Abstract
Background: Both classical severe low-flow low-gradient aortic stenosis (LFLG-AS) and severe normal-flow low-gradient aortic stenosis (NFLG-AS) patients undergo transcatheter aortic valve implantation (TAVI). However, few studies have compared outcomes between the two groups. Our study aimed to assess short term post-TAVI outcomes between classical LFLG-AS and NFLG-AS.
Methods: We conducted a retrospective, single-center, analysis of 206 patients who underwent TAVI between January 2011 to September 2020. Of these, 44 (males: 33) had classical LFLG-AS and 162 (males: 89) had NFLG-AS. Six-month primary outcomes included all-cause and cardiovascular mortality. Multiple secondary outcomes were assessed including stroke, myocardial infarction (MI), new-onset atrial fibrillation, acute kidney injury (AKI), major bleeding, vascular complications, and repeat hospitalizations from cardiac causes.
Results: The cumulative six-month all-cause and cardiovascular mortality were similar between classical severe LFLG-AS and NFLG-AS patients (6.81 % vs. 4.32 %, P = 0.49 and 2.27 % vs. 2.47 %, P = 0.93 respectively). All secondary outcomes were also similar, except for a statistically significant higher rate of AKI in patients with classical LFLG-AS (P = < 0.0001).
Conclusion: Except for a higher incidence of AKI in patients with severe classical LFLG-AS, six-month post-TAVI outcomes did not differ between classical LFLG-AS and NFLG-AS.
Keywords
TAVI, Aortic stenosis, Low flow low gradient severe aortic stenosis, Normal flow low gradient severe aortic stenosis
Abbreviations
NFLG-AS: Normal-Flow Low-Gradient Aortic Stenosis; LFLG-AS: Low-Flow Low-Gradient Aortic Stenosis; TAVI: Transcatheter Aortic Valve Implantation; SAVR: Surgical Aortic Valve Replacement; AS: Aortic Stenosis; MI: Myocardial Infarction; AKI: Acute Kidney Injury; SVI: Stroke Volume Index; LV: left ventricular; LVOT: Left ventricular outflow tract; CT-AVC: Computed Tomography Aortic Valve Calcium Scoring
Highlights
- Controversy exists over the appropriate management of patients with severe normal-flow low-gradient aortic stenosis.
- Post-transcatheter aortic valve implantation outcome data is limited in patients with severe normal-flow low-gradient aortic stenosis.
- Patients with severe normal-flow low-gradient aortic stenosis and classical severe low-flow low-gradient aortic stenosis have similar all-cause and cardiovascular mortality at 6 months following transcatheter aortic valve implantation.
- Patients with classical severe low-flow low-gradient aortic stenosis have a higher incidence of AKI at 6 months following transcatheter aortic valve implantation compared to patient with normal-flow low-gradient aortic stenosis.
- Patients with severe normal-flow low-gradient aortic stenosis and classical severe low-flow low-gradient aortic stenosis equally benefit from transcatheter aortic valve implantation.
Introduction
Severe normal flow-high gradient aortic stenosis (NFHG-AS) is defined as an aortic valve area (AVA) < 1.0 cm2, a mean gradient > 40 mmHg, and a peak velocity > 4 m/sec [1]. The diagnosis and management of severe NFHG-AS is well defined in the literature [1]. However, many patients with symptomatic aortic stenosis (AS) have been found to have an AVA < 1.0 cm2 with discordance in mean gradient or stroke volume index (SVI). This discordance has led to the classification of variant forms of AS.
The management of AS has become more challenging with the identification and widespread acceptance of the variant forms of severe AS. The most common variants of severe AS are low flow and/or low gradient. Recently, focus has been placed on the diagnosis and management of patients with low gradient severe AS [2-4]. Patients with low gradient severe AS are separated into classical low flow, paradoxical low flow, and normal flow [2]. Classical severe low flow-low gradient aortic stenosis (LFLG-AS) is found in the setting of reduced left ventricular (LV) dysfunction or a low LV outflow state with an SVI < 35 ml/m2, whereas paradoxical LFLG-AS is associated with preserved LV function with an SVI < 35 ml/m2 [5]. In clinical practice, the management of classical LFLG-AS is more widely agreed upon amongst experts when compared to normal flow-low gradient aortic stenosis (NFLG-AS) [5].
