Abstract
Diabetes is one of the most common metabolic conditions that can significantly impair the overall quality of life. Diabetic nephropathy is a diabetes-associated renal damage condition and one of the most common diabetes-induced renal complications. The hyperglycaemia associated with diabetes is considered one of the key factors associated with the renal damage. The endocannabinoid system is a complex biological process involved in various physiological activities, while its impairment is directly associated with numerous pathological conditions as well. The components of the endocannabinoid system are widely distributed in the body, including in the renal tissues. Hence, alteration in the renal endocannabinoid system is associated with chronic kidney dysfunction conditions. Palmitoylethanolamide (PEA) is an endogenous fatty acid amide that is produced on demand by almost all the cells in the body. Due to its myriad molecular targets, PEA is able to produce potent anti-inflammatory and antioxidant effects. While much focus has highlighted the potential therapeutic efficacy of exogenous PEA therapy in various painful conditions, its beneficial effect in diabetes-induced renal damage conditions is still largely unexplored. The current review study aimed to highlight the potential effect of PEA therapy in renal damage conditions, with particular emphasis on the molecular mechanisms involved for the same. The current review supports the use of PEA in diabetic conditions due to its myriad protective effects on renal tissues; thereby, the need for well-designed clinical studies to establish PEA’s renal protective efficacy in the diabetic population is warranted.
Keywords
Diabetes, Chronic kidney disease, Palmitoylethanolamide, PPAR, TRPV, Nephropathy, Narrative review.
Introduction
Diabetes mellitus is a chronic metabolic condition usually associated with significantly raised blood glucose level (hyperglycaemia), reduced insulin sensitivity (insulin resistance), or both. Due to the progressively increasing prevalence, diabetes is one of the major and most prevalent non-communicable metabolic disease conditions that affect millions of people globally [1,2]. With the exact prevalence being unidentified, it was estimated that in the year 2021, approximately 537 million adults will have diabetes, and with the current progression rate, the prevalence is estimated to reach up to 783 million adults by the year 2045 [2,3]. Diabetes is one of the major conditions responsible for the high rate of morbidity and increased hospital expenditure rate among the middle-to-older adult population. It can pose a significant threat to overall quality of life, as untreated diabetes can lead to numerous serious complications [2]. Similarly, the mortality rate estimated due to diabetes is also considerably high, with around 8 deaths per minute directly associated with diabetes [2,4,5].
Diabetic nephropathy (DN) is the most common renal complication of diabetes. As the pathogenesis of DN is not completely understood, the treatment strategies for the management of DN are limited [6]. Additionally, it has been observed that the agents responsible for reducing blood sugar levels are unable to prevent the progression of DN to end-stage kidney disease and chronic kidney disease (CKD), which makes DN management more complex [6]. Various studies have demonstrated that a chronic systemic inflammatory state along with increased oxidative stress play an important role in the progression of DN to CKD [7]. The increased inflammation and associated oxidative stress increases nuclear factor kappa-B (NF-κB) and transforming growth factor (TGF)-ß levels in renal tissues, which further causes an increase in renal cytokines and chemokines, increased inducible nitric oxide synthase expression and resident mast cell activation, and an increase in collagen and matrix metalloproteinase (MMP) production, further causing glomerular hypertension, an increase in urinary albumin excretion, tubular-interstitial damage, and renal fibrosis, ultimately causing the progression of CKD [8].
