Abstract
The modulation of the innate immune system has been a significant area of interest in research, as it represents the body’s first line of defense against pathogens. Heat shock proteins (HSPs), particularly small HSP, like HSPB1 (HSP27), are emerging as potent therapeutic options for preventing and managing inflammatory conditions due to their ability to modulate the immune system. Studies have shown that exogenous HSPB1 (exHSPB1) can stimulate macrophages and other immune cells to initiate an inflammatory response, offering several potential advantages over existing treatments; and given the strong anti-inflammatory effects that has exHSPB1 upon macrophage stimulation, exHSPB1 could be used as a powerful tool in managing and mitigating inflammatory diseases.
The results obtained demonstrated that exHSPB1 can induce the secretion of both pro-inflammatory and anti-inflammatory cytokines in THP-1 macrophages. Furthermore, preincubation with specific TLR blocking peptides confirmed the presence of the TLRs on the surface of macrophages and showed that exHSPB1- induced cytokine secretion depends on TLR interaction with external stimuli. Additionally, the intracellular signaling proteins MYD88, MAPK P38, and NF-κβ P65 were found to be activated within the exHSPB1 treated cells, with NF-κβ gene expression significantly upregulated.
Given these results, exHSPB1 proved to be a promising therapeutic agent for controlling inflammation and could be a valuable agent in the development of treatments. For example, exHSPB1 can enhance the activity of immune cells like macrophages and T cells in inflammatory diseases or cancer.
Keywords
Cytokines, HSPB1, Immune response, THP-1, TLRs
Introduction
Heat shock proteins (HSPs) are a group of extensively conserved intracellular proteins present in both prokaryotic and eukaryotic organisms [1]. Over the years, the functions of HSPs as intracellular molecules have been well-known. Primarily, they act as molecular chaperones, facilitating the correct folding and stabilization of cellular proteins [2]. Numerous studies have reported that under severe stressful conditions, HSPs can also be found circulating extracellularly, released through apoptosis, necrosis, or alternative pathways [3,4]. In this extracellular environment, they perform different functions compared to their intracellular roles [5].
HSPs are classified according to their molecular weight into two main categories: small HSPs (15-43 kDa), which are ATP independent, and large HSPs (50-100 kDa), which are ATP-dependent [6].
HSPB1 (or HSP27) is one of the well-studied small HSPs along with HSPB5 and HSP20 [7]. Like all HSPs, HSPB1’s major biological function is to capture and hold misfolded polypeptides caused by stress, preventing them from aggregation [8]. HSPB1 is also known to play a crucial role in regulating cytoskeletal organization, as it interacts with actin filaments and microtubules, stabilizing the cytoskeleton under stress conditions and facilitating its reorganization [9]. HSPB1 is also widely recognized for its protective properties, including antiapoptotic and antioxidant effects [8,9]. It exhibits potent anti-apoptotic effects by inhibiting key components of the apoptotic machinery, such as cytochrome c release from mitochondria and caspase activation [6,7,9]. Furthermore, HSPB1 contributes to cellular defense against oxidative stress by modulating reactive oxygen species (ROS) levels, either through direct interaction with ROS or by upregulating antioxidant enzymes [2,4,5,9].
Interest in HSPB1 has grown recently due to its consistent expression across various tissues, especially under pathological conditions [10]. For example, HSPB1 is highly expressed in various tissues, including lung, colon, and brain as well as in many cancer cells [11]. In fact, Banerjee et al. [12] reported that circulating HSPB1 levels are significantly upregulated in breast cancer patients. This elevation is associated with tumorigenesis, resistance to apoptosis and chemoresistance leading to poor prognosis [13]. Similar HSPB1 upregulation was found in many other types of cancer including hepatic and pancreatic cancer [14]. Nevertheless, the role of exogenous HSPB1 and their impact on the innate immune response has attracted significant attention and interest in research [15].
The modulation of the innate immune system has been a significant area of interest as it represents the body’s first line of defense against pathogens. Research studies have demonstrated that exogenous HSPB1 (exHSPB1) can stimulate the innate immune system in vitro [16,17].
Cells of the innate immune system, some of which are known as antigen presenting cells (APCs), such as macrophages, dendritic cells and neutrophils are key factors in both induction and resolution of inflammation [18]. These APCs rely on pattern recognition receptors (PRRs), including Toll-like receptors (TLRs) and scavenger receptors (SRs), to quickly detect and respond to pathogen and danger associated molecular patterns (PAMPs and DAMPs) [19]. Even though these PRRs have different properties on reacting to different PAMPs and DAMPs, they exhibit numerous similarities in their subsequent signaling cascades [20].
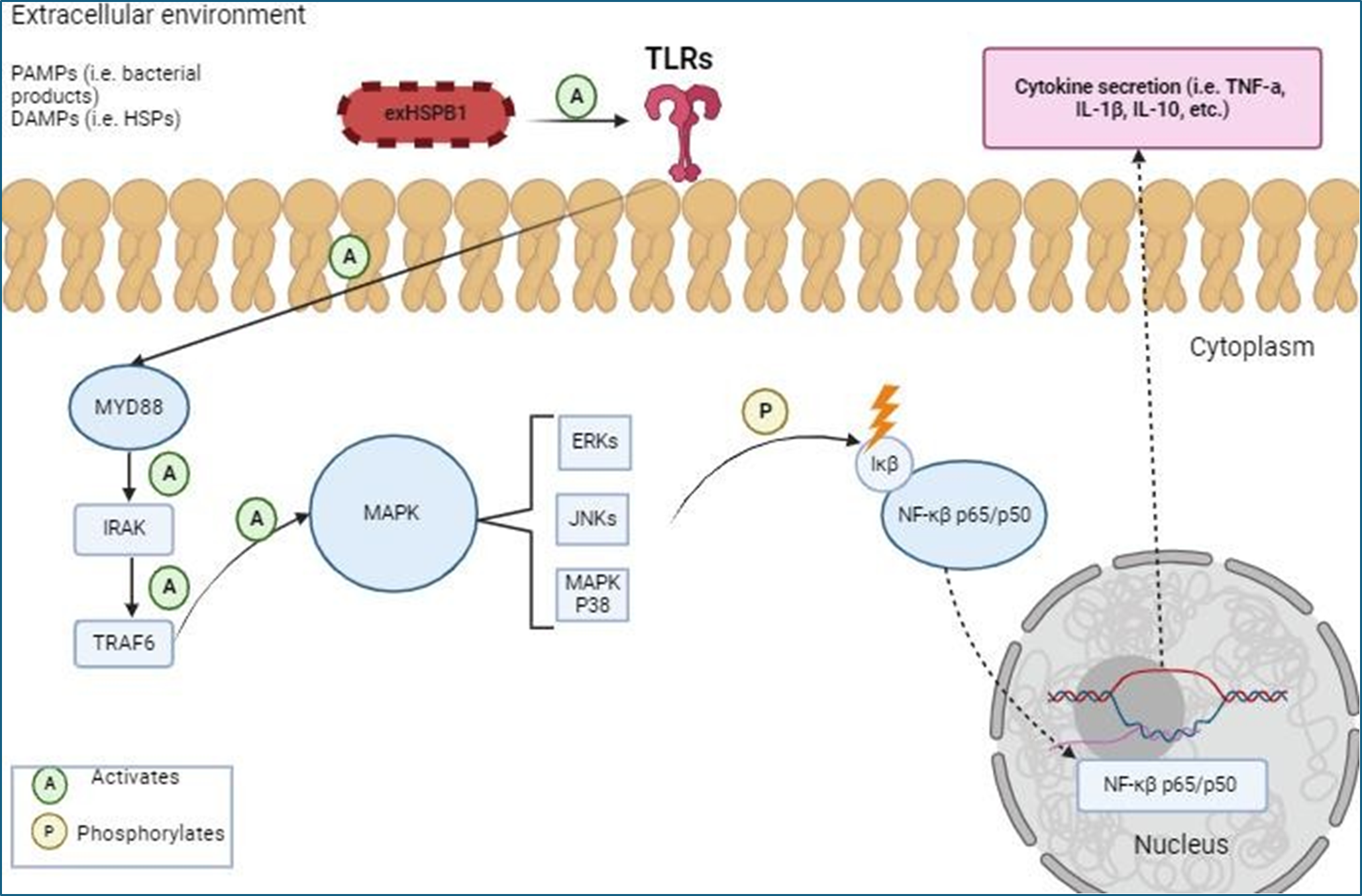
Recent data revealed that exHSPB1 can act as DAMPs to activate macrophages and develop an inflammatory response [21]. This activation occurs through interactions with the PRRs present in the APCs surfaces, mainly TLRs and SRs leading to cytokine release [22]. In fact, exHSPB1, has been shown to promote the production of anti-inflammatory cytokines, particularly interleukin 10 (IL-10) over pro-inflammatory cytokines to help control inflammation [23]. While exHSPB1 is primarily identified for its anti-inflammatory behavior [24], studies by Salari et al. [25] and Ogbodo et al. [26] in THP-1 and U937 monocytes, respectively have shown that HSPB1 can interact with TLRs and SRs to trigger a variety of signal transduction pathways, resulting in an increased transcription of genes encoding both pro- and anti-inflammatory cytokines such as interleukin 1β (IL-1β), IL-10 and tumor necrosis factor α (TNF-α). The Myeloid differentiation Primary Response 88 (MyD88) / mitogen- activated protein kinase (MAPK)/ Nuclear Factor κβ (NF-κβ) signaling cascade plays an important role in cytokine secretion via TLRs (Figure 1) [27].