Severe NFLG-AS is defined as an AVA < 1.0 cm2, a mean gradient < 40 mmHg, and an SVI ≥ 35 ml/m2, in the setting of preserved LV function (ejection fraction ≥ 50%) [2]. According to recent studies, NFLG-AS is the most prevalent variant of low-gradient severe AS [3,4]. However, there is controversy over the appropriate severity classification and management of patients with NFLG-AS [3,4,6]. As a result, indications for surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI) in patients with symptomatic NFLG-AS are inconsistent [5,7]. Prior studies demonstrate that SAVR in patients with severe NFLG-AS have improved survival when compared with medical management [8-10]. While TAVI has been widely accepted in the management of AS, including patients with low gradient AS, few studies have evaluated the post-TAVI short-term outcomes of patients with severe NFLG-AS [11-14]. In a recent study, patients with severe NFLG-AS and patients with severe NFHG-AS were found to have similar all-cause and cardiovascular mortality at six months following TAVI [15]. Our study aimed to assess short-term (6 month) post-TAVI outcomes in patients with severe NFLG-AS compared to patients with severe LFLG-AS.
Methods
Study design
A retrospective analysis was performed on patients that had undergone TAVI at our large academic medical center between January 2011 to August 2020. The study was approved by the institutional review board (IRB Registration 00006910). Informed consent was waived by IRB, given the retrospective nature of the study. The study was conducted in compliance with the ethical standards of the institution and the revised Helsinki Declaration.
Patient and public involvement: Patients and the public were not (or will not) be involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient population
All patients with classical LFLG-AS and NFLG-AS who underwent TAVI between January 2011 to August 2020 were included in the study. NFLG-AS was defined as a left ventricular ejection fraction (LVEF) ≥ 50 %, AVA < 1 cm2, mean gradient across aortic valve < 40 mmHg, peak velocity (V max) < 4 m/s, and SVI ≥ 35 ml/m2. LFLG-AS was defined as a as LVEF < 50 %, AVA < 1 cm2, mean gradient across aortic valve < 40 mmHg, peak velocity < 4 m/s and SVI < 35 ml/m2. Patients with paradoxical LFLG-AS, defined as LVEF ≥ 50 %, AVA < 1 cm2, mean gradient across aortic valve < 40 mmHg, peak velocity (V max) < 4 m/s, and SVI < 35 ml/m2, were excluded from the study. Patients that required transfer to an outside facility for TAVI were also excluded from this study.
Patient selection for TAVI
Patients were selected for TAVI through the utilization of a multidisciplinary TAVI heart team. Our institutional heart team consists of a cardiothoracic surgeon, interventional/structural cardiologist, general cardiologist, valve coordinator, catheterization lab/operating room staff and echocardiographers. Patients included in this study had symptomatic AS prior to TAVI, with the majority having NYHA Class III-IV heart failure symptoms. All patients in the study underwent echocardiogram, which were independently interpreted by two cardiologists in accordance with the American Society of Echocardiography (ASE) clinical recommendations. Echocardiograms were obtained to assess aortic valve mean pressure gradient, peak velocity (V max), and AVA. Additional echocardiographic measurements were calculated including LVEF and left ventricular SVI. Through these measurements patients were categorized into their appropriate severe AS variant. For this study, only patients with NFLG-AS and classical LFLG-AS were selected. With the utilization of continuous-wave Doppler ultrasound, the peak velocity across aortic valve was measured in multiple acoustic windows. The simplified Bernoulli equation was used to measure mean pressure gradient across the aortic valve. AVA was calculated using the continuity equation. Left ventricular outflow tract (LVOT) diameter was measured from the inner edge to inner edge, parallel and adjacent to the aortic valve in the parasternal long axis view with zoom and adjusted gain. LVOT velocity was assessed using pulse-wave Doppler in the apical long axis view with sample volume positioned 1 cm on the left ventricle side of the aortic valve.
Patients with classical LFLG-AS and some patients with NFLG-AS underwent low dose dobutamine stress echocardiogram to evaluate for true versus pseudo severe AS. A peak stress mean gradient ≥ 40 mmHg or the composite of peak stress mean gradient ≥ 40 mmHg and peak stress AVA ≤ 1.0 cm2 during dobutamine testing were consistent with true severe AS. Low dose dobutamine stress echocardiograms were performed in compliance with European Association of cardiovascular imaging in the American Society of echocardiography updated recommendations. In patients with poor-quality echocardiographic images that precluded an accurate Doppler assessment, a left heart catheterization was performed. Left ventricular and aortic pressure were simultaneously measured to determine a peak and mean transaortic pressure. The AVA was calculated using the Gorlin formula. Of note, some classical LFLG and NFLG patients underwent dobutamine challenge during cardiac catheterization to assess the true severity of AS.
Computed tomography aortic valve calcium scoring (CT-AVC) was also performed in patients with classical LFLG-AS as well as NFLG-AS to determine the true severity of AS. True severe AS was considered when the aortic valve calcium score was > 1200 AU in females and > 2000 AU in males. However, not all patients underwent CT-AVC as it was not available during the initial years of TAVI at our institution. Therefore, for the purposes of this study, CT-AVC was not used to differentiate true vs pseudo severe AS. Rather, the diagnosis was made using accurate echocardiographic measurements and the use of dobutamine stress echocardiogram or dobutamine challenge during cardiac catheterization.