Endocannabinoid System
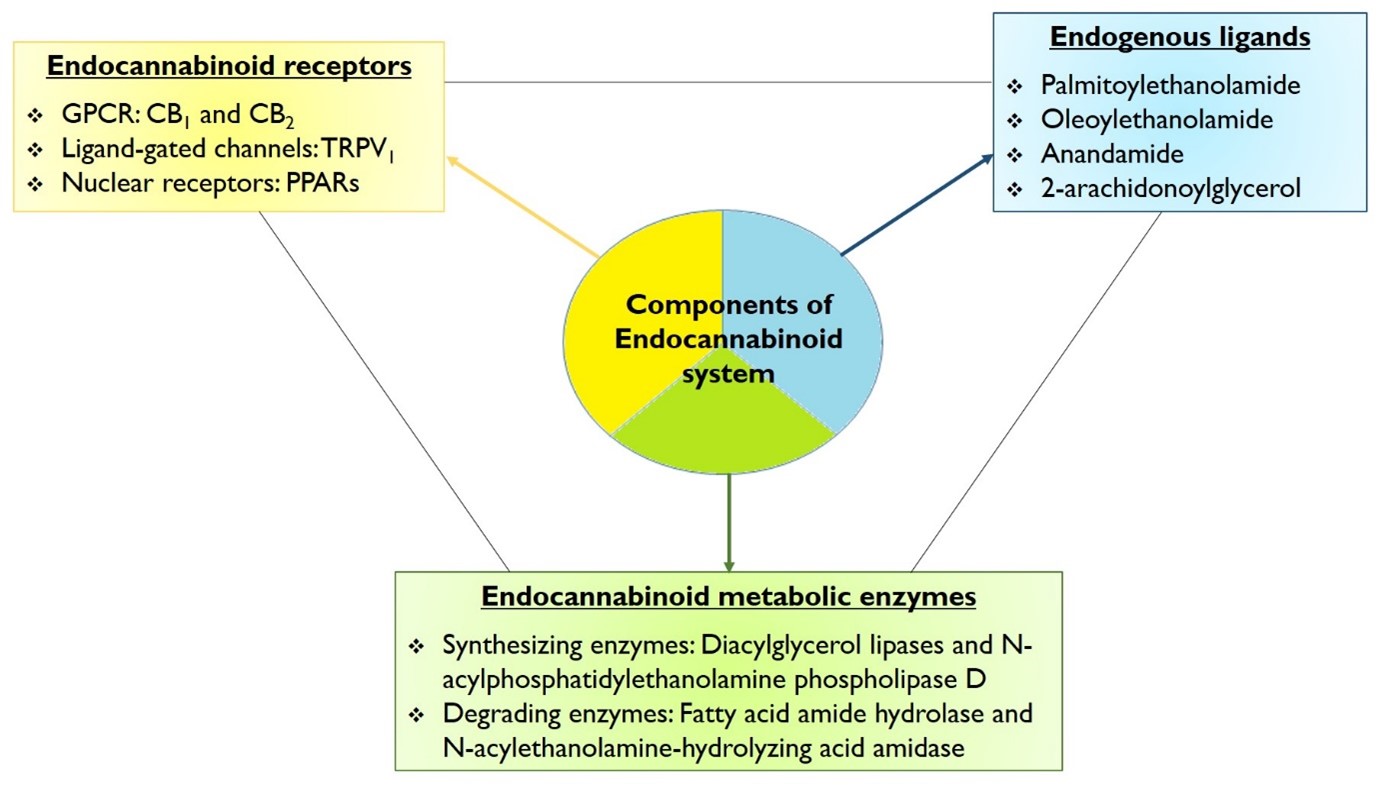
First discovered in 1988 by Howlett and Devane, the endocannabinoid (EC) system is a complex molecular/biological process that plays a crucial role in multiple physiological processes [9,10]. The term “endocannabinoid” system was coined based on the molecule ?1-tetrahydrocannabinol (THC), which is the active ingredient found in cannabis/marijuana and has a long history of human use. The complete EC system comprises three components as follows (Figure 1) [9]:
- Endocannabinoid receptors: There are mainly 3 family of receptors present in EC system
- The G-Protein Coupled Receptors (GPCRs): these include the cannabinoid receptors type 1 (CB1) and type 2 (CB2).
- Ligand-sensitive ion channels: including Transient Receptor Potential Vanilloid-1 (TRPV-1).