Figure 1: Simplified illustration of exHSPB1 interaction with TLRs and downstream activation of the MyD88/MAPK/NF-κβ signaling cascade in THP-1 macrophages to produce an inflammatory response (created with BioRender.com). ExHSPB1 is recognized by surface TLRs, leading to the activation of MyD88 and subsequent activation of the MAPK signaling pathway, and phosphorylation of NF-κβ, resulting in the transcription of the pro-and anti-inflammatory genes.
In this study TLR2, TLR4, TLR5, and TLR7 were selected to study how exHSPB1 interacts with these TLRs to induce cytokine secretion in THP-1 macrophages. TLR2 and TLR4 were chosen due to their broad role in recognizing bacterial cell wall components such as Lipoteichoic acid (LTA) and lipopolysaccharides (LPS), respectively [28]. As for TLR5 and TLR7, these were selected for their ability recognize Gram-negative bacterial flagellin protein [29] and viral or abnormal DNA [30], respectively.
Given this, HSPB1 can be considered a potent factor for modulating the immune system and thus holds significant potential as a target for therapeutic interventions aimed at regulating inflammatory responses [31]. By influencing both pro- and anti-inflammatory pathways, HSPB1 could be used to develop treatments for various inflammatory and autoimmune conditions as well as to improve immune responses in diseases such as cancer and chronic infections [32]. For example, ExHSPB1's ability to induce pro-inflammatory cytokines (e.g., IL-1β, TNF-α) can be leveraged to boost immune responses against infections or cancer by enhancing the activity of immune cells like macrophages and T cells [33,34]. Moreover, its anti-inflammatory cytokine induction (e.g., IL-10) could help in treating chronic inflammatory or autoimmune diseases by suppressing excessive immune responses [35,36].
The primary aim of this research is to examine the capacity of exHSPB1 to trigger an inflammatory response in THP-1 macrophages in vitro, using pro-inflammatory cytokines (IL-1β, TNF-α) and anti-inflammatory cytokines (IL-4, IL-10) as biomarkers. Additionally, the study investigates the role of exHSPB1 in cytokine secretion through interactions with surface receptors (TLR2, TLR4, TLR5) and the intracellular receptor TLR7, employing TLR-blocking peptides. It also explores the involvement of key signaling pathways (MYD88, MAPK p38, and NF-κB p65) in cytokine secretion and their activation.
Methods
Cell culture
Human acute monocytic leukemia cell line THP-1 was obtained from American Type Culture Collection (ATCC, TIB-202). The cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma Aldrich, R0883) with 10% fetal bovine serum (FBS, Gibco™, A5256701) and 1% penicillin-streptomycin (Pen-Strep) (Gibco™, 15140122). The culture environment was maintained in a humified atmosphere at 37°C with 5% CO2 (pH 7.0-7.6). Cell count and viability were performed using the Trypan blue exclusion method. The cell density was maintained at 5x105 cells/ml for optimal growth.
THP-1 cell preparation and differentiation
THP-1 cells were centrifuged at 1500 rpm for 5 min, and the supernatant was discarded. The cell pellet underwent three washes RPMI 1640 with 10% heat- inactivated FBS and 1% Pen-Strep (HI-RPMI 1640). HI-RPMI 1640 was prepared by heating the RPMI 1640 complete media in a 60°C water bath for 25-30 min to deactivate the growth factors. Subsequently, the THP-1 cells were resuspended in HI- RPMI media, seeded at a density of 5x105 cells/ml and treated with 10 mg/ml phorbol 12-myristate 13-acetate (PMA) for 24h (37°C with 5% CO2) to adhere and generate THP-1-derived macrophages. Cell differentiation was monitored by a light microscope.
PMA-induced differentiation of THP-1 cells is a cost-effective and standardized method for generating macrophage-like cells from monocytes that are suitable for many in vitro studies by activating protein kinase C (PKC) and the downstream signaling cascades [37]. Even though this method is widely used to study the immune response and recapitulates relevant phenotypes and functions of macrophages (ie. CD14 and CD36), PMA cannot fully mimic the heterogeneity, plasticity, and tissue- specific functions of macrophages in vivo [38,39]. Therefore, complementary use of in vivo models is recommended for more comprehensive insights [39].
THP-1 macrophages treatment
THP-1-derived macrophages were washed with HI-RPMI to remove residual PMA prior to treatment. THP-1 macrophages were first preincubated (30 minutes) with 100 ng/ml TLR-2/-4/-5 and -7 blocking peptides to confirm binding specificity and to prevent nonspecific binding with extracellular receptors (Sigma-Aldrich; SBP3135, SBP3500765, SBP3500374 and SBP3269). Subsequently, 1 ug/ml recombinant HSPB1 (purity >95%) (R&D Systems, 1580-HS-050) was then added to the cells and incubated for 18 h (37°C with 5% CO2). The treatments on THP-1 macrophages were added either alone or in combination (control samples) to investigate the individual effects of each treatment on cytokine secretion.
Although, a dose-response curve was not conducted to determine the optimal concentration for TLRs inhibition, the use of 100 ng/ml of TLR blocking peptides in this study was based on concentrations tested in similar research studies that explored different dosages to assess their effects in cytokine secretion. For example, Ogbodo et al. [40] tested concentrations as high as 20,000 ng/ml of TLR blocking peptides in their research, while Ultaigh et al. [41] only used 1,000 ng/ml. Given the range of concentrations explored in the literature, this study aimed to investigate the efficacy of 100 ng/ml of TLR blocking peptides and determine if this lower concentration could achieve similar effects to those observed at higher concentrations.
Cytokine ELISA
Following the TLR blocking peptides/ HSPB1 treatment, the cell suspensions media were collected and centrifuged at 1500 rpm for 5 min to remove cell debris. The supernatants were then analyzed to determine IL-4, IL-10, IL-1β, and TNF-α concentrations using sandwich Human ELISA kits (Invitrogen™; KHC0041, EHIL10, KHC0011 and KHC3011) following the manufacturer’s instructions. The absorbance was measured at 450 nm (optimal density) by the LabTech™ LT-4500 microplate reader. The absorbance values were converted to cytokine concentration using GraphPad Prism software 10.2.3. The adherent THP-1 macrophages were kept in HIRPMI-1640 media and maintained in a humified atmosphere at 37°C with 5% CO2.
Cell extraction and lysis
Adherent THP-1 macrophages were washed with PBS. The cells were then incubated for 5 minutes with Trypsin (Sigma Aldrich, T4674) to detach the cells from the flask. The cells were then collected and centrifuged three times (1000 rpm/ 10 minutes), each time discarding the supernatant and resuspending the cell pellet in PBS. In the final round of centrifugation, the cells were suspended in RIPA buffer to lyse the cells and kept 30 minutes on ice. The lysed cells were then centrifuged (9000 rpm/ 10 minutes) to pellet the debris. The supernatants (containing the protein) were transferred to new Eppendorf tubes for intracellular ELISA analysis.
Intracellular ELISA
Human MyD88 Sandwich ELISA (Abcam, ab171341), Human p38 MAPK (Total) Sandwich ELISA (Invitrogen™, KHO0061) and Human NF-κβ p65 InstantOne ELISA (Invitrogen™, 85-86083-11) were used to determine the concentration of the intracellular signaling pathways proteins, following the manufacturer’s instructions. The absorbance was measured at 450 nm (optimal density), and the values were converted to protein concentrations using GraphPad Prism software 10.2.3.
RNA isolation and qRT-PCR Analysis
Total ribonucleic acid (RNA) from THP-1 macrophages treated with 100 ng/ml TLR bp and 1 μg/ml HSPB1 were extracted using the RNeasy® Mini QIAcube® Kit (Qiagen, 74104) according to the manufacturer’s instructions. Complementary deoxyribonucleic acids (cDNAs) were then synthetized using the Reverted First Strand cDNA Synthesis Kit (ThermoScientific™, K1621) following the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR) reactions were carried out using Qiagen™ Rotor-GeneQ PCR systems. The primer pairs used were as follow: forward 5’- GCGCTTCTCTGCCTTCCTTA-3’ and reverse 5’-TCTTCAGGTTTGATGCCCCC-3’ for NF- κβ gene; forward 5’- GAGTCAACGGATTTGGTCGT-3’ and reverse 5’- GACAAGCTTCCCGTTCTCAG-3’ for GAPDH gene. Amplification conditions consisted of 40 cycles of denaturation at 95°C for 5 seconds and annealing at 60°C for 30 seconds. Relevant expression of NF-κβ was normalized to GAPDH using the 2-ΔΔCT method, where Ct represents the threshold cycle.