Procedural details for TAVI
Patients eligible for aortic valve intervention underwent a detailed risk assessment for consideration of aortic valve replacement. Based on multiple factors including fragility, age, comorbidities, major organ dysfunction, patient preference, and STS score the choice of valve intervention (SAVR or TAVI) was selected. TAVI eligible patients underwent a detailed evaluation by our institution’s multidisciplinary heart team for consideration. Patients included in this study underwent TAVI with a balloon expandable Edwards SAPIEN valve system (SAPIEN XT or SAPIEN 3) or a self-expanding Medtronic CoreValve. Following TAVI, patients were placed on dual antiplatelet therapy with aspirin 81 mg and Plavix 75 mg for total of 6 months if no bleeding complications occurred. Following 6 months of dual antiplatelet therapy, patients were continued on aspirin monotherapy. Patients on oral anticoagulants such as warfarin, rivaroxaban or apixaban, were continued on either their anticoagulant alone or anticoagulant plus aspirin combination following TAVI.
Study outcome measures
Clinical outcomes were assessed at six months following TAVI. Primary outcomes included all-cause mortality and cardiovascular mortality. Secondary outcomes included myocardial infarction, new onset atrial fibrillation, stroke, acute kidney injury, vascular complications, and major bleeding complications (Bleeding Academic Research Consortium (BARC) Type 3 or Type 5 bleeding). Additionally, repeat cardiac disease-related hospitalizations were also included as a secondary outcome in the study.
Statistical analysis
The Shapiro-Wilk normality test was used to evaluate the normal distribution of variables. Continuous variables were expressed as means ± standard deviation and the difference between the two groups was tested with an unpaired t-test. Categorical variables were expressed as frequencies and percentages, and the difference between the two groups was tested with a Chi-square test or Fisher's exact test. The Kaplan-Meier method was used to estimate survival probability and the difference between the two groups was assessed by two-sided log-rank test. A 2 tailed p-value of <0.05 was considered statistically significant for this study. Statistical analysis was performed using GraphPad Prism, Version 9.
Results
A total of 206 patients who underwent TAVI for severe AS were included in the study. Of those, 44 patients had LFLG-AS, and 162 patients had NFLG-AS.
Baseline characteristics
In our study population, the balloon-expandable Edwards Sapien valve was mostly commonly used. The mean age of the classical LFLG-AS population was 76.84 ± 9.6, and 33 (75%) were males. The mean age of the NFLG-AS population was 77.98 ± 8.87, and 89 (54.94%) were males. Compared to NFLG-AS, patients with classical LFLG-AS had a higher percentage of peripheral vascular disease (52.27 % in LFLG-AS vs. 24.69 % in NFLG-AS, P = 0.004), coronary artery disease (88.63 % in LFLG-AS vs. 66.66 % in NFLG-AS, P = 0.004), previous MI (50 % in LFLG-AS vs. 19.12 % in NFLG-AS, P ≤ 0.0001), and pervious coronary artery bypass grafting (47.72 % in LFLG-AS vs. 22.84 % in NFLG-AS, P = 0.001). Most patients in both groups had NYHA class III-IV symptoms (93.18% in LFLG-AS vs. 96.91% in NFLG-AS). There was a statistically significant difference in echocardiographic ejection fraction (p < 0.0001) or stroke volume index (p < 0.0001) between the two groups. There was no statistically significant difference in AVA, peak velocity (V max), or mean gradient between the two groups. Baseline characteristics including age, sex, clinical history, and echocardiographic parameters are presented in Table 1.
