Abstract
The ongoing pandemic of the novel coronavirus of 2019 (COVID-19) has resulted in over 1 million deaths, primarily affecting older patients with chronic ailments. Multiple sclerosis (MS) patients have been deemed particularly vulnerable given their high rates of disability and increased susceptibility to infections. There have also been concerns regarding disease-modifying therapy (DMT) during the pandemic as many DMTs may increase the risk of infection due to some of their immunosuppressive properties. Furthermore, due to MS-related chronic inflammatory damage within the central nervous system, there have been concerns for worsening neurological injury by COVID-19. This has resulted in an alarmingly high level of anxiety and stress among the MS community leading to a lack of compliance with medications and routine check-ups, and even failure to obtain treatment for relapse. However, there is currently substantial evidence that MS and most DMT usage is not associated with increased COVID-19 severity. MS patients who suffer worse outcomes were more likely to be older and suffer from significant disabilities and comorbid conditions, which would also be expected from those in the general population. Likewise, there is little if any evidence demonstrating an increased susceptibility of MS patients to COVID-19-related neurological complications. Therefore, we aim to summarize the most recent findings related to COVID-19 and MS demonstrating that MS and most DMTs do not appear as risk factors for severe COVID-19.
Introduction
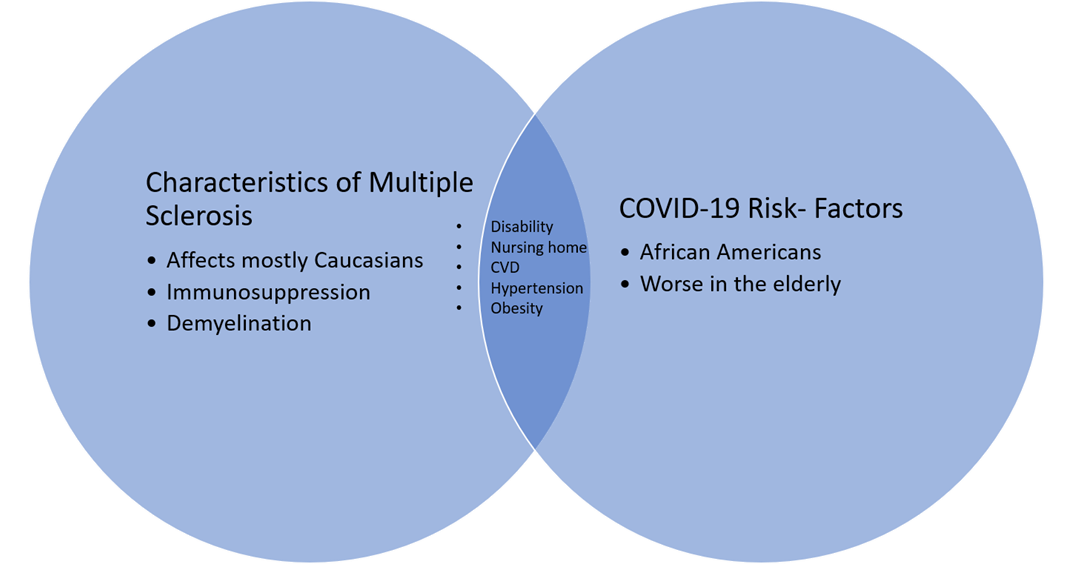
Severe respiratory syndrome coronavirus-2 (SARSCoV- 2) is the virus responsible for the novel coronavirus disease of 2019 (COVID-19) and has resulted in the death of over one million people around the world [1]. COVID- 19’s presentation is highly heterogeneous as cases range from asymptomatic to rapidly progressive resulting in low survival rates [2,3]. Specifically, patients that are older, have multiple comorbidities (i.e hypertension, lung disease, diabetes, obesity), and reside in nursing homes are more likely to succumb to COVID-19 [1-4]. Black/ African Americans have a higher risk of COVID-19 severity [5]. This is most likely multifactorial, stemming from healthcare disadvantages associated with socioeconomic disadvantages suffered among this population. Therefore, we have become particularly concerned during this pandemic for our patients with chronic debilitating diseases who are more likely to possess these risk factors.
Multiple sclerosis (MS) is a common chronic immunemediated demyelinating disease of the central nervous system(CNS) [6]. MS immunopathogenesis involves CNS inflammation, blood-brain-barrier (BBB) disruption, and autoreactive lymphocytes thereby requiring diseasemodifying therapies (DMT) of immunomodulation, immunosuppression, cell depletion and/or alteration of inflammatory cell trafficking [6]. MS is categorized into several phenotypes including primary progressive MS (PPMS), relapsing-remitting MS (RRMS), and secondary progressive MS (SPMS) [7]. PPMS is characterized by a continuously steady progression and a higher rate of disability when compared to that seen in RRMS. SPMS patients tend to be older in age than RRMS patients since it evolves from RRMS over the course of 10-20 years and is characterized by the gradual worsening of the disease. Furthermore, due to the chronic and disabling nature of MS, many patients will eventually reside in long-term care facilities [8].
Consequently, the combination of pathogenesis, treatment and natural history most likely explains the four-fold greater risk for contracting serious infections in MS patients when compared to that seen in the general population [9]. We would thus reasonably expect that MS patients are at higher risk of contracting COVID-19 and have worse outcomes [10]. Additionally, due to the compromise of the CNS by MS and the growing evidence of COVID-19 neurological manifestations, there are concerns for higher rates of neurological complications in MS patients [10,11].
Cardiovascular disease and hypertension are significantly associated with COVID-19 risk [12,13]. Patients with MS are at a significantly increased risk for these risk-factors when compared to the general population [14,15]. This is most likely due to higher rates of disability resulting in less healthy life-styles (i.e smoking, obesity, reduced physical activity) [16]. These higher rates of comorbidities could also contribute to worse COVID-19 outcomes in MS patients (Figure 1). Early studies were contradicting as survey reports from China, found no increased risk of COVID-19 in MS patients [10]. Conversely, studies from Spain and France found that MS patients are at a higher risk for poor COVID-19 outcomes when compared to the seen from the general population, but not necessarily at a higher risk to contract COVID-19 [17,18].
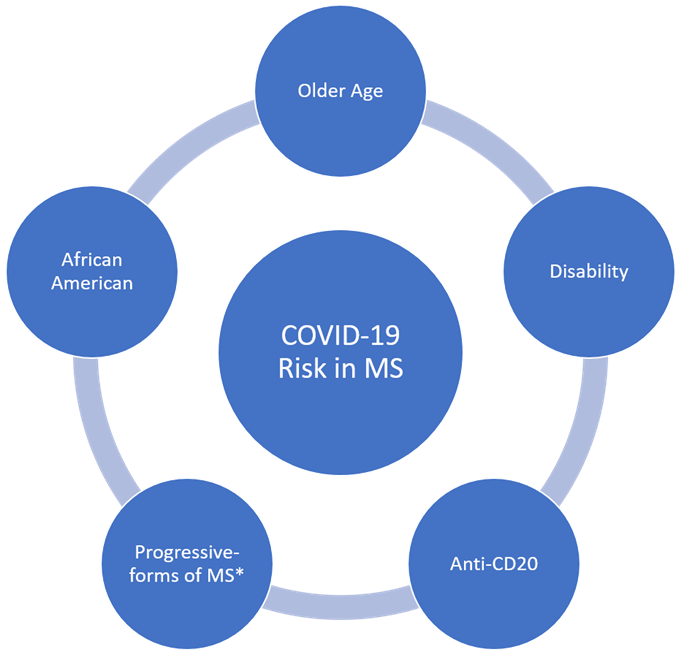
Recently, though, evidence from a larger cohort in the United States, Canada, and the United Kingdom actually showed that the risk of severe outcomes from COVID-19 was similar to that seen from the general population [19]. Regardless, all patients with more severe COVID-19 cases were older, and had higher levels of disability [17,18,20]. Additionally, the Black/African American race was also associated with worse COVID-19 outcomes [19]. As mentioned before, these risk factors have been found in the general population as well. Patients with progressive forms of MS were also more likely to succumb to severe infection, but this has yet to be found statistically significant in further larger studies [19,20]. This sub-group, due to the nature of the disease, tend to have worse disabilities and have poorer health, which most likely contributes to COVID-19 severity [21].
Contrary to what was originally thought, DMTs and higher levels of immunosuppression were not independent risk factors for worse COVID-19 outcomes in MS, with the possible exception of anti-CD20 therapy [17,19,20]. Likewise, in other immune-mediated diseases requiring chronic immunosuppression, immunosuppressive therapy has failed to significantly increase the severity of COVID-19 [22-24]. Furthermore, MS patients had symptoms similar to that experienced in the general population, without a higher rate of neurological complications. The concern for MS patients during the COVID-19 pandemic is still warranted with respect to those who are older, have more comorbidities and have significant disability, but we should bring into question MS and DMTs as severe COVID-19 risk factors (Figure 2). In this review, we aim to summarize clinical and basic science data surrounding COVID-19 and MS.
COVID-19 Pathogenesis
Virology
SARS-CoV-2 is an RNA-virus of the same genus as the severe acute respiratory syndrome coronavirus of 2002- 2003 (SARS-CoV) and targets angiotensin-converting enzyme-2 (ACE2) with its spike-glycoprotein [25]. SARS-CoV spike-glycoprotein utilizes the serine protease TMPRSS2 for priming to enter ACE2+ cells [26]. Targeting of ACE2 with spike-glycoprotein results in significant acute lung injury via an excessive inflammatory response mediated by an imbalance of Th17/Treg cells and an overproduction of proinflammatory cytokines [27-29].
Possible cytokine storm
This cytokine-storm-like response in COVID-19 was supported by early reports demonstrating COVID-19 severity was associated with increased plasma levels of cytokines such as IL-6 [30-32]. Postmortem studies of COVID-19-infected lungs also found significant signs of an inflammatory response dominated by lymphocytes resulting in severe lung damage [33,34]. Furthermore, preliminary results from clinical trials provide evidence that glucocorticoids can significantly improve COVID-19 outcomes in moderately and severely affected individuals, possibly by reducing this inflammatory response [35,36].
The cytokine-storm hypothesis stemming from COVID-19, however, has recently been brought into question. It was shown in a small cohort of patients that cytokine levels in severe COVID-19 cases were in fact lower than that seen from patients with bacterial sepsis and similar to that seen in other critically ill patients [37,38]. Furthermore, targeting IL-6 with tocilizumab was found not to be effective in preventing severe COVID-19 disease [39].
Nonetheless, IL-1 inhibition with Anakinra was associated with reduced rates of severe COVID-19, but clinical trials are still ongoing (NCT04603742) [40]. Cytokine storms are multifaceted with many different mediators, both cytokine and non-cytokine in nature. Indeed, the failure of an inhibitor of one of many proinflammatory cytokines does not negate the idea that a mixture of proinflammatory cytokines produces deleterious clinical outcomes. Therefore, even though there is evidence for a cytokine storm-like response in COVID-19, it may not adhere to the same pathogenesis typically seen in a cytokine storm.
DMT and Infection Risk
Types of MS therapies
There are many different treatment options with different mechanisms of action that are used for MS [41]. Relapse treatments include glucocorticoids and occasionally plasmapheresis in corticosteroid failures; however, these are typically reserved for acute relapses of MS, thus are administered only for a short term. DMTs, on the other hand, are administered chronically to help prevent relapse thereby slowing down MS progression. Detailed reviews of DMTs have previously been written [41,42]. DMTs are known to reduce relapses and slow the progression of disabilities by adjusting and/or suppressing the immune system, thus reducing the formation of new CNS lesions.
Classification of DMTs, albeit imperfect and sometimes not fully understood as many DMTs have many different mechanisms of action, can be divided into: 1) immunomodulation: interferon-beta-1 (IFN-b1), glatiramer acetate (GA), and fumarates (i.e dimethyl fumarate), 2) cell trafficking alterations molecules like S1P receptor modulators (i.e fingolimod), and natalizumab, an anti-α4-integrin antibody, 3) cell depletion (anti-CD20 antibodies [i.e ocrelizumab, rituximab, ofatumumab], cladribine, and anti-CD52 antibodies [i.e. alemtuzumab]), and 4) systemic immunosuppression (i.e teriflunomide) [41,42].
DMTs and overall infection risk
Among DMTs, GA and IFN-b1 were associated with a 50% increase risk of overall serious infections (defined as an infection resulting in hospitalization) in MS patients in comparison to that seen from age-/sex-matched non-MS patients [43]. However, this study most likely showed the effect of the higher risk of infection seen in MS patients as increases in overall infection was not prominent in clinical trials of these agents studied only in MS patients [44-46]. Furthermore, GA and IFN-b1 usage were not associated with higher rates of physician-reported infection-related claims for MS patients when compared to that seen from no DMT usage [47].
In head-to-head comparison studies, the anti-CD20 antibody rituximab had a significantly higher rate of overall severe infection in comparison to that seen from GA and IFN-b1 in MS patients [43]. There was also a slightly higher rate of overall severe infection with rituximab when compared to that seen in natalizumab and fingolimod treated MS patients, but this was not statistically significant [43]. Similarly, MS patients treated with the newer anti-CD20 antibody, ocrelizumab, were twice as likely to suffer from an upper respiratory infection compared to placebo-treated patients [48]. Fingolimod and natalizumab also had higher rates of severe infection when compared to IFN-b1 and GA, but not statistically significant [43]. Natalizumab was associated, with a 59% higher relative risk of infection-related physician claims compared to that seen from MS patients not on DMT [47]. Herpes zoster infections were more frequent in the cladribine-treated arm compared to that seen in the placebo-arm in an MS clinical trial [49]. Herpes simplex infections were more frequent in MS patients treated with the anti-CD52 antibody, alemtuzumab, in comparison to those treated with IFN-b1 [50].
DMT exposure and COVID-19 risk
Given that immunosuppression by some DMTs further increases the risk of infection in MS patients, it was reasonable to assume that this would pertain to COVID-19 as well. Rituximab and ocrelizumab were especially concerning due to their well-established infection-risk profile [43]. By targeting CD-20, B-cells are largely eliminated in the peripheral immune system, reducing B-cell cytokines and availability of B cells to act as antigenpresenting cells. Reduction of B-cell development and differentiation to plasma cells also occurs, but the early therapeutic effects do not seem to involve the reduction of serum and cerebrospinal fluid (CSF) IgG [51]. Importantly, anti-CD20 antibodies reduce type-II pneumocyte response to infections and prevents CD4+ T-cell priming, thereby attenuating the clearance of viral infections from the respiratory tract [51,52].
Recent evidence, however, fails to show that most DMTs are independent risk factors for COVID-19 incidence and severity [17,19,20,53]. In fact, some hypothesized that DMTs may in fact be protective against COVID-19 by attenuating the cytokine-storm-like response [54,55]. Additionally, certain DMTs (i.e GA, fumaric acid, fingolimod) are associated with an increased expression of circulating natural killer cells possibly allowing for a better defense against COVID-19 [56]. These theories, though, are still purely speculative and subgroup analyses of different DMT therapies, albeit small in sample size, have yet to show protection from COVID-19 [17,20,53].
Analysis of a larger dataset of COVID-19 MS patients did, however, reveal that anti-CD20 treatment may portend worse COVID-19 outcomes [19]. Rituximab-usage resulted in significantly higher hospital admissions (aPRs=1.58, P<0.05), ICU admissions (aPR=4.12, P<0.05), and mechanical ventilation (aPR=7.27, P<0.05) when compared to that seen with dimethyl fumarate-usage among MS patients [19]. Ocrelizumab was only associated with more ICU admissions (aPR=3.53, P<0.05) when compared to dimethyl fumarate, but not associated with more total hospital admissions and mechanical ventilations [19]. Overall, pooled-frequencies of hospitalizations, ICU admissions and ventilations were significantly higher for MS patients treated with anti-CD20 antibodies when compared to those treated with other DMTs (aPR=1.49, 2.55, and 3.05 respectively, all P<0.05). The relatively higher association of rituximab with poorer COVID-19 outcomes in comparison to that seen with ocrelizumab, admittedly not a head-to-head study, could be because rituximab has a significantly higher affinity, resulting in more deleterious effects on B-cell numbers [57].
Anti-CD20 therapies’ possible effect on the COVID-19 vaccine
There are many COVID-19 vaccines under development, however, Pfizer’s BNT162b vaccine was the first one approved for administration in the U.S and has been shown to be highly effective [58]. Since anti-CD20 antibodies deplete B-cells, there is some concern regarding their potential to attenuate humoral immune responsiveness to COVID-19 vaccines [59]. Both rituximab and ocrelizumab have been shown to blunt influenza vaccine seroconversion [60,61]. BNT162b, conversely though, is a unique mRNA-based vaccine resulting in a robust CD8+ T-cell response in addition to a humoral response, thereby possibly circumventing the attenuating effects from anti- CD20 antibodies [62]. Consequently, we would expect that BNT162b would still be highly efficacious despite anti-CD20 treatment. Even if anti-CD20 antibodies blunt BNT162b response, the risks associated with BNT162b are minimal, thus the benefit of BNT162b would most likely still far-exceed the risks [58]. Moderna’s mRNA-1273 vaccine was also approved for use in the US and has been confirmed to be effective against COVID-19. It is also an mRNA virus and would work in a similar fashion as the BNT162b vaccine, thus, having a similar risk-benefit ratio for MS patients [63].
Neurological Implications of COVID-19 in MS
COVID-19-associated neuropathogenesis
SARS viruses have been known to invade the brain [64].
ACE2 is expressed along the cerebrovasculature comprising part of the BBB, facilitating SARS transport via spikeprotein targeting [65]. In vitro models show that SARSCoV- 2 spike protein, once bound to ACE-2, can disrupt the BBB by increasing the pro-inflammatory response by endothelial cells [66]. Specifically, endothelial cells when exposed to the spike-protein increased expression of ICAM- 1 and VCAM-1 allowing increased leukocyte adhesion to the endothelium, and also increased expression of IL-1b, IL-6 and CCL5. It does not appear that the spike-protein is cytotoxic to the endothelial cells itself, but it does increase BBB permeability most likely due to increased expression of matrix metallo-proteinases (MMPs), thereby possibly permitting invasion by inflammatory cells.
There have been an alarming number of neurological manifestations of COVID-19 ranging from mild, such as headaches and myalgia, to severe, such as stroke and encephalopathy [67,68]. Whether direct infection or secondary consequences of COVID-19 such as its known hyper-coagulopathic effect is the reason for some of its neurologic-implications remains to be explored [69]. Nonetheless, the potential implications of COVID-19 on the CNS could theoretically exacerbate pre-existing neurologic injury stemming from MS.
COVID-19 in the MS Brain
BBB breakdown is a part of the pathogenesis of MS. The breakdown of the BBB in MS is mediated by inflammatory injury and the release of MMPs [70]. The breakdown of the BBB results in immune cell infiltration promoting demyelination. COVID-19 results in a systemic inflammatory response and induces vascular damage, thereby potentially exacerbating BBB breakdown and worsening MS progression [71]. Case reports/case-series have detected COVID-19 in MS patients with possible relapse, but only one case detected SARS-CoV-2 within the CSF [72-74]. Furthermore, disability only increased mildly (2-3 points on the Extended Disability Status Score), there were no MRI-confirmed findings of relapse, and all patients made a full neurological recovery. Therefore, these were likely not a true relapse, but what has been termed as pseudo-relapse. Pseudo-relapse is the transient worsening of MS symptoms due to something other than true-demyelinating pathogenesis [75]. They are triggered by other processes such as fever, heat exposure or infection/inflammation [76].
It is possible that it is too early to tell, and studies have been too small to detect a noticeable increase in neurological symptoms. However, this means that the chance of neurological complications in MS patients is low, and probably like that seen from the general population.
Viral molecular mimicry in MS
Certain viruses have been implicated in MS. The risk of MS has been shown to significantly increase in those with certain genetic susceptibilities who have had EBV infection [77]. Therefore, it is hypothesized that EBV infection in certain genetically susceptible patients can cause a molecular mimicry autoimmune response leading to MS, though this has yet to be substantiated [78].
The role of molecular mimicry by COVID-19 leading to neurological injury has been considered because of the reported association between COVID-19 and Guillain-Barré syndrome(GBS), an immune-mediated demyelinating disease of the peripheral nervous system [79,80]. Due to the significant relationship between GBS and infections, molecular mimicry has been strongly implicated in the pathogenesis of GBS, but most notably with bacterial infections of Campylobacter jejuni. However, the potential relationship between COVID-19 and GBS is only based on small case-series/studies and require larger epidemiological analysis [81]. Nonetheless, the potential molecular mimicry from COVID-19 has gained tremendous attention [81-83]. Recently, a computational analysis found that 20 human peptides were uniquely mimicked by SARS-CoV-2 [84]. Four of these peptides were mapped onto different human leukocyte antigen (HLA) phenotypes. The most pertinent neurological protein was annexin A7 (ANXA7, also called synexin), which was found to be expressed along with endothelial cells across many different organs including the brain and found to be expressed on oligodendrocytes. Annexins are a family of calcium-binding proteins that are involved in a diverse array of cellular mechanisms vesicle transport to apoptosis [85,86]. ANXA7, specifically, plays a role in vesicle exocytosis, the release of glutamate, and NMDA trafficking [87]. In the presence of intracerebral hemorrhage (ICH), ANXA7 exacerbates the release of glutamate and the trafficking of NMDA receptors, thereby potentially worsening glutamate excitotoxicity and secondary brain injury. This could support a theory that there can be molecular mimicry of SARS-CoV-2 targeting ANXA7-expressing cells in the brain, resulting in neurological injury [88].
The spike protein of SARS-CoV binds not only to ACE2, but also to sialic acid-containing glycoproteins and gangliosides on the cellular membrane (i.e GM1) [81,89]. It has been speculated that there could be cross-reactivity between epitopes at the spike-ganglioside interface and glycolipids along peripheral nerves [81]. This mechanism of molecular mimicry has been shown as a trigger for GBS following Campylobacter jejuni infection [90]. Even though the presence of mimicked peptides does not necessitate an inflammatory response, the role of molecular mimicry in demyelination/neurologic injury stemming from SARS-CoV-2 warrants further research.
Conclusion
The ongoing COVID-19 pandemic has created a lot of anxiety among MS patients, their families and physicians who care for patients with MS. In comparison to the general population, MS patients have had higher rates of depression, worse sleep quality, and fatigue during the pandemic [91]. Additionally, many MS patients are concerned about their DMT-regimens during the pandemic and some have even stopped it [92].
MS patients were believed to represent a particularly vulnerable group to COVID-19; however, mounting evidence allows us to reassess their presumed vulnerability. MS patients that are at increased risk tend to be older, have more disability, and have comorbidities such as obesity, hypertension, cardiovascular disease, diabetes and others that come with increasing age, which are similar to that seen in the general population. Currently, these risk factors appear to represent the consequences of MS and patients’ associated comorbidities rather than MS itself. Except for some anti-CD20 therapies, the use of DMT does not seem to be a risk factor for COVID-19 in MS. Therefore, MS and its treatments are most likely not strong risk factors for COVID-19, contrary to what was once thought. Instead, we should start tailoring our risk-models to look at the characteristics of our MS patients individually.
Acknowledgements
This work was supported by National Institutes of Health (NIH) grants, T32AI007527 to Dr. Robert P Lisak.
References
2. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020.
3. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052-2059.
4. Shi SM, Bakaev I, Chen H, Travison TG, Berry SD. Risk Factors, Presentation, and Course of Coronavirus Disease 2019 in a Large, Academic Long-Term Care Facility. J Am Med Dir Assoc. 2020;21(10):1378-1383 e1371.
5. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. New England Journal of Medicine. 2020;382(26):2534-2543.
6. Hauser SL, Oksenberg JR. The Neurobiology of Multiple Sclerosis: Genes, Inflammation, and Neurodegeneration. Neuron. 2006;52(1):61-76.
7. Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology. 2006;66(2):172-177.
8. Noyes K, Bajorska A, Weinstock-Guttman B, Mukamel DB. Is There Extra Cost of Institutional Care for MS Patients? Mult Scler Int. 2013;2013:713627.
9. Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. European Journal of Neurology. 2013;20(8):1153-1160.
10. Fan M, Qiu W, Bu B, et al. Risk of COVID-19 infection in MS and neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. 2020;7(5).
11. Barzegar M, Mirmosayyeb O, Nehzat N, Sarrafi R, Khorvash F, Maghzi AH, et al. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurology(R) Neuroimmunology & Neuroinflammation. 2020;7(4):e753.
12. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical Characteristics of Covid-19 in New York City. New England Journal of Medicine. 2020.
13. Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020; Jul 1;180(7):934-43.
14. Palladino R, Marrie RA, Majeed A, Chataway J. Evaluating the Risk of Macrovascular Events and Mortality Among People With Multiple Sclerosis in England. JAMA Neurology. 2020;77(7):820-828.
15. Saroufim P, Zweig SA, Conway DS, Briggs FBS. Cardiovascular conditions in persons with multiple sclerosis, neuromyelitis optica and transverse myelitis. Multiple Sclerosis and Related Disorders. 2018;25:21-25.
16. Marrie R, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. High frequency of adverse health behaviors in multiple sclerosis. Mult Scler. 2009;15(1):105-113.
17. Louapre C, Collongues N, Stankoff B, et al. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurology. 2020.
18. Castillo Álvarez F, López Pérez M, Marzo Sola ME. Risk of SARS-CoV-2 infection and clinical outcomes in multiple sclerosis patients in La Rioja (Spain). Med Clin (Barc). 2020;155(8):362-363.
19. Salter A GA. COViMS registry and UKMSR. 8th Joint European Committee for Treatment and Research in Multiple Sclerosis-Americas Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS-ACTRIMS) 2020; 2020; Washington DC, United States.
20. Chaudhry F, Bulka H, Rathnam AS, et al. COVID-19 in multiple sclerosis patients and risk factors for severe infection. J Neurol Sci. 2020;418:117147.
21. Eriksson M, Andersen O, Runmarker B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler. 2003;9(3):260-274.
22. Faye AS, Lee KE, Laszkowska M, Kim J, Blackett JW, McKenney AS, et al. Risk of Adverse Outcomes in Hospitalized Patients with Autoimmune Disease and COVID-19: A Matched Cohort Study from New York City. J Rheumatol. 2020; Nov 1.
23. Haberman R, Axelrad J, Chen A, Castillo R, Yan D, Izmirly P, et al. Covid-19 in Immune-Mediated Inflammatory Diseases — Case Series from New York. New England Journal of Medicine. 2020 Jul 2;383(1):85-8.
24. Liu M, Gao Y, Zhang Y, Shi S, Chen Y, Tian J. The association between severe or dead COVID-19 and autoimmune diseases: A systematic review and metaanalysis. The Journal of Infection. 2020;81(3):e93-e95.
25. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nature Medicine. 2020 Apr;26(4):450-2.
26. Ou X, Liu Y, Lei X, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1):1620.
27. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112-116.
28. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875-879.
29. Li Y, Zeng Z, Li Y, Huang W, Zhou M, Zhang X, et al. Angiotensin-converting enzyme inhibition attenuates lipopolysaccharide-induced lung injury by regulating the balance between angiotensin-converting enzyme and angiotensin-converting enzyme 2 and inhibiting mitogenactivated protein kinase activation. Shock. 2015;43(4):395- 404.
30. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet. 2020;395(10223):507-513.
31. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954.
32. Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020 Jun;69(6):599-606.
33. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Apr 1;8(4):420-2.
34. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J Thorac Oncol. 2020 May 1;15(5):700-4.
35. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. New England Journal of Medicine. 2020.
36. Group TWREAfC-TW. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Metaanalysis. JAMA. 2020;324(13):1330-1341.
37. Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Internal Medicine. 2020;180(9):1152-1154.
38. Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA. 2020;324(15):1565-1567.
39. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. New England Journal of Medicine. 2020 Dec 10;383(24):2333-44.
40. Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. The Lancet Rheumatology. 2020;2(7):e393-e400.
41. Winkelmann A, Loebermann M, Reisinger EC, Hartung H-P, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nature Reviews Neurology. 2016;12(4):217-233.
42. Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BA, Gronseth GS, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):789-800.
43. Luna G, Alping P, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, et al. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. 2019;77(2).
44. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, doubleblind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45(7):1268-1276.
45. Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebocontrolled study of the effects of glatiramer acetate on magnetic resonance imaging--measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol. 2001;49(3):290-297.
46. Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. The Lancet Neurology. 2009;8(11):987-997.
47. Wijnands JMA, Zhu F, Kingwell E, Fisk JD, Evans C, Marrie RA, et al. Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. Journal of Neurology, Neurosurgery & Psychiatry. 2018:jnnp- 2017-317493.
48. Montalban X, Hauser SL, Kappos L, Arnold DL, Bar- Or A, Comi G, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. New England Journal of Medicine. 2016;376(3):209-220.
49. Cook S, Leist T, Comi G, Montalban X, Giovannoni G, Nolting A, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Mult Scler Relat Disord. 2019;29:157-167.
50. Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. The Lancet. 2012;380(9856):1829-1839.
51. Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120(1):214-222.
52. Elsegeiny W, Eddens T, Chen K, Kolls JK. Anti-CD20 antibody therapy and susceptibility to Pneumocystis pneumonia. Infect Immun. 2015;83(5):2043-2052.
53. Sormani MP. An Italian programme for COVID-19 infection in multiple sclerosis. The Lancet Neurology.
54. Berger JR, Brandstadter R, Bar-Or A. COVID-19 and MS disease-modifying therapies. Neurol Neuroimmunol Neuroinflamm. 2020;7(4).
55. Novi G, Mikulska M, Briano F, Toscanini F, Tazza F, Uccelli A, et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord. 2020;42:102120.
56. Al-Ani M, Elemam NM, Hundt JE, Maghazachi AA. Drugs for Multiple Sclerosis Activate Natural Killer Cells: Do They Protect Against COVID-19 Infection? Infect Drug Resist. 2020;13:3243-3254.
57. Morschhauser F, Marlton P, Vitolo U, Lindén O, Seymour JF, Crump M, et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol. 2010;21(9):1870-1876.
58. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020 Dec 31;383(27):2603-15.
59. Houot R, Levy R, Cartron G, Armand P. Could anti- CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? European Journal of Cancer. 2020;136:4-6.
60. Bar-Or A, Calkwood JC, Chognot C, Evershed J, Fox EJ, Herman A, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis. Neurology. 2020;95(14):e1999.
61. Kim W, Kim S-H, Huh S-Y, Kong SY, Choi YJ, Cheong HJ, et al. Reduced antibody formation after influenza vaccination in patients with neuromyelitis optica spectrum disorder treated with rituximab. European Journal of Neurology. 2013;20(6):975-980.
62. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-599.
63. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2020;384(5):403-416.
64. Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011-1033.
65. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637.
66. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol Dis. 2020;146:105131.
67. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. The Lancet Neurology. 2020;19(9):767-783.
68. Fifi JT, Mocco J. COVID-19 related stroke in young individuals. The Lancet Neurology. 2020;19(9):713-715.
69. Aid M, Busman-Sahay K, Vidal SJ, Maliga Z, Bondoc S, Starke C, et al. Vascular Disease and Thrombosis in SARSCoV- 2-Infected Rhesus Macaques. Cell. 2020;183(5):1354- 1366.e1313.
70. Losy J. Is MS an inflammatory or primary degenerative disease? J Neural Transm (Vienna). 2013;120(10):1459- 1462.
71. Lin C, Arevalo YA, Nanavati HD, Lin DM. Racial differences and an increased systemic inflammatory response are seen in patients with COVID-19 and ischemic stroke. Brain, Behavior, & Immunity - Health. 2020;8:100137.
72. Thornton JR, Harel A. Negative SARS-CoV-2 antibody testing following COVID-19 infection in Two MS patients treated with ocrelizumab. Multiple Sclerosis and Related Disorders. 2020;44.
73. Domingues RB, Mendes-Correa MC, de Moura Leite FBV, Sabino EC, Salarini DZ, Claro I, et al. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. Journal of Neurology. 2020;267(11):3154-3156.
74. Kataria S, Tandon M, Melnic V, Sriwastava S. A case series and literature review of multiple sclerosis and COVID-19: Clinical characteristics, outcomes and a brief review of immunotherapies. eNeurologicalSci. 2020;21:100287-100287.
75. Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;1(8441):1313-1315.
76. Confavreux C. Infections and the risk of relapse in multiple sclerosis. Brain. 2002;125(5):933-934.
77. Jacobs BM, Giovannoni G, Cuzick J, Dobson R. Systematic review and meta-analysis of the association between Epstein-Barr virus, multiple sclerosis and other risk factors. Multiple sclerosis (Houndmills, Basingstoke, England). 2020;26(11):1281-1297.
78. Tengvall K, Huang J, Hellström C, Kammer P, Biström M, Ayoglu B, et al. Molecular mimicry between Anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proceedings of the National Academy of Sciences. 2019;116(34):16955-16960.
79. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. New England Journal of Medicine. 2020;382(26):2574-2576.
80. Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barre syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020 Aug 25:1-38.
81. Dalakas MC. Guillain-Barré syndrome: The first documented COVID-19-triggered autoimmune neurologic disease: More to come with myositis in the offing. Neurology(R) Neuroimmunology & Neuroinflammation. 2020;7(5):e781.
82. Angileri F, Legare S, Marino Gammazza A, Conway de Macario E, Jl Macario A, Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun Rev. 2020;19(8):102591-102591.
83. Cappello F. COVID-19 and molecular mimicry: The Columbus’ egg? J Clin Neurosci. 2020;77:246-246.
84. Venkatakrishnan AJ, Kayal N, Anand P, Badley AD, Church GM, Soundararajan V. Benchmarking evolutionary tinkering underlying human-viral molecular mimicry shows multiple host pulmonary-arterial peptides mimicked by SARS-CoV-2. Cell Death Discov. 2020;6:96.
85. Kawai H, Chaudhry F, Shekhar A, Petrov A, Nakahara T, Tanimoto T, et al. Molecular Imaging of Apoptosis in Ischemia Reperfusion Injury With Radiolabeled Duramycin Targeting Phosphatidylethanolamine: Effective Target Uptake and Reduced Nontarget Organ Radiation Burden. JACC: Cardiovascular Imaging. 2018;11(12):1823-1833.
86. Li H, Liu S, Sun X, Yang J, Yang Z, Shen H, et al. Critical role for Annexin A7 in secondary brain injury mediated by its phosphorylation after experimental intracerebral hemorrhage in rats. Neurobiol Dis. 2018;110:82-92.
87. Li H, Liu S, Sun X, Yang J, Yang Z, Shen H, et al. Critical role for Annexin A7 in secondary brain injury mediated by its phosphorylation after experimental intracerebral hemorrhage in rats. Neurobiology of Disease. 2018;110:82-92.
88. Melmed KR, Cao M, Dogra S, Zhang R, Yaghi S, Lewis A, et al. Risk factors for intracerebral hemorrhage in patients with COVID-19. Journal of Thrombosis and Thrombolysis. 2020 Sep 24:1-8.
89. Tortorici MA, Walls AC, Lang Y, Wang C, Li Z, Koerhuis D, et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nature Structural & Molecular Biology. 2019;26(6):481-489.
90. Dalakas MC. Pathogenesis of immune-mediated neuropathies. Biochim Biophys Acta. 2015;1852(4):658-666.
91. Motolese F, Rossi M, Albergo G, Stelitano D, Villanova M, Di Lazzaro V, et al. The Psychological Impact of COVID-19 Pandemic on People With Multiple Sclerosis. Frontiers in Neurology. 2020;11(1255).
92. Alnajashi H, Jabbad R. Behavioral practices of patients with multiple sclerosis during Covid-19 pandemic. PLOS ONE. 2020;15(10):e0241103.