ortega Á, Cebrián ME, Hernández- Garciadiego L, Gómez-Ruiz H, Gamboa-Loira B, et al. Exposure to Bisphenol A and diabetes risk in Mexican women. Environmental Science and Pollution Research. 2019 Sep 1;26(25):26332-8.
24. Wang B, Li M, Zhao Z, Lu J, Chen Y, Xu Y, et al. Urinary bisphenol A concentration and glucose homeostasis in non-diabetic adults: a repeated-measures, longitudinal study. Diabetologia. 2019 Sep 1;62(9):1591-600.
25. Duan Y, Yao Y, Wang B, Han L, Wang L, Sun H, et al. Association of urinary concentrations of bisphenols with type 2 diabetes mellitus: a case-control study. Environmental Pollution. 2018 Dec 1;243:1719-26.
26. Bellavia A, Cantonwine DE, Meeker JD, Hauser R, Seely EW, McElrath TF, et al. Pregnancy urinary bisphenol-A concentrations and glucose levels across BMI categories. Environment International. 2018 Apr 1;113:35- 41.
27. Hong SH, Sung YA, Hong YS, Ha E, Jeong K, Chung H, et al. Urinary bisphenol A is associated with insulin resistance and obesity in reproductive-aged women. Clinical Endocrinology. 2017 Apr;86(4):506-12.
28. Chailurkit LO, Tengpraettanakorn P, Chanprasertyotin S, Ongphiphadhanakul B. Is bisphenol A exposure associated with the development of glucose intolerance and increased insulin resistance in Thais?. Nutrition and Health. 2017 Sep;23(3):185-91.
29. Tai X, Chen Y. Urinary bisphenol A concentrations positively associated with glycated hemoglobin and other indicators of diabetes in Canadian men. Environmental research. 2016 May 1;147:172-8.
30. Aekplakorn W, Chailurkit LO, Ongphiphadhanakul B. Relationship of serum bisphenol A with diabetes in the Thai population, National Health Examination Survey IV, 2009 No. 4 The Second National Health Survey. Journal of Diabetes. 2015 Mar;7(2):240-9.
31. Shu X, Tang S, Peng C, Gao R, Yang S, Luo T, et al. Bisphenol A is not associated with a 5-year incidence of type 2 diabetes: a prospective nested case–control study. Acta Diabetologica. 2018 Apr 1;55(4):369-75.
32. Wu S, Wang F, Lu S, Chen Y, Li W, Li Z, et al. Urinary bisphenol A and incidence of metabolic syndrome among Chinese men: a prospective cohort study from 2013 to 2017. Occupational and Environmental Medicine. 2019 Oct 1;76(10):758-64.
33. Ndaw S, Remy A, Jargot D, Robert A. Occupational exposure of cashiers to Bisphenol A via thermal paper: urinary biomonitoring study. International Archives of Occupational and Environmental Health. 2016 Aug 1;89(6):935-46.
34. Sogorb MA, Estévez J, Vilanova E. Case study: Is bisphenol S safer than bisphenol A in thermal papers?. Archives of Toxicology. 2019 Jul 1;93(7):1835-52.
35. Liu X, Miao M, Zhou Z, Gao E, Chen J, Wang J, et al. Exposure to bisphenol-A and reproductive hormones among male adults. Environmental Toxicology and Pharmacology. 2015 Mar 1;39(2):934-41.
36. Metwally FM, Hasheesh A, Zaid MM, El Sharkawy SA, Mohamed FA, Fattah A, et al. Bisphenol A levels among workers in plastic processing industry and their relations to thyroid hormones. Journal of Applied Pharmaceutical Science. 2019 Nov;9(11):107-11.
37. González N, Cunha SC, Monteiro C, Fernandes JO, Marquès M, Domingo JL et al. Quantification of eight bisphenol analogues in blood and urine samples of workers in a hazardous waste incinerator. Environmental Research. 2019 Sep 1;176:108576.
38. Miao M, Yuan W, Yang F, Liang H, Zhou Z, Li R, et al. Associations between bisphenol A exposure and reproductive hormones among female workers. International Journal of Environmental Research and Public Health. 2015 Oct;12(10):13240-50.
39. Kim SI, Yang YJ, Hong YP, Myung SC, Kim SC. Distribution of serum bisphenol A diglycidyl ether and its metabolite in Korean adult men and its association with reproductive hormone levels. Molecular & Cellular Toxicology. 2015 Mar 1;11(1):71-8.
40. Zhuang W, Wu K, Wang Y, Zhu H, Deng Z, Peng L, et al. Association of serum bisphenol-A concentration and male reproductive function among exposed workers. Archives of Environmental Contamination and Toxicology. 2015 Jan 1;68(1):38-45.
41. Vahedi M, Saeedi A, Poorbaghi SL, Sepehrimanesh M, Fattahi M. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environmental Science and Pollution Research. 2016 Dec 1;23(23):23546-50.
42. Rochester JR. Bisphenol A and human health: a review of the literature. Reproductive Toxicology. 2013 Dec 1;42:132-55.
43. Newbold RR. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones. 2010 Jul 1;9(3):206-17.
44. De Coster S, Van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. Journal of Environmental and Public Health. 2012 Oct;2012.
45. Stahlhut RW, Myers JP, Taylor JA, Nadal A, Dyer JA, vom Saal FS. Experimental BPA exposure and glucosestimulated insulin response in adult men and women. Journal of the Endocrine Society. 2018 Oct;2(10):1173-87.
46. Hagobian T, Bird A, Stanelle S, Williams D, Schaffner A, Phelan S. Effects of varying doses of oral bisphenol A consumption on type 2 diabetes risk markers in healthy adults. Journal of the Endocrine Society. 2019;3(3):643.
47. Hwang S, Lim JE, Choi Y, Jee SH. Bisphenol A exposure and type 2 diabetes mellitus risk: a meta-analysis. BMC Endocrine Disorders. 2018 Dec 1;18(1):81.
48. Lind PM, Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018 Jul 1;61(7):1495-502.