|
|
LFLG-AS (n = 44) |
NFLG-AS (n = 162) |
P value |
|
Age (Yrs) |
76.84 ± 9.6 |
77.98 ± 8.87 |
ns |
|
Male |
33 (75%) |
89 (54.94%) |
0.016 |
|
Clinical history |
|||
|
Diabetes Mellitus |
26 (59.1%) |
78 (48.15%) |
ns |
|
Hypertension |
41 (93.18%) |
143 (88.27) |
ns |
|
Peripheral vascular disease |
23 (52.27%) |
40 (24.69%) |
0.004 |
|
Stroke/TIA |
6(13.63%) |
28 (17.28%) |
ns |
|
COPD |
12 (27.27%) |
44 (27.16%) |
ns |
|
Atrial fibrillation |
18 (40.90%) |
65 (40.12%) |
ns |
|
Previous permanent pacemaker |
9 (20.45%) |
27 (16.67%) |
ns |
|
Chronic kidney disease |
26(59.09%) |
79 (48.76%) |
ns |
|
Coronary artery disease |
39(88.63%) |
108 (66.66%) |
0.004 |
|
Previous myocardial infarction |
22 (50%) |
31 (19.13%) |
<0.0001 |
|
Previous CABG |
21 (47.72%) |
37 (22.84%) |
0.001 |
|
BMI (kg/m2) |
28.66 ± 6.2 |
28.99 ± 6.38 |
ns |
|
NYHA class III-IV symptoms |
41 (93.18%) |
157 (96.91%) |
ns |
|
GFR (ml/min) |
57.47 ± 21.69 |
58.35 ± 22.59 |
ns |
|
Echocardiographic parameters |
|||
|
Left ventricular ejection fraction (%) |
29.70 ± 6.36 |
56.61 ± 8.16 |
<0.0001 |
|
Aortic valve area (cm2) |
0.76 ± 0.19 |
0.79 ± 0.15 |
ns |
|
Peak velocity (Vmax) (cm/s) |
3.67 ± 0.59 |
3.62 ± 0.58 |
ns |
|
Mean gradient (MG) (mm Hg) |
31.72 ± 13.13 |
31.12 ± 7.36 |
ns |
|
Stroke volume index (ml/m2) |
25.68 ± 5.01 |
40.17 ± 5.07 |
<0.0001 |
|
Abbreviations: LFLG-AS: Low Flow Low Gradient Severe Aortic Stenosis; NFLG-AS: Normal Flow Low Gradient Severe Aortic Stenosis; TIA: Transient Ischemic Attack; COPD: Chronic Obstructive Pulmonary Disease; CABG: Coronary Artery Bypass Graft; BMI: Body Mass Index; NYHA: New York Heart Association; GFR: Glomerular Filtration Rate; TAVI: Transcatheter Aortic Valve Implantation; ns: non-significant. Data is expressed as mean ± standard deviation or proportion (percentages). P < 0.05 indicates the difference between the two groups is statistically significant. |
|||
Clinical outcomes
There was no statistically significant difference in all-cause mortality or cardiovascular mortality between the two groups. Cumulative six-month incidence of all-cause mortality was 6.81 % for patients with classical LFLG-AS and 4.32 % for patients with NFLG-AS (P = 0.49). Cumulative six-month incidence of cardiovascular mortality was 2.27 % for patients with classical LFLG-AS and 2.47 % for patients with NFLG-AS (P = 0.93). There was no difference in all-cause mortality and cardiovascular mortality between the two groups using Kaplan-Meier survival analysis (Figure 1 and Figure 2). In review of secondary outcomes, there was no statistically significant difference between the two groups for stroke (4.54 % in LFLG-AS vs 6.17 % in NFLG-AS, P = 0.81), MI (0 % in LFLG-AS vs 0.62 % in NFLG-AS, P = 0.60), new onset atrial fibrillation (13.63 % in LFLG-AS vs 6.17 % in NFLG-AS, P = 0.11), vascular complications (9.09 % in LFLG-AS vs 9.88 % in NFLG-AS, P = 0.72 major bleeding (6.81 % in LFLG-AS vs 9.26 % in NFLG-AS, P = 0.57), or repeat hospitalizations for cardiac causes (13.63 % in LFLG-AS vs 19.14 % in NFLG-AS, P = 0.07). However, in comparison to NFLG-AS, patients with classical LFLG-AS had a higher incidence of acute kidney injury at six months following TAVI (36.36 % in LFLG-AS vs 9.88 % in NFLG-AS, P < 0.0001). Primary and secondary six-month outcomes are summarized in Table 2.