- Nuclear receptors: including Peroxisome proliferator-activated receptors (PPARs).
- Endogenous ligands including palmitoylethanolamide (PEA), oleoylethanolamide (OEA), N-arachidonoyl ethanolamine (anandamide; AEA), and 2-arachidonoylglycerol (2-AG).
- Endocannabinoid metabolic enzymes
- Endocannabinoid synthesizing enzymes including Diacylglycerol lipases and N-acylphosphatidylethanolamine phospholipase D.
- Endocannabinoid degrading enzymes including fatty acid amide hydrolase (FAAH), N-acylethanolamine-hydrolyzing acid amidase (NAAA), Alpha/Beta-hydrolase domain type 6 and type 12, and Monoacylglycerol lipase.
Figure 1: Components of the endocannabinoid system.
Role of Endocannabinoid System in Renal Physiology
The kidneys are one of the major organs of the renal system, which is involved in various processes including the elimination of metabolism by-products and toxins from the systemic circulation, regulating body volume status, maintaining systemic electrolyte balance, producing certain hormones, and maintaining normal body homeostasis [11]. With the constant rise in the prevalence of acute kidney disease and chronic kidney disease (CKD), the search for new therapies for improving renal health has also increased in recent decades. Although the majority of studies have focused on the role of the EC system in CNS tissues, recent studies have demonstrated the presence of CB-receptors in renal tissues as well, where they are responsible for maintaining normal renal hemodynamics and overall health, while the alteration in the expression level of endocannabinoids in the kidney is associated with various renal complications, including CKD, diabetic nephropathy, and various types of kidney injuries [11]. The presence of CB-receptors in the renal tissues is found to modulate the glomerular filtration rate and modulate the renal vascular contractility, thereby influencing the overall renal homeostasis and physiological activity [11]. Moreover, various evidences have suggested that the alteration in the renal EC system activity is involved in the development and/or progression of various acute and chronic kidney disease conditions, while the activators of the EC system demonstrate positive effects in such conditions as well [12-14].
Palmitoylethanolamide
PEA is a bioactive endocannabinoid mediator belonging to the N-acyl ethanolamine (NAE) fatty acid amide family (Figure 2). First discovered in 1950, PEA has been the center for various explorative studies, primarily in the fields of neuroscience and pain modulation [15]. PEA, the naturally occurring derivative of ethanolamine and palmitic acid, is synthesized on cellular demand within the lipid bilayer and is found in almost all tissues of the body [16,17]. Similar to other NAE, PEA is never stored in the body and is produced largely as part of the body’s protective mechanism against any cellular injury or in response to certain disease conditions [15].
Figure 2: Molecular structure of palmitoylethanolamide.
PEA: Molecular Mechanism of Action
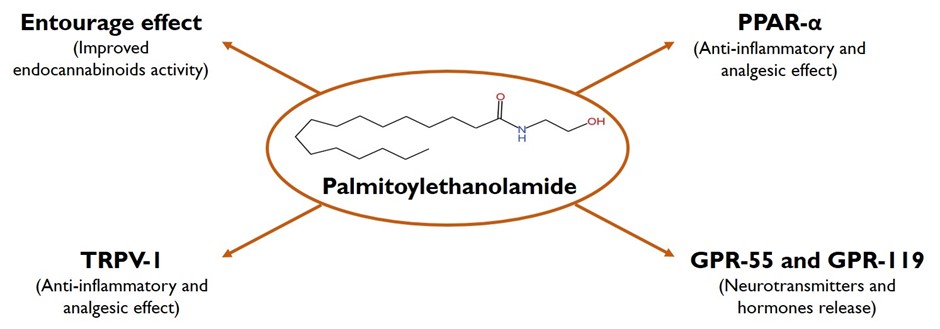
The myriad of actions of PEA is due to the multi-faceted molecular mechanism of actions of PEA, as depicted in Figure 3. The most studied and widely accepted mechanism of action of PEA involves the activation of the PPAR family, activation of GPR-55 and GPR-119, and indirect activation of the endocannabinoid receptors (CB1 and CB2) by a mechanism called the “entourage effect” via increasing the level and effect of AEA and increasing the activation level, leading to desensitization of TRPV-1 [18].