Data analysis
The data is presented as mean ± SD, n=3. The statistical tests included both, One- way and Two-way ANOVA depending on the specific requirements of the analysis, followed by Bonferroni’s correction post-hoc test to further investigate significant differences between groups. Statistics were performed using GraphPad Prism™ software version 10.2.3. Statistical significance was determined at three levels: P<0.05 (*), P<0.01 (**) and P<0.001 (***). Results were considered statistically non-significant (ns) if P>0.05 (Table 1).
|
Treatments |
THP-1 macrophages cytokine secretions |
|||
|
IL-4 |
IL-10 |
IL-1β |
TNF-α |
|
|
Control vs exHSPB1 only at 18h |
P<0.001 (***) |
P<0.001 (***) |
P<0.001 (***) |
P<0.001 (***) |
|
exHSPB1 only vs exHSPB1+ TLR2 |
P<0.01 (**) |
P<0.05 (*) |
P<0.05 (*) |
P<0.05 (*) |
|
exHSPB1 only vs exHSPB1+ TLR4 |
P<0.01 (**) |
P<0.05 (*) |
P<0.05 (*) |
P<0.05 (*) |
|
exHSPB1 only vs exHSPB1+ TLR5 |
P<0.01 (**) |
P<0.05 (*) |
P<0.05 (*) |
P<0.05 (*) |
|
exHSPB1 only vs exHSPB1+ TLR7 |
P<0.01 (**) |
P<0.05 (*) |
P<0.05 (*) |
P<0.05 (*) |
|
Treatments |
THP-1 macrophages intracellular signaling pathways |
|||
|
MyD88 |
MAPK P38 |
NF-κβ p65 activity |
||
|
Control vs exHSPB1 only |
ns |
P < 0.05 (*) |
ns |
|
|
exHSPB1 only vs exHSPB1+ TLR2 |
ns |
ns |
ns |
|
|
exHSPB1 only vs exHSPB1+ TLR4 |
ns |
ns |
ns |
|
|
exHSPB1 only vs exHSPB1+ TLR5 |
ns |
P<0.01 (**) |
ns |
|
|
exHSPB1 only vs exHSPB1+ TLR7 |
ns |
ns |
ns |
|
Results
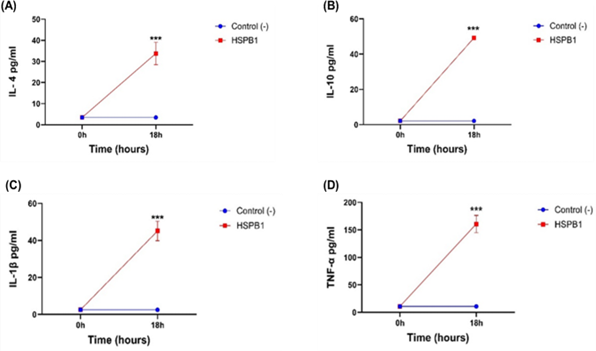
THP-1 macrophages incubated with exHSPB1 for 18 h, lead to a considerable secretion of IL-4 (Figure 2A), IL-10 (Figure 2B), IL-1β (Figure 2C), and TNF-α (Figure 2D) compared to the treated THP-1 macrophages at 0h (P<0.001). While, both pro-inflammatory and anti-inflammatory cytokines were secreted in the media following 1 µg/ml exHSPB1 treatment, the most pronounced secretions were observed in IL-10 with 49.25 pg/ml and TNF-α with 160.81 pg/ml, indicating a strong pro- and anti-inflammatory response.
Figure 2: THP-1 cells cytokine secretion at 0 h and 18 h post-treatment with HSPB1. Differentiated THP-1 cells suspended in HI-RPMI complete media were incubated with 1 ug/ml HSPB1 for 18 h. After incubation, the supernatants were collected and cytokine concentration (pg/ml) was measured at 0 h (blue) and at 18 h (red) incubation using ELISA (450 nm): (A) IL-4, (B) IL-10, (C) IL-1β, and (D) TNF-α. Control samples consisted of HI-RPMI media without any treatment on differentiated THP-1 cells. The data is reported as mean ± SD, n=3. Statistical analysis was by two-way ANOVA with Bonferroni’s post-hoc test. The significance differences between HSPB1 induced cytokine secretion at 18 h and the control (0 h) are shown as P<0.001 (***).
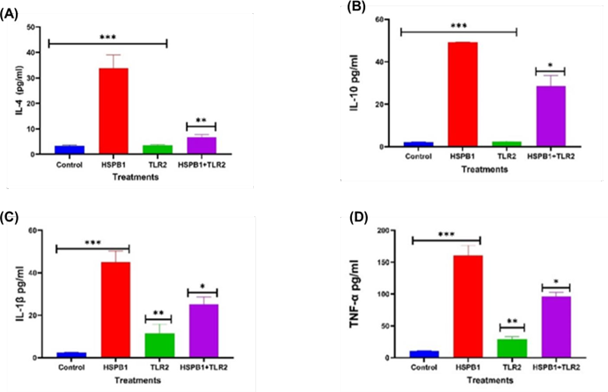
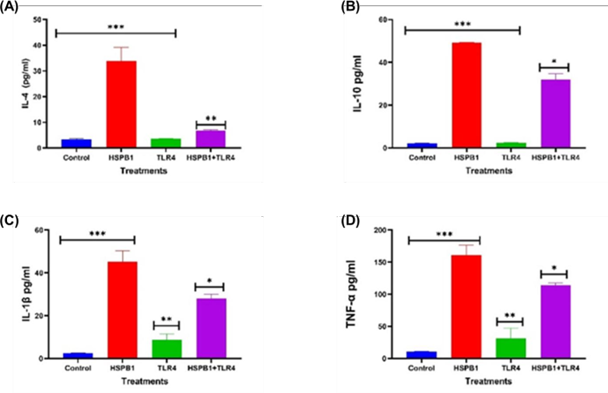
The effects of TLR2 (Figure 3), TLR4 (Figure 4), TLR5 (Figure 5), and TLR7 (Figure 6) blocking peptides on exHSPB1-induced cytokine secretion (IL-4, IL-10, IL-1β and TNF-α) were investigated.
Figure 3: Effects of TLR2 BP on HSPB1 induced cytokine secretion in THP-1 macrophages. Differentiated THP-1 cells suspended in HI-RPMI complete media were pre-incubated 30 minutes with 100 ng/ml of TLR2 BP, followed by treatment with 1 ug/ml HSPB1 for 18 hours. The supernatants were collected, and cytokine concentrations (pg/ml) were measured using ELISA (450 nm): (A) IL-4, (B) IL- 10, (C) IL-1β, and (D) TNF-α. Control samples consisted of just HI-RPMI media without treatments. The data is reported as mean ± SD, n=3. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The significant differences in cytokine secretion between the control, HSPB1- only and the combination of TLR2 BP with HSPB1 are indicated: P<0.05 (*), P< 0.01 (**), and P< 0.001 (***).
Figure 4: Effects of TLR4 BP on HSPB1 induced cytokine secretion in THP-1 macrophages. Differentiated THP-1 cells suspended in HI-RPMI complete media were pre-incubated 30 minutes with 100 ng/ml of TLR4 BP, followed by treatment with 1 ug/ml HSPB1 for 18 hours. The supernatants were collected, and cytokine concentrations (pg/ml) were measured using ELISA (450 nm): (A) IL-4, (B) IL- 10, (C) IL-1β, and (D) TNF-α. Control samples consisted of just HI-RPMI media without treatments. The data is reported as mean ± SD, n=3. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The significant differences in cytokine secretion between the control, HSPB1- only and the combination of TLR4 BP with HSPB1 are indicated: P<0.05 (*), P< 0.01 (**), and P< 0.001 (***).
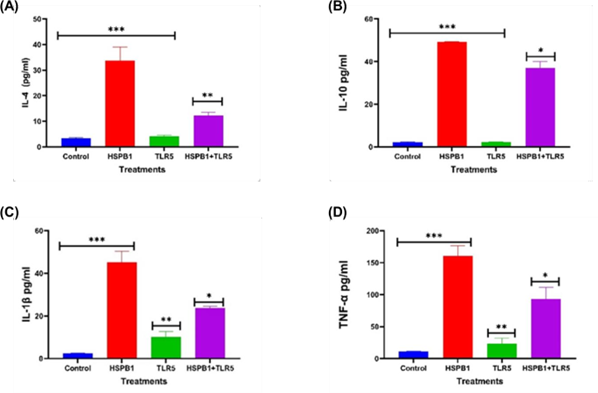
Figure 5: Effects of TLR5 BP on HSPB1 induced cytokine secretion in THP-1 macrophages. Differentiated THP-1 cells suspended in HI-RPMI complete media were pre-incubated 30 minutes with 100 ng/ml of TLR5 BP, followed by treatment with 1 ug/ml HSPB1 for 18 hours. The supernatants were collected, and cytokine concentrations (pg/ml) were measured using ELISA (450 nm): (A) IL-4, (B) IL- 10, (C) IL-1β, and (D) TNF-α. Control samples consisted of just HI-RPMI media without treatments. The data is reported as mean ± SD, n=3. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The significant differences in cytokine secretion between the control, HSPB1- only and the combination of TLR5 BP with HSPB1 are indicated: P<0.05 (*), P<0.01 (**), and P<0.001 (***).
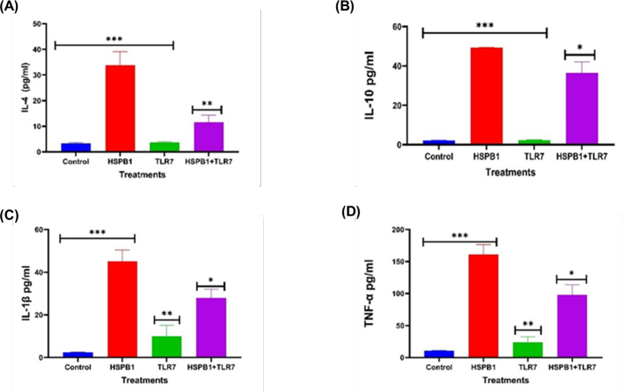
Figure 6: Effects of TLR7 BP on HSPB1 induced cytokine secretion in THP-1 macrophages. Differentiated THP-1 cells suspended in HI-RPMI complete media were pre-incubated 30 minutes with 100 ng/ml of TLR7 BP, followed by treatment with 1 ug/ml HSPB1 for 18 hours. The supernatants were collected, and cytokine concentrations (pg/ml) were measured using ELISA (450 nm): (A) IL-4, (B) IL- 10, (C) IL-1β, and (D) TNF-α. Control samples consisted of just HI-RPMI media without treatments. The data is reported as mean ± SD, n=3. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The significant differences in cytokine secretion between the control, HSPB1- only and the combination of TLR7 BP with HSPB1 are indicated: P<0.05 (*), P<0.01 (**), and P<0.001 (***).
The preincubation of THP-1 macrophages with TLR2 BP (Figure 3) followed by exHSPB1 treatment resulted in a significant decrease in IL-4 (Figure 3A), IL-10 (Figure 3B), IL-1β (Figure 3C), TNF-α (Figure 3D) secretion, compared to exHSPB1 treatment alone. The most substantial reduction in cytokine levels was observed in IL-4, which decreased to 6.68 pg/ml compared to 33.8 pg/ml in cells treated with exHSPB1 alone (P<0.01, Figure 3A). This was followed by a reduction in IL-1β levels to 25.2 pg/ml, compared to 45.1 pg/ml with exHSPB1 alone (P< 0.05, Figure 3C). Similarly, IL-10 levels deceased to 28.5 pg/ml compared to 49.2 pg/ml (P< 0.05, Figure 3B), and TNF-α levels decreased to 96.1 pg/ml compared to 160.8 pg/ml in the exHSPB1-alone group (P<0.05, Figure 3D).
A similar reduction in cytokine levels was observed in THP-1 macrophages preincubated with TLR4 BP prior to exHSPB1 treatment (Figure 4). This preincubation significantly decreased the levels of IL-4 (Figure 4A), IL-10 (Figure 4B), IL-1β (Figure 4C), and TNF-α (Figure 4D) compared to cells treated with exHSPB1 alone. The most pronounced reduction was in IL-4, which decreased to 6.7 pg/ml (P<0.01, Figure 4A). This was followed by reductions in IL-1β to 28.0 pg/ml (P<0.05, Figure 4C), IL-10 to 31.8 pg/ml (P<0.05, Figure 4B), and TNF-α to 114.0 pg/ml (P<0.05, Figure 4D).
Preincubation with TLR5 BP before exHSPB1 treatment resulted in a significant reduction in cytokine secretion (Figure 5). Specifically, IL-4 levels decreased to 12.3 pg/ml (P<0.01, Figure 5A), IL-10 to 36.9 pg/ml (P<0.05, Figure 5B), IL-1β to 23.8 pg/ml (P<0.05, Figure 5C), and TNF-α to 93.0 pg/ml (P<0.05, Figure 5D), compared to the cells treated with exHSPB1 alone.
Preincubation with TLR7 BP prior to exHSPB1 treatment similarly resulted in a significant reduction in cytokine levels (Figure 6). Specifically, IL-4 levels decreased to 11.6 pg/ml (P<0.01, Figure 6A), IL-10 to 36.5 pg/ml (P<0.05, Figure 6B), IL-1β to 27.9 pg/ml (P<0.05, Figure 6C), and TNF-α to 97.7 pg/ml (P<0.05, Figure 6D), compared to cells treated with exHSPB1 alone.
The primary signaling pathways of MYD88 (Figure 7), MAPK p38 (Figure 8), and NF-κB p65 (Figure 9), involved in cytokine secretion were analyzed to understand how their activation and concentrations influence THP-1 macrophages treated with TLR BP and exHSPB1.
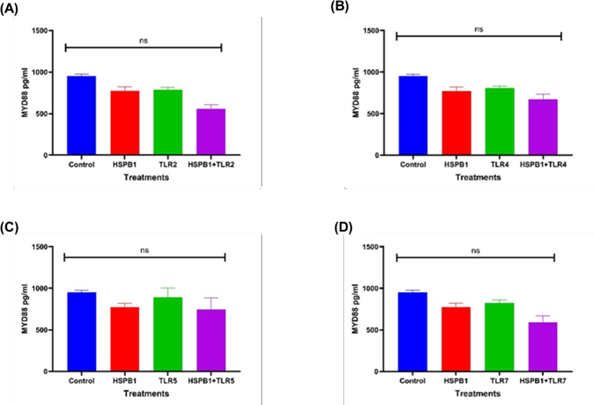
Figure 7: Effects of HSPB1- induced cytokine secretion and TLRs BP on MyD88 protein. Differentiated THP-1 cells were preincubated 30 minutes with 100 ng/ml of TLRs BP and treated with 1ug/ml HSPB1 for 18 hours. The cells were then lysed to extract the intracellular MyD88 protein. Control samples consisted of lysed differentiated THP-1 cells without treatment. MyD88 protein concentration (pg/ml) were then measured by ELISA (450nm): (A) TLR2, (B) TLR4, (C) TLR5, and (D) TLR7. The data is reported as mean ± SD, n=3. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The statistical significance in protein concentration between the control, HSPB1 and the combination of TLRs BPs with HSPB1 are indicated: P < 0.05 (*) and ns (P>0.05).
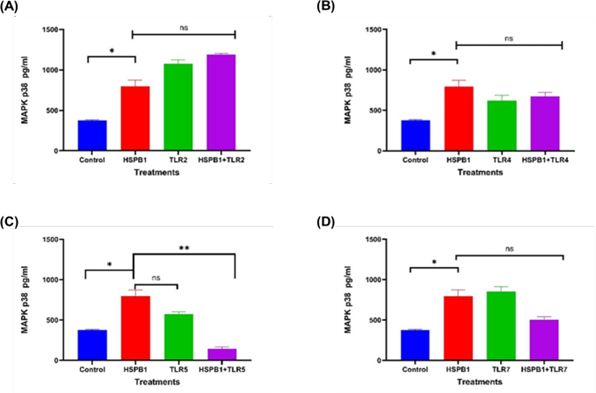
Figure 8: Effects of HSPB1- induced cytokine secretion and TLRs BP on MAPK p38 protein. Differentiated THP-1 cells were preincubated 30 minutes with 100 ng/ml of TLRs BP and treated with 1ug/ml HSPB1 for 18 hours. The cells were then lysed to extract the intracellular MAPK p38 protein. Control samples consisted of lysed differentiated THP-1 cells without treatment. MAPK p38 protein concentration (pg/ml) were then measured by ELISA (450 nm): (A) TLR2, (B) TLR4, (C) TLR5, (D) TLR7. The data is reported as mean ± SD, n=3. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The statistical significance in protein concentration between the control, HSPB1 and the combination of TLRs BPs with HSPB1 are indicated: P<0.05 (*) and ns (P>0.05).
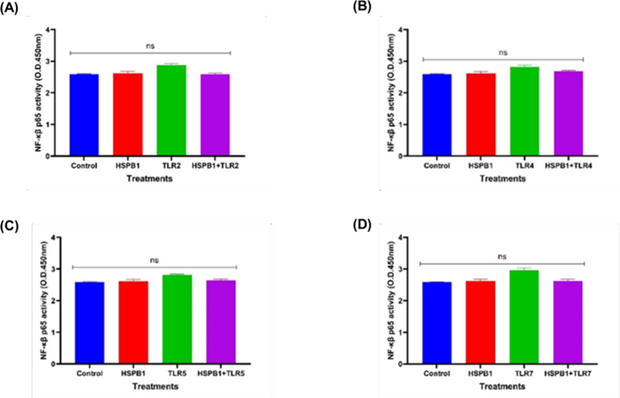
Figure 9: Effects of HSPB1- induced cytokine secretion and TLRs BP on NF-κβ p65 protein. Differentiated THP-1 cells were preincubated 30 minutes with 100 ng/ml of TLRs BP and treated with 1ug/ml HSPB1 for 18 hours. The cells were then lysed to extract the intracellular NF-κβ p65 protein. Control samples consisted of lysed differentiated THP-1 cells without treatment. NF-κβ p65 protein activity was reported as optimal density (450 nm): (A) TLR2, (B) TLR4, (C) TLR5, (D) TLR7. The data is reported as mean ± SD, n=3. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The statistical significance in protein concentration between the control, HSPB1 and the combination of TLRs BPs with HSPB1 are indicated as ns (P>0.05).
Preincubation of THP-1 macrophages with TLR BP prior to exHSPB1 treatment led to modest reductions in MyD88 concentrations compared to exHSPB1 treatment alone, though the differences were not statistically significant (ns, Figure 7). Specifically, MyD88 levels decreased to 559.4 pg/ml with TLR2 BP (Figure 7A), 674.0 pg/ml with TLR4 BP (Figure 7B) and 590.5 pg/ml with TLR7 BP (Figure 7D), compared to 772.0 pg/ml in the exHSPB1-alone group. MyD88 concentrations were slightly increased in TLR5 with 774.0 pg/ml. An increase in MyD88 levels was also observed in the cells without treatment (control groups) with 952pg/ml compared to the exHSPB1 alone treatment (772.0 pg/ml) (ns) (Figures 7A-D).
Regarding MAPK p38 concentrations (Figure 8), preincubation of THP-1 macrophages with TLR2 BP (Figure 8A) followed by exHSPB1 treatment resulted in an increase in MAPK p38 levels to 1186 pg/ml, compared to 795.0 pg/ml observed with exHSPB1 treatment alone (ns). Furthermore, MAPK p38 levels were significantly higher in cells treated with exHSPB1 alone (795.0 pg/ml) compared to the untreated control (376 pg/ml, P<0.05). In contrast, preincubation with TLR4 (Figure 8B), TLR5 (Figure 8C), and TLR7 (Figure 8D) BPs prior to exHSPB1 treatment led to a decrease in MAPK p38 concentrations, ranging from 673.2 pg/ml to 145.5 pg/ml, compared to 795.0 pg/ml in the exHSPB1-alone group (ns to P<0.01).
NF-κB p65 activity (Figure 9) remained comparable to control levels in THP-1 macrophages preincubated with TLR2 (Figure 9A), TLR4 (Figure 9B), TLR5 (Figure 9C), or TLR7 (Figure 9D) blocking peptides followed by exHSPB1 treatment, as well as in cells treated with either exHSPB1 or the TLR blocking peptides alone. No significant changes were observed.
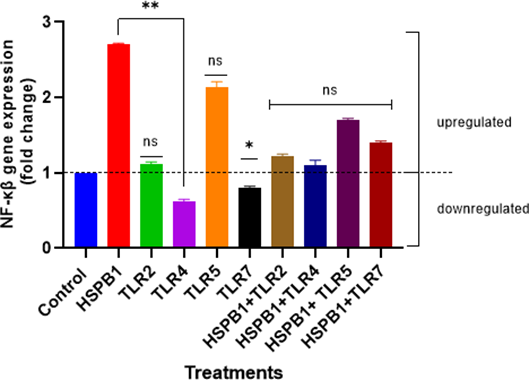
Given NF-κβ’s role in regulating the inflammatory genes, NF-κβ gene expression was also investigated to assess the impact pf exHSPB1-induced cytokine secretion in THP-1 macrophages (Figure 10). The results showed a significant upregulation (2.7-fold increase) in NF-κβ gene expression in differentiated THP-1 cells treated with exHSPB1 alone, compared to the control (ns). Similar upregulation was observed with TLR2 and TLR5 BPs alone, with increases of 1.1-fold and 2.2-fold, respectively. Differently, treatments with the TLR4 and TLR7 BPs alone led to a downregulation of NF-κβ gene expression, with decreases of 1.56-fold and a 1.22-fold, respectively, compared to the control. Compared to the exHSPB1-only treatment, TLR4 and TLR7 BPs showed a 2.1-fold and 1.9-fold decrease, respectively (P<0.01 and P<0.05). Combination treatments also resulted in NF-κβ gene upregulation, although to a lesser extent than ex-HSPB1alone (ns). The most notable increase among the combinations was observed with HSPB1+TLR5, which resulted in a 1.7-fold increase compared to the control. While NF-κβ gene expression was upregulated in both, individual (except TLR4 and TLR7) and combination treatments, the most substantial effect was observed when the cells were treated with exHSPB1-alone.
Figure 10: NF-κβ gene expression in differentiated THP-1 cells treated with HSPB1 and TLR BPs. Differentiated THP-1 pre-incubated with 100 ng/ml of TLR BPs (2,4,5 and 7) and then treated with 1 ug/ml HSPB1 for 18 hours were lysed to extract the total RNA. The extracted RNA was then converted to cDNA and qPCR was performed to detect NF-κβ gene expression. The CT values were generated for each sample, and the fold change in NF-κβ gene expression was calculated using the 2-ΔΔCT method, with normalization to the GAPDH gene. Control samples consisted of cells with HI-RPMI without treatment. No template samples were also included, generating no CT values. NF-κβ gene expression was classified as upregulated if the fold change values were ≥ 1, and downregulated if the values were <1. Statistical analysis was by one-way ANOVA with Bonferroni’s correction post-hoc test. The statistical significance in fold change expression between HSPB1-only and the downregulated TLR BPs (TLR4 and TLR7) are indicated as P<0.05 (*) and P<0.01 (**); as for the HSPB1-only and the combinations are indicated as ns (P>0.05).
Discussion
The aim of this research was to investigate the role of 1 ug/ml exHSPB1 in inducing the secretion of IL-4, IL-10, IL-1β, and TNF-α cytokines in THP-1 macrophages, and to determine how this secretion is mediated through interactions with TLR2, TLR4, TLR5, and TLR7. Additionally, the project also examined the influence of exHSPB1- induced cytokine secretion on the activation and concentration of MYD88, MAPK p38, and NF-κβ p65 signaling pathway proteins as well as, the impacts of exHSPB1 and TLR BPs treatments on the expression of the NF-κβ gene in THP-1 macrophages.
Recent findings reported that exposure to exHSPB1 can stimulate the production of both pro- and anti-inflammatory cytokines in macrophages [43,44]. While earlier studies reported that cytokine secretion induced by exogenous HSPs is attributed to bacterial contamination due to their isolation from Escherichia coli [44,45], this issue has been addressed and recent researchers reported that exHSPB1 can induce cytokine secretion regardless of contamination [46].
The findings from this research project demonstrated that 1 μg/ml exHSPB1 induced the secretion of both pro-inflammatory and anti-inflammatory cytokines following an 18-hour incubation with THP-1 macrophages (Figure 2).
Many studies reported that exHSPB1 is known for its strong anti-inflammatory response. For example, De et al. [47] found that 2 ug/ml HSPB1 induced a 10-fold increase in IL-10 secretion compared to the untreated control after only 16 h of incubation with primary human monocytes, while the secretion of TNF-α was minimal. This was mainly due to the IL-10 secretion was dependent on MAPK p38 phosphorylation, while the secretion of the pro-inflammatory cytokines was dependent on the phosphorylation of both MAPK p38 and MAPK ERK [48]. Similarly, Burut et al. [49] revealed that when exHSPB1 was incubated with murine J774 macrophages, TNF-α secretion was minimal even after 48 hours, whereas significant IL-10 production was observed as early as 8 hours of incubation. Moreover, another study by Salari et al. [25], using THP-1 macrophages but higher exHSPB1 concentrations (250 ug/ml) reported that IL-10 levels were significantly secreted after 24 h incubation (>150 pg/ml).
In this research, it was found that IL-10 secretion in media increased 22.5-fold after 18h incubation with exHSPB1 (Figure 2B). Nevertheless, IL-1β secretion was also increased 18.8-fold after HSPB1 treatment, indicating a strong pro- and anti-inflammatory response. In fact, similar findings were reported by Ogbodo et al. [26] in U937 macrophages and by Jin et al. [50] in human/mouse coronary vascular endothelial cells treated with 1 ug/ml and 4 ug/ml exHSPB1 for 24 h, respectively. These findings suggest that the effects of HSPB1- induced cytokine secretion can vary depending on the cell lineage and the concentrations of HSPB1 used, indicating that exHSPB1 can either induce excessive pro- or anti-inflammatory cytokines, or both [51]. However, several factors could have influenced cytokine production during the experiment. For instance, PMA, used for cell differentiation, can affect cytokine production and potentially lead to misleading results in pro-inflammatory cytokine secretion [52]. Although the cells were washed with HI-RPMI to remove PMA-induced cytokines, complete removal is not guaranteed, this could potentially result in inaccurate cytokine concentrations being detected by ELISA, potentially attributable to PMA rather than exHSPB1.
It is noteworthy that the pro-inflammatory cytokines TNF-α and IL-1β were rapidly detected after adding the TMB solution in ELISA assay, unlike the anti-inflammatory cytokines IL-4 and IL-10. This observation may be partially due to the experimental factors mentioned above and also can be attributed to the dual role of macrophages in inflammation when stimulated with exHSPB1 [53]. Initially, macrophages adopt the classically activated (M1) phenotype, rapidly releasing pro-inflammatory cytokines to combat pathogens [54]. Subsequently, they switch to the alternatively activated (M2) phenotype, which secretes anti-inflammatory cytokines like IL-10 to promote healing and tissue repair [55]. In fact, the production of anti-inflammatory cytokines is often controlled by feedback mechanisms that become active only after the initial inflammatory response is established [56]. Therefore, this regulatory delay likely explains the slower detection of anti-inflammatory responses in THP-1 macrophages treated with exHSPB1.
ExHSPB1 can function as DAMPs [21] and they interact with extracellular TLRs to trigger an inflammatory response [57,58]. In the presented study TLR BPs were used to determine how HSPB1 cytokine secretion is mediated through interactions with TLR2, TLR4, TLR5, and TLR7 in THP-1 macrophages. For example, Jin et al. [50] demonstrated that TLR2 and TLR4 are crucial receptors for activating the NF-κβ pathway to initiate the expression of the pro-inflammatory cytokines induced by exHSPB1 in mouse coronary vascular endothelial cells. They found that blocking TLR2 and TLR4 significantly reduced the pro-inflammatory effects of exHSPB1, indicating that exHSPB1 interacts with TLR2 and/or TLR4 to trigger an immune response. Moreover, Yusuf et al. [59] conducted an experiment with dendritic cells derived from murine bone marrow and reported similar findings. They observed that exHSPB1 induced cytokine secretion, particularly IL-1β and TNF-α, primarily occurred though TLR4 signaling. This conclusion was supported by the significant reduction in cytokine secretion when TLR4-deficient cells were treated with exHSPB1, compared to the cells with fully functional TLR4 signaling. In another study, Hirata et al. [60] reported that cooperative signaling via TLR2 and TLR4 in murine conventional dendritic cells significantly induced IL-10 production by activating the p38 MAPK pathways. Furthermore, Shi et al. [22] also reported that the interaction of exHSPB1 with TLR4 is required to activate NF-κβ signaling and generate an inflammatory response in THP-1 macrophages.
Figures 2 and 3 demonstrate that blocking TLR2 and TLR4 significantly reduced the productions of both pro-inflammatory cytokines (Figure 3C/4C; Figure 3D/4D) and the anti- inflammatory cytokines (Figure 3A/4A; Figure 3B/4B) compared to the exHSPB1 treatment alone in THP-1 macrophages. This indicates the presence of these receptors on the surface of THP-1 macrophages and suggests that the use of TLR BPs inhibits the optimal cytokine response induced by exHSPB1 [61]. However, despite the use of TLR BPs, substantial levels of both pro- and anti-inflammatory cytokines were still produced. This suggests that exHSPB1 may interact with other receptors to mediate cytokine secretion [57,62]. For example, Shi et al. [22] reported that exHSPB1 can also engage with scavenger receptors such as SR-A and CD36 to generate an inflammatory response.
TLR5 is specifically known to recognize gram-negative bacterial flagellin protein [63]. Nishi et al. [64] reported that in epithelial A549 cells, TLR5 activation by flagellin triggers the MAPK p38 signaling, leading to subsequent signal transduction cascades and secretion of the pro-inflammatory cytokines such as IL-6 and IL-12. Another study by Hyang et al. [65] demonstrated that in THP-1 cell lines TLR5 is highly expressed, and large HSPs, particularly HSP90 interacts with TLR5 to activate the NF-κβ p65 signaling pathway resulting in a pro-inflammatory response. While the results presented in Figure 5 indicate that TLR5 is also present in THP-1 macrophages and that HSPB1 can act as TLR5 agonist to induce an inflammatory response, limited research exists on the interactions between TLR5 and HSPB1 in cytokine secretion [66]. Nevertheless, these findings suggest that HSPB1 might interact with TLR5 in a manner similar to other TLRs, but further research is needed to clarify this interaction [67,81].
TLR7 is known for detecting viral or abnormal DNA [68]. It is primarily located in endolysosmes, where upon activation it induces secretion of large amounts of type I and II interferons (IFNs) as well as many pro-inflammatory cytokines [69]. This process is reported to initiate an innate antiviral response through the MYD88- dependent pathway [70]. Nevertheless, studies by Kanno et al. [71] reported that TLR7 can also be expressed on the surface of human macrophages and monocytes and can initiate various signaling pathways to induce an immune response. Despite this finding, the mechanisms by which TLR7 triggers an immune response or whether it can interact with HSPB1 to induce cytokine secretion remain unclear [72]. Even though in the present study HSPB1 showed interactions with TLR7 to induce both pro- and anti-inflammatory cytokines (Figure 6), further research is needed to determine these mechanisms and the potential role of surface TLR7 in response to HSPB1.
Studies reported that exHSPB1 induces cytokine secretion through the activation of the MYD88, MAPK p38, and NF-κβ p65 signaling pathway [25,61]. In this research, 1 ug/ml exHSPB1 incubation with THP-1 macrophages, resulted in the activation of MyD88, MAPK p38 and NF-κβ p65 proteins (Figures 7-9). Additionally, the NF-κβ gene expression was also detected to verify that the observed NF-κβ p65 activity corresponded to the upregulation/downregulation at the gene level (Figure 10).
The levels of MyD88 significantly decreased when THP-1 macrophages were preincubated with TLR-2/4/5/7 BPs before treatment with exHSPB1, compared to treatment with exHSPB1 alone. This reduction happened because most TLRs, except TLR3, initiate their downstream signaling via the MyD88-dependent pathway [73]. Thus, blocking these receptors likely accounts for the observed decrease in MyD88 concentration (Figure 7).
Similar results were observed in MAPK p38 concentrations when blocking TLR-4, 5, and 7 prior to exHSPB1 treatment (Figure 8). However, when TLR2 BP was used in combination with exHSPB1, MAPK p38 levels were significantly higher than with exHSPB1 alone (Figure 8A). This increase could be attributed to an insufficient concentration of the TLR2 blocking peptide used, leading to incomplete inhibition of TLR2 mediated signaling [74]. Alternatively, the activation of MAPK p38 could be driven by other receptors that remain active such as scavenger receptors or other TLRs not tested in this research, highlighting the complexity of signaling pathways and the potential for crosstalk between different receptors in this context [75].
Regarding NF-κB p65 (Figure 9), substantial activity was observed, all close to the control. This observation was further supported by gene expression data (Figure 10), which showed upregulation of the NF-κB gene in both exHSPB1 alone and in combination with TLR BP treatments. However, the use of TLR BPs resulted in a reduction of NF-κB gene expression compared to the control, which could be attributed to similar reasons mentioned above [22]. Various factors can modulate NF-κB activity apart from exHSPB1. For example, PMA, used for cell differentiation, is a potent activator of protein kinase C (PKC), which can initiate a cascade of phosphorylation events, leading to the activation of downstream signaling pathways [76]. Through PKC activation, the MAPK pathway is triggered, resulting in the upregulation of NF-κB and the transcription of inflammatory genes [77]. This is evident in the negative control (HI- RPMI with cells), where considerable levels of all signaling proteins were observed (Figures 7-9).
While this study produced valuable results, several limitations were observed. For example, PMA treatment which was used to induce the differentiation of monocytes into macrophages, was only monitored by observing cell morphology under a light microscope. Although this method is valid, a stronger confirmation would involve checking for macrophage-specific CD markers, such as CD11β, CD14 and CD36 by flow cytometry [78]. This would provide clearer evidence of successful differentiation. Moreover, the incubation period for HSPB1 treatment was limited to 18 hours, whereas the more commonly used incubation period in similar research is 24 hours [25]. Even though it was not tested in this research, extending the incubation time to at least 24 h could potentially optimize the interaction between exHSPB1 and THP-1 macrophages. Furthermore, while only NF-κβ gene expression was measured in this study, assessing the gene expression of MyD88 and MAPK whether with Western Blot or RT-qPCR, would provide a more comprehensive understanding of HSPB1 effects on these signaling pathways [79,80].
Conclusion
In conclusion, this research project demonstrated that 1 μg/ml exHSPB1 can induce both pro- and anti-inflammatory responses, with a predominant anti-inflammatory effect. The use of TLR BPs revealed that exHSPB1 interacts with the TLRs covered in this study, influencing cytokine secretion and activating important signaling pathways in the innate immune system. However, while cytokine secretion decreased when THP-1 macrophages were treated with TLR BPs and exHSPB1, considerable cytokine levels were still observed. This may be attributed to the concentration of TLR BPs used (100 ng/ml), which might not have been sufficient to ensure specific binding and effectively block extracellular receptors; or there is a strong possibility that exHSPB1- induced cytokine secretion involved other receptors not covered in this research. Therefore, further research is necessary to confirm these findings and explore more macrophage receptors that could play a role in triggering immune responses through exHSPB1 interactions, thus solidifying the potential of HSPB1 as a therapeutical target for modulating the immune system in many diseases.
Authorship Contribution Statement
EO and HM conceived the study and designed the experiments. EO and HM supervised the study. RKE executed the experiments and analyzed the data. EO, HM, NJH, and CRW supported in the experiments. KE, EO, and HM interpreted the data and planned the publication. RKE wrote the first draft of the manuscript, and EO edited and approved the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
School of Life Sciences, Coventry University, UK.
References
2. Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011 Jul 20;475(7356):324-32.
3. Reddy VS, Madala SK, Trinath J, Reddy GB. Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress Chaperones. 2018 May;23(3):441-54.
4. Evdonin AL, Martynova MG, Bystrova OA, Guzhova IV, Margulis BA, Medvedeva ND. The release of Hsp70 from A431 carcinoma cells is mediated by secretory-like granules. Eur J Cell Biol. 2006 Jun;85(6):443-55.
5. Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J Biol Chem. 2005 Jun 17;280(24):23349-55.
6. Jee H. Size dependent classification of heat shock proteins: a mini-review. J Exerc Rehabil. 2016 Aug 31;12(4):255-9.
7. Ungelenk S, Moayed F, Ho CT, Grousl T, Scharf A, Mashaghi A, et al. Small heat shock proteins sequester misfolding proteins in near-native conformation for cellular protection and efficient refolding. Nat Commun. 2016 Nov 30;7:13673.
8. Salminen A, Paimela T, Suuronen T, Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: regulation of the IKK complex by HSP70 and HSP90. Immunol Lett. 2008 Apr 15;117(1):9-15.
9. Arrigo AP, Gibert B. Protein interactomes of three stress inducible small heat shock proteins: HspB1, HspB5 and HspB8. Int J Hyperthermia. 2013 Aug;29(5):409-22.
10. Scarlato M, Viganò F, Carrera P, Previtali SC, Bolino A. A novel heat shock protein 27 homozygous mutation: widening of the continuum between MND/dHMN/CMT2. J Peripher Nerv Syst. 2015 Dec;20(4):419-21.
11. Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol. 2013 Jan;87(1):19-48.
12. Banerjee S, Lin CF, Skinner KA, Schiffhauer LM, Peacock J, Hicks DG, et al. Heat shock protein 27 differentiates tolerogenic macrophages that may support human breast cancer progression. Cancer Res. 2011 Jan 15;71(2):318-27.
13. Kaigorodova EV, Zavyalova MV, Bogatyuk MV, Tarabanovskaya NA, Slonimskaya EM, Perelmuter VM. Relationship between the expression of phosphorylated heat shock protein beta-1 with lymph node metastases of breast cancer. Cancer Biomark. 2015;15(2):143-50.
14. Melle C, Ernst G, Escher N, Hartmann D, Schimmel B, Bleul A, et al. Protein profiling of microdissected pancreas carcinoma and identification of HSP27 as a potential serum marker. Clin Chem. 2007 Apr;53(4):629-35.
15. Zininga T, Ramatsui L, Shonhai A. Heat Shock Proteins as Immunomodulants. Molecules. 2018 Nov 1;23(11):2846.
16. Borges TJ, Wieten L, van Herwijnen MJ, Broere F, van der Zee R, Bonorino C, et al. The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012 May 4;3:95.
17. Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. J Immunol. 2014 Dec 15;193(12):5765-71.
18. Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011 Jul;31(7):1506-16.
19. Roh JS, Sohn DH. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018 Aug 13;18(4):e27.
20. Spiering R, van der Zee R, Wagenaar J, van Eden W, Broere F. Mycobacterial and mouse HSP70 have immuno-modulatory effects on dendritic cells. Cell Stress Chaperones. 2013 Jul;18(4):439-46.
21. Shi C, Ulke-Lemée A, Deng J, Batulan Z, O'Brien ER. Characterization of heat shock protein 27 in extracellular vesicles: a potential anti-inflammatory therapy. FASEB J. 2019 Feb;33(2):1617-30.
22. Shi C, Deng J, Chiu M, Chen YX, O'Brien ER. Heat shock protein 27 immune complex altered signaling and transport (ICAST): Novel mechanisms of attenuating inflammation. FASEB J. 2020 Nov;34(11):14287-301.
23. Miller-Graziano CL, De A, Laudanski K, Herrmann T, Bandyopadhyay S. HSP27: an anti-inflammatory and immunomodulatory stress protein acting to dampen immune function. Novartis Found Symp. 2008;291:196-208; discussion 208-11, 221-4.
24. van Noort JM, Bsibsi M, Nacken P, Gerritsen WH, Amor S. The link between small heat shock proteins and the immune system. Int J Biochem Cell Biol. 2012 Oct;44(10):1670-9.
25. Salari S, Seibert T, Chen YX, Hu T, Shi C, Zhao X, et al. Extracellular HSP27 acts as a signaling molecule to activate NF-κB in macrophages. Cell Stress Chaperones. 2013 Jan;18(1):53-63.
26. Ogbodo E, Michelangeli F, Williams JHH. Exogenous heat shock proteins HSPA1A and HSPB1 regulate TNF-α, IL-1β and IL-10 secretion from monocytic cells. FEBS Open Bio. 2023 Oct;13(10):1922-40.
27. Bayer AL, Alcaide P. MyD88: At the heart of inflammatory signaling and cardiovascular disease. J Mol Cell Cardiol. 2021 Dec;161:75-85.
28. Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021 Aug 4;6(1):291.
29. Hajam IA, Dar PA, Shahnawaz I, Jaume JC, Lee JH. Bacterial flagellin-a potent immunomodulatory agent. Exp Mol Med. 2017 Sep 1;49(9):e373.
30. Mishra H, Schlack-Leigers C, Lim EL, Thieck O, Magg T, Raedler J, et al. Disrupted degradative sorting of TLR7 is associated with human lupus. Sci Immunol. 2024 Feb 23;9(92):eadi9575.
31. Calderwood SK, Gong J, Murshid A. Extracellular HSPs: The Complicated Roles of Extracellular HSPs in Immunity. Front Immunol. 2016 Apr 25;7:159.
32. Dukay B, Csoboz B, Tóth ME. Heat-Shock Proteins in Neuroinflammation. Front Pharmacol. 2019 Aug 27;10:920.
33. Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci. 2019 Nov 28;20(23):6008.
34. Li R, Qian J, Zhang W, Fu W, Du J, Jiang H, et al. Human heat shock protein-specific cytotoxic T lymphocytes display potent antitumour immunity in multiple myeloma. Br J Haematol. 2014 Sep;166(5):690-701.
35. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32(1):23-63.
36. Feng CG, Kullberg MC, Jankovic D, Cheever AW, Caspar P, Coffman RL, et al. Transgenic mice expressing human interleukin-10 in the antigen-presenting cell compartment show increased susceptibility to infection with Mycobacterium avium associated with decreased macrophage effector function and apoptosis. Infect Immun. 2002 Dec;70(12):6672-9.
37. Veh J, Mangold C, Felsen A, Ludwig C, Gerstner L, Reinhardt P, et al. Phorbol-12-myristate-13-acetate is a potent enhancer of B cells with a granzyme B+ regulatory phenotype. Front Immunol. 2023 Jul 27;14:1194880.
38. Sun JX, Xu XH, Jin L. Effects of Metabolism on Macrophage Polarization Under Different Disease Backgrounds. Front Immunol. 2022 Jul 14;13:880286.
39. Tedesco S, De Majo F, Kim J, Trenti A, Trevisi L, Fadini GP, et al. Convenience versus Biological Significance: Are PMA-Differentiated THP-1 Cells a Reliable Substitute for Blood-Derived Macrophages When Studying in Vitro Polarization? Front Pharmacol. 2018 Feb 22;9:71.
40. Ogbodo E, Michelangeli F, Williams JH. Exogenous Heat Shock Proteins HSPA1A and HSPB1 Interact with TLR2, TLR4, TLR5, and TLR7 on Differentiated U937 Monocytic Cells. Journal of Cancer Immunology. 2024 Nov 12;7(1):1-14.
41. Ultaigh SN, Saber TP, McCormick J, Connolly M, Dellacasagrande J, Keogh B, et al. Blockade of Toll-like receptor 2 prevents spontaneous cytokine release from rheumatoid arthritis ex vivo synovial explant cultures. Arthritis Res Ther. 2011 Feb 23;13(1):R33.
42. Hulina-Tomašković A, Somborac-Bačura A, Grdić Rajković M, Bosnar M, Samaržija M, Rumora L. Effects of extracellular Hsp70 and cigarette smoke on differentiated THP-1 cells and human monocyte-derived macrophages. Mol Immunol. 2019 Jul;111:53-63.
43. Shan R, Liu N, Yan Y, Liu B. Apoptosis, autophagy and atherosclerosis: Relationships and the role of Hsp27. Pharmacol Res. 2021 Apr;166:105169.
44. Wallin RP, Lundqvist A, Moré SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002 Mar;23(3):130-5.
45. Han MJ, Park SJ, Park TJ, Lee SY. Roles and applications of small heat shock proteins in the production of recombinant proteins in Escherichia coli. Biotechnol Bioeng. 2004 Nov 20;88(4):426-36.
46. Hulina-Tomašković A, Somborac-Bačura A, Grdić Rajković M, Bosnar M, Samaržija M, Rumora L. Effects of extracellular Hsp70 and cigarette smoke on differentiated THP-1 cells and human monocyte-derived macrophages. Mol Immunol. 2019 Jul;111:53-63.
47. De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000 Oct 1;165(7):3951-8.
48. Ferns G, Shams S, Shafi S. Heat shock protein 27: its potential role in vascular disease. Int J Exp Pathol. 2006 Aug;87(4):253-74.
49. Burut DP, Shafi S, Oviedo-Orta E, Ferns G. Heat shock protein 27 and the macrophage immune response. Atherosclerosis. 2010 Nov 1;213(1):e8.
50. Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, et al. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Molecular medicine. 2014 Jan;20:280-9.
51. Arrigo AP, Gibert B. Protein interactomes of three stress inducible small heat shock proteins: HspB1, HspB5 and HspB8. Int J Hyperthermia. 2013 Aug;29(5):409-22.
52. Gatto F, Cagliani R, Catelani T, Guarnieri D, Moglianetti M, Pompa PP, et al. PMA-Induced THP-1 Macrophage Differentiation is Not Impaired by Citrate-Coated Platinum Nanoparticles. Nanomaterials (Basel). 2017 Oct 17;7(10):332.
53. Mills CD. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol. 2012;32(6):463-88.
54. Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007 May 14;204(5):1057-69.
55. Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011 Oct 25;11(11):750-61.
56. da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol. 2015 Feb;51(1):19-31.
57. Murshid A, Theriault J, Gong J, Calderwood SK. Investigating receptors for extracellular heat shock proteins. Methods Mol Biol. 2011;787:289-302.
58. Hofmann U, Ertl G, Frantz S. Toll-like receptors as potential therapeutic targets in cardiac dysfunction. Expert Opin Ther Targets. 2011 Jun;15(6):753-65.
59. Yusuf N, Nasti TH, Huang CM, Huber BS, Jaleel T, Lin HY, et al. Heat shock proteins HSP27 and HSP70 are present in the skin and are important mediators of allergic contact hypersensitivity. J Immunol. 2009 Jan 1;182(1):675-83.
60. Hirata N, Yanagawa Y, Ebihara T, Seya T, Uematsu S, Akira S, et al. Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells. Mol Immunol. 2008 May;45(10):2734-42.
61. Jiang SJ, Tsai PI, Peng SY, Chang CC, Chung Y, Tsao HH, et al. A potential peptide derived from cytokine receptors can bind proinflammatory cytokines as a therapeutic strategy for anti-inflammation. Sci Rep. 2019 Feb 19;9(1):2317.
62. Whittall T, Wang Y, Younson J, Kelly C, Bergmeier L, Peters B, et al. Interaction between the CCR5 chemokine receptors and microbial HSP70. Eur J Immunol. 2006 Sep;36(9):2304-14.
63. El-Zayat SR, Sibaii H, Mannaa FA. Toll-like receptors activation, signaling, and targeting: an overview. Bulletin of the National Research Centre. 2019 Dec;43(1):1-2.
64. Nishi H, Maeda N, Izumi S, Higa-Nakamine S, Toku S, Kakinohana M, et al. Differential regulation of epidermal growth factor receptor by hydrogen peroxide and flagellin in cultured lung alveolar epithelial cells. European Journal of Pharmacology. 2015 Feb 5;748:133-42.
65. Na BH, Hoang TX, Kim JY. Hsp90 Inhibition Reduces TLR5 Surface Expression and NF-κB Activation in Human Myeloid Leukemia THP-1 Cells. Biomed Res Int. 2018 Feb 14;2018:4319369.
66. Das N, Dewan V, Grace PM, Gunn RJ, Tamura R, Tzarum N, et al. HMGB1 Activates Proinflammatory Signaling via TLR5 Leading to Allodynia. Cell Rep. 2016 Oct 18;17(4):1128-40.
67. Jang GY, Lee JW, Kim YS, Lee SE, Han HD, Hong KJ, et al. Interactions between tumor-derived proteins and Toll-like receptors. Exp Mol Med. 2020 Dec;52(12):1926-35.
68. Saito K, Kukita K, Kutomi G, Okuya K, Asanuma H, Tabeya T, et al. Heat shock protein 90 associates with Toll-like receptors 7/9 and mediates self-nucleic acid recognition in SLE. Eur J Immunol. 2015 Jul;45(7):2028-41.
69. Delanghe JR, Speeckaert MM. Host polymorphisms and COVID-19 infection. Adv Clin Chem. 2022;107:41-77.
70. Fallerini C, Daga S, Mantovani S, Benetti E, Picchiotti N, Francisci D, et al. Association of Toll-like receptor 7 variants with life-threatening COVID-19 disease in males: findings from a nested case-control study. Elife. 2021 Mar 2;10:e67569.
71. Kanno A, Tanimura N, Ishizaki M, Ohko K, Motoi Y, Onji M, et al. Targeting cell surface TLR7 for therapeutic intervention in autoimmune diseases. Nat Commun. 2015 Feb 4;6:6119.
72. Mielcarska MB, Bossowska-Nowicka M, Toka FN. Cell Surface Expression of Endosomal Toll-Like Receptors-A Necessity or a Superfluous Duplication? Front Immunol. 2021 Feb 1;11:620972.
73. Shah M, Anwar MA, Kim JH, Choi S. Advances in Antiviral Therapies Targeting Toll-like Receptors. Expert Opin Investig Drugs. 2016;25(4):437-53.
74. Mukherjee S, Karmakar S, Babu SP. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. 2016 Mar-Apr;20(2):193-204.
75. Kwon HK, Patra MC, Shin HJ, Gui X, Achek A, Panneerselvam S, et al. A cell-penetrating peptide blocks Toll-like receptor-mediated downstream signaling and ameliorates autoimmune and inflammatory diseases in mice. Exp Mol Med. 2019 Apr 26;51(4):1-19.
76. Damascena HL, Silveira WAA, Castro MS, Fontes W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells. 2022 Sep 16;11(18):2889.
77. Holden NS, Squires PE, Kaur M, Bland R, Jones CE, Newton R. Phorbol ester-stimulated NF-kappaB-dependent transcription: roles for isoforms of novel protein kinase C. Cell Signal. 2008 Jul;20(7):1338-48.
78. Baxter EW, Graham AE, Re NA, Carr IM, Robinson JI, Mackie SL, et al. Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M(IFNγ+LPS), M(IL-4) and M(IL-10) phenotypes. J Immunol Methods. 2020 Mar;478:112721.
79. Qin C, Zhang B, Zhang L, Zhang Z, Wang L, Tang L, et al. MyD88-dependent Toll-like receptor 4 signal pathway in intervertebral disc degeneration. Exp Ther Med. 2016 Aug;12(2):611-8.
80. Lu Z, Priya Rajan SA, Song Q, Zhao Y, Wan M, Aleman J, Skardal A, et al. 3D scaffold-free microlivers with drug metabolic function generated by lineage-reprogrammed hepatocytes from human fibroblasts. Biomaterials. 2021 Feb;269:120668.
81. Pullikuth AK, Routh ED, Zimmerman KD, Chifman J, Chou JW, Soike MH, et al. Bulk and Single-Cell Profiling of Breast Tumors Identifies TREM-1 as a Dominant Immune Suppressive Marker Associated With Poor Outcomes. Front Oncol. 2021 Dec 8;11:734959.