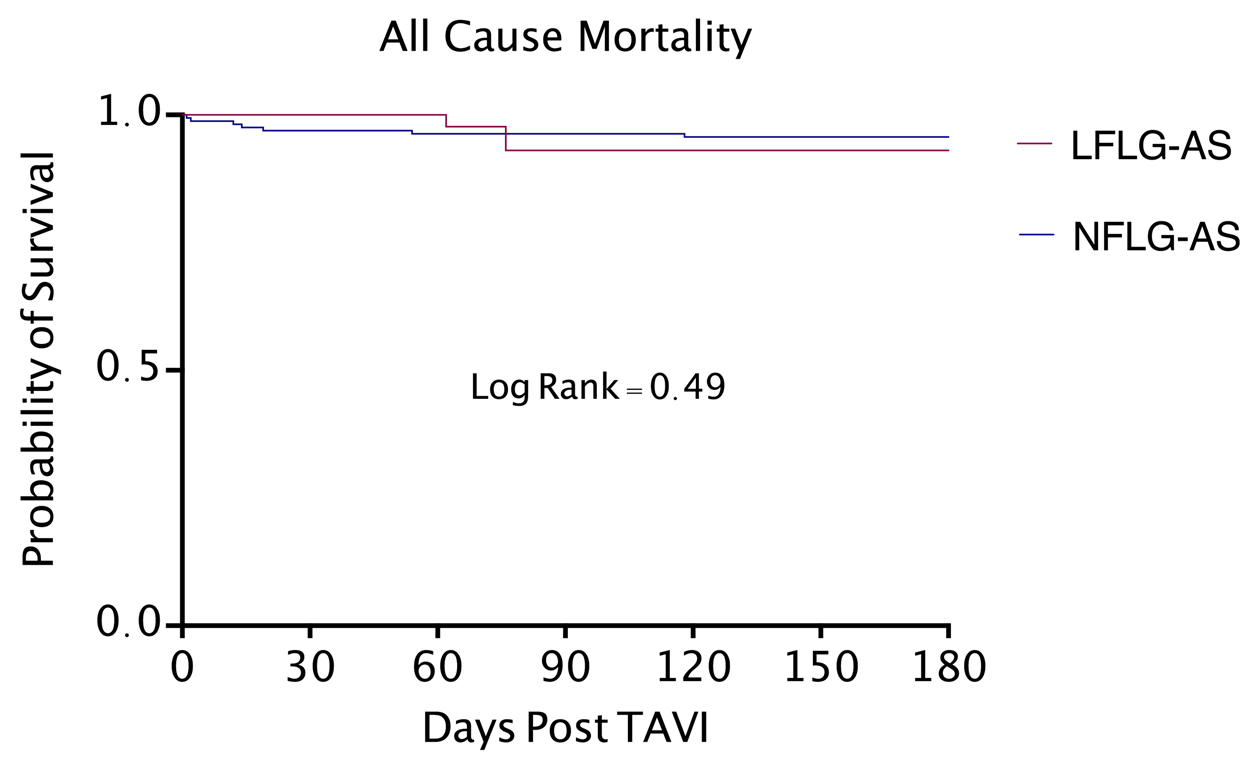
Figure 1. Kaplan-Meier curve for all-cause mortality. LFLG-AS: Low Flow Low Gradient Severe Aortic Stenosis; NFLG-AS: Normal Flow Low Gradient Severe Aortic Stenosis; TAVI: Transcatheter Aortic Valve Implantation.
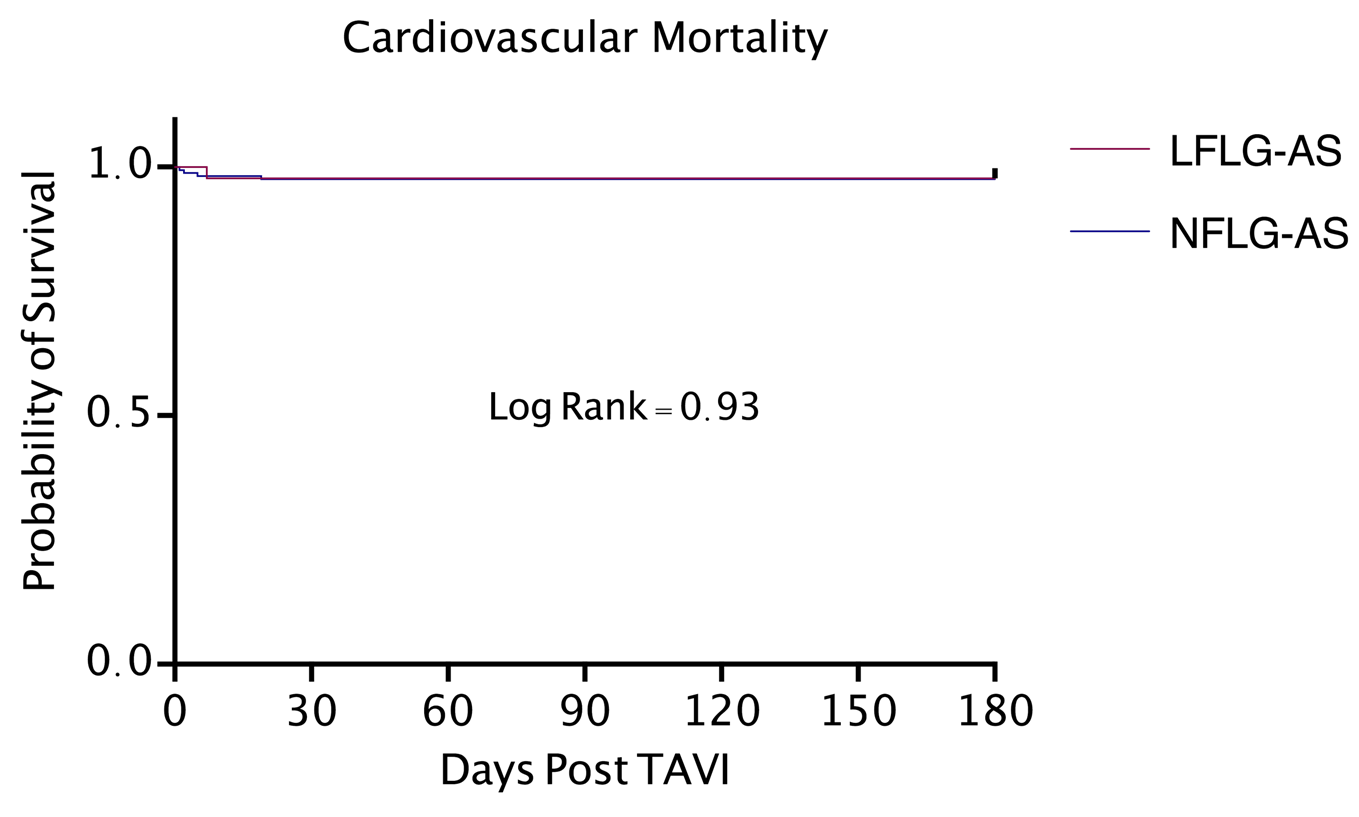
Figure 2. Kaplan-Meier curve for cardiovascular mortality. LFLG-AS: Low Flow Low Gradient Severe Aortic Stenosis; NFLG-AS: Normal Flow Low Gradient Severe Aortic Stenosis; TAVI: Transcatheter Aortic Valve Implantation.
|
Clinical Outcomes |
LFLG-AS (n = 44) |
NFLG-AS (n = 162) |
P Value |
|
Primary outcomes |
|||
|
All-cause mortality |
3 (6.81%) |
7 (4.32%) |
0.49 |
|
Cardiovascular mortality |
1 (2.27%) |
4 (2.47%) |
0.93 |
|
Secondary outcomes |
|||
|
Stroke |
2 (4.54%) |
10 (6.17%) |
0.81 |
|
Myocardial infarction |
0 (0%) |
1 (0.62%) |
0.60 |
|
New onset atrial fibrillation |
6 (13.63%) |
10 (6.17%) |
0.11 |
|
Major bleeding |
3 (6.81%) |
15 (9.26%) |
0.57 |
|
Vascular complication |
4 (9.09%) |
16 (9.88%) |
0.72 |
|
Acute kidney injury |
16 (36.36%) |
16 (9.88%) |
<0.0001 |
|
Repeat hospitalizations |
6 (13.63%) |
31 (19.14%) |
0.07 |
|
Abbreviations: TAVI: Transcatheter Aortic Valve Implantation; LFLG-AS: Low Flow Low Gradient Severe Aortic Stenosis; NFLG-AS: Normal Flow Low Gradient Severe Aortic Stenosis. Data is expressed as mean ± standard deviation or proportion (percentages). P < 0.05 indicates the difference between the two groups is statistically significant. |
|||
Discussion
The goal of this study was to compare six-month outcomes of patients with severe NFLG-AS and severe classical LFLG-AS who underwent TAVI. In our cohort of 206 patients that underwent TAVI, we found that all-cause mortality and cardiovascular mortality between the two groups were similar. Furthermore, we concluded that patients with severe classical LFLG-AS have a higher rate of AKI compared to patients with NFLG-AS. However, all other secondary outcomes were found to be similar between the two groups.
In comparison to NFHG-AS and classical LFLG-AS, there are guideline inconsistencies and debate amongst clinicians regarding the treatment of severe NFLG-AS [3-6]. The pathophysiology of severe NFLG-AS is not fully understood, which has contributed to the uncertainty regarding its management. Prior studies that have compared severe NFLG-AS to the hemodynamics and outcomes of patients with moderate AS [4,6]. In contrast, some experts have classified severe NFLG-AS as a transitional stage between moderate and severe AS with a high propensity to rapidly progress to severe LFLG-AS [3]. In addition, several potential risk factors of severe NFLG-AS have been identified including increased systolic pressure, reduced arterial compliance, low transvalvular flow rates, and prolonged LV ejection time [2,16]. Despite its prevalence and the known causative factors, there is limited data regarding the diagnosis, management strategy, and clinical outcomes of patients with severe NFLG-AS.
Recent emphasis has been placed on the appropriate diagnosis and management of patients with low gradient severe AS, including classical and paradoxical LFLG-AS as well as NFLG-AS [17-19]. Prior studies have demonstrated improved survival and better outcomes with AVR in patients with low flow severe AS including those with NFLG-AS [5,8,9,20]. However, referral for surgical management has remained inconsistent in patients with NFLG-AS [9]. When compared to normal flow AS, patients with low flow AS are found to have higher cardiac mortality and lower survival [21]. Poor outcomes in patients with classical LFLG-AS are associated with impaired functional capacity, increased severe valve stenosis, and a reduced peak stress left ventricular ejection fraction [17]. In our study, patients with LFLG-AS were found to be more complex given their higher prevalence of comorbidities (peripheral vascular disease, coronary artery disease, previous MI, pervious coronary artery bypass grafting) at baseline.
More recently, experts have evaluated the use of TAVI in patients with low gradient AS including those with severe NFLG-AS. Studies have shown that patients with low gradient severe AS with preserved LV function have comparable post-TAVI hemodynamic changes and clinical outcomes to patients with severe NFHG-AS post-TAVI [14,22]. The use of TAVI in patients with severe NFLG-AS has been previously presented in the literature, however few large studies have assessed the short-term outcomes in patients with severe NFLG-AS following TAVI. In a recent study, we concluded that patients with severe NFLG-AS have comparable six months post-TAVI outcomes to patients with classic severe NFHG-AS [15].
Our current study sought to compare short term (six-month) outcomes between patients with classical LFLG-AS and patients with NFLG-AS undergoing TAVI. We concluded that these two groups have comparable all-cause mortality and cardiovascular mortality. While patients with LFLG-AS had a greater incidence of acute kidney injury at six months following TAVI, all additional secondary outcomes were comparable between the two groups. Other studies have also reported higher incidences of acute kidney injury in patients with LFLG-AS [23-25]. While patients with LFLG-AS are pre-disposed to kidney injury given their baseline low flow state, overly cautious fluid recusation post-TAVI may further contribute to renal dysfunction in this patient population [25]. Notably, NFLG-AS and classical LFLG-AS were also found to have no difference in cardiac disease-related hospitalizations six months following TAVR. We conclude that six-month post-TAVI outcomes do not differ between patients with severe classical LFLG-AS and severe NFLG-AS. However, we stress the importance of accurate diagnosis and differentiation of NFLG-AS and classical LGLF-AS with precise echocardiographic assessment of valve area and hemodynamics as well as the appropriate utilization of dobutamine stress echocardiography and CT-AVC.
Limitations
Limitations to this study including a small sample size and its retrospective nature. Furthermore, this is a single center study that evaluated only short term (six month) outcomes. The nonrandomization of the study could have resulted in outcome assessment bias. Although efforts were made to obtain accurate echocardiographic measurements, small variations in LVOT diameter and underestimation in LVOT area exist given its natural elliptical shape. While studies have demonstrated the utility of CT-AVC to evaluate the severity of AS, not all patients in this study had a CT performed to differentiate true vs pseudo severe AS. To appropriately differentiate pseudo-severe LFLG-AS and true severe LFLG-AS the results of dobutamine stress echocardiogram or dobutamine challenge during cardiac catheterization were used. Lastly, outcome comparison was not performed between patients receiving an Edwards SAPIEN valve systems (SAPIEN XT or SAPIEN 3) or Medtronic CoreValves.
Conclusion
Except for a higher incidence of AKI in patients with severe classical LFLG-AS, six-month post-TAVI outcomes including all-cause and cardiovascular mortality, did not differ between classical LFLG-AS and NFLG-AS. This suggests that despite having a lower ejection fraction and low flow state, patients with classical LFLG-AS benefit equally from TAVI compared to patients with NFLG-AS.
Declaration of Interest/Funding
None to declare.
Conflicts of Interest
The authors report no financial relationships or conflicts of interest regarding the content herein.
Acknowledgements
We would like to express our gratitude to all those who helped us during the writing of this manuscript. We would also like to thank all the peer reviewers for their opinions and suggestions.
References
2. Clavel M-A, Guzzetti E, Annabi M-S, Salaun E, Ong G, Pibarot P. Normal-Flow Low-Gradient Severe Aortic Stenosis: Myth or Reality? Structural Heart. 2018;2(3):180-7.
3. Chadha G, Bohbot Y, Lachambre P, Rusinaru D, Serbout S, Altes A, et al. Progression of Normal Flow Low Gradient "Severe" Aortic Stenosis With Preserved Left Ventricular Ejection Fraction. Am J Cardiol. 2020;128:151-8.
4. Chadha G, Bohbot Y, Rusinaru D, Maréchaux S, Tribouilloy C. Outcome of Normal-Flow Low-Gradient Severe Aortic Stenosis With Preserved Left Ventricular Ejection Fraction: A Propensity-Matched Study. J Am Heart Assoc. 2019;8(19):e012301.
5. Clavel MA, Magne J, Pibarot P. Low-gradient aortic stenosis. Eur Heart J. 2016;37(34):2645-57.
6. Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, et al. Outcome of patients with low-gradient "severe" aortic stenosis and preserved ejection fraction. Circulation. 2011;123(8):887-95.
7. Barasch E, Fan D, Chukwu EO, Han J, Passick M, Petillo F, et al. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis. 2008;17(1):81-8.
8. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. High- Versus Low-Gradient Severe Aortic Stenosis: Demographics, Clinical Outcomes, and Effects of the Initial Aortic Valve Replacement Strategy on Long-Term Prognosis. Circ Cardiovasc Interv. 2017;10(5):e004796.
9. Saeed S, Vamvakidou A, Seifert R, Khattar R, Li W, Senior R. The impact of aortic valve replacement on survival in patients with normal flow low gradient severe aortic stenosis: a propensity-matched comparison. Eur Heart J Cardiovasc Imaging. 2019;20(10):1094-101.
10. Zusman O, Pressman GS, Banai S, Finkelstein A, Topilsky Y. Intervention Versus Observation in Symptomatic Patients With Normal Flow Low Gradient Severe Aortic Stenosis. JACC: Cardiovascular Imaging. 2018;11(9):1225-32.
11. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597-1607.
12. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374(17):1609-1620.
13. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380(18):1695-1705.
14. Biner S, Birati EY, Topilsky Y, Steinvil A, Ben Assa E, Sadeh B, et al. Outcome of transcatheter aortic valve implantation in patients with low-gradient severe aortic stenosis and preserved left ventricular ejection fraction. Am J Cardiol. 2014;113(2):348-54.
15. Baljepally R, Tahir H, Goodwin RP, Livesay J, Fogelson B, Patel C, et al. Comparison of Transcatheter Aortic Valve Implantation Outcomes Between Normal-Flow, Low-Gradient Severe Aortic Stenosis and Normal-Flow, High-Gradient Severe Aortic Stenosis. Cardiovasc Revasc Med. 2022;39:12-7.
16. Guzzetti E, Pibarot P, Clavel MA. Normal-flow low-gradient severe aortic stenosis is a frequent and real entity. Eur Heart J Cardiovasc Imaging. 2019;20(10):1102-4.
17. Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, et al. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS Study. Circulation. 2008;118(14 Suppl):S234-42.
18. Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, et al. Transcatheter Aortic Valve Replacement in Patients With Low-Flow, Low-Gradient Aortic Stenosis: The TOPAS-TAVI Registry. J Am Coll Cardiol. 2018 Mar 27;71(12):1297-1308.
19. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, et al. High- Versus Low-Gradient Severe Aortic Stenosis: Demographics, Clinical Outcomes, and Effects of the Initial Aortic Valve Replacement Strategy on Long-Term Prognosis. Circ Cardiovasc Interv. 2017 May;10(5):e004796.
20. Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, et al. Predictors of mortality and outcomes of therapy in low-flow severe aortic stenosis: a Placement of Aortic Transcatheter Valves (PARTNER) trial analysis. Circulation. 2013;127(23):2316-26.
21. Eleid MF, Michelena HI, Nkomo VT, Nishimura RA, Malouf JF, Scott CG, et al. Causes of death and predictors of survival after aortic valve replacement in low flow vs. normal flow severe aortic stenosis with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2015;16(11):1270-5.
22. Salaun E, Clavel MA, Hahn RT, Jaber WA, Asch FM, Rodriguez L, et al. Outcome of Flow-Gradient Patterns of Aortic Stenosis After Aortic Valve Replacement: An Analysis of the PARTNER 2 Trial and Registry. Circ Cardiovasc Interv. 2020;13(7):e008792.
23. Ram P, Mezue K, Pressman G, Rangaswami J. Acute kidney injury post-transcatheter aortic valve replacement. Clin Cardiol. 2017;40(12):1357-62.
24. Beohar N, Doshi D, Thourani V, Jensen H, Kodali S, Zhang F, et al. Association of Transcatheter Aortic Valve Replacement With 30-Day Renal Function and 1-Year Outcomes Among Patients Presenting With Compromised Baseline Renal Function: Experience From the PARTNER 1 Trial and Registry. JAMA Cardiol. 2017;2(7):742-9.
25. Hein AM, Scialla JJ, Edmonston D, Cooper LB, DeVore AD, Mentz RJ. Medical Management of Heart Failure With Reduced Ejection Fraction in Patients With Advanced Renal Disease. JACC: Heart Failure. 2019;7(5):371-82.