Figure 3. Palmitoylethanolamide mechanism of action.
PPAR is a ligand-activated nuclear receptor superfamily comprised of three structurally similar receptors, namely PPAR-α, PPAR-γ, and PPARβ/δ. Among these subtypes, PEA action is widely studied on the activity of the PPAR-α receptor [19]; certain evidence has suggested that PEA can stimulate other PPARs as well to produce its classical anti-inflammatory effect [20]. The binding of PEA and other endogenous and exogenous ligands to PPAR-α causes the internalization of the receptor and subsequent heterodimerization with retinoid X-receptors in the cellular nucleus and thereby activates or suppresses the gene expression level of various PPAR-response elements. Additionally, activation of PPAR-α causes activation of various other co-activators, which in combination with PPAR-α forms the transcriptionally active PPAR-α interacting cofactor complex. These co-factors can induce transcription activity, independent of PPAR-α itself [21]. The activation of PPAR-α by PEA leads to the repression of transcription genes related to pro-inflammatory cytokines/chemokines (including nuclear factor kappa-B (NF-κB)) which leads to a reduction in the level of inflammatory cytokines like tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 [19,21]. As a wide range of preclinical studies have evaluated the PPAR-α agonistic effect of PEA, the current scientific world expects PEA to be clinically equivalent to fibrates with better tolerance and a lower side effect profile [19]. Other than anti-inflammatory action, the PPAR-α agonistic activity of PEA is also responsible for its rapid analgesic action. While the PPAR-α activation-dependent transcription process might not provide rapid analgesic action because transcription is a time-consuming process, the rapid analgesic action is thought to be due to the PPAR-α activation-dependent non-transcriptional (non-genomic) activity [22]. It is hypothesized that the PPAR-α activation complex along with other proteins involved in the signaling pathway, leads to modulation of medium and large calcium channels, which might be associated with the rapid analgesic activity of PEA [22]. However, this particular assumption is too preliminary and needs to be verified by conducting intensive research.
PEA is also studied to increase the activation of the GPR-55 receptor [23]. GPR-55 is an orphan and novel cannabinoid receptor with PEA as one of its ligands. While major studies have focused the activity of PEA on PPAR-α, the exploration of the GPR-55-mediated action of PEA is still in its infancy. The GPR-55 is studied for its central effect on presynaptic terminals to modulate the intracellular calcium signaling and modulate the release of glutamate and dopamine in the hippocampus region of the brain [24]. In a proof-of-concept pre-clinical study, PEA was studied for its GPR-55 modulatory activity in the brain, where PEA-indued GPR-55 activation caused significant modulation in social interaction and recognition memory, spatial location memory, and formation of fear memory. This action of PEA was due to the hyper-dopaminergic state produced in the mesolimbic system due to the activation of GPR-55, which was characterized by an increase in the firing and bursting rate of dopaminergic neurons in the ventral tegmental area that altered the synaptic transmission rate and resulted in the above-observed effects [24]. GPR-55 is a ubiquitous receptor that is widely distributed in all the body tissues. Hence, the effect of GPR-55 in conditions including anxiety, bone development, cancer, inflammation, metabolic disturbance, and pain modulation has been extensively studied. However, the effect of PEA in all above-stated conditions (due to its GPR-55 agonist activity) needs to be confirmed [23]. GPR-119, initially cloned as an orphan receptor, is predominantly expressed in the pancreatic cells and the enteroendocrine cells [23,25]. As GPR-119 activation is associated with the release of incretin hormones, including GLP-1, extensive research is currently being carried out for the development of ligands that specifically target activation of GPR-119; and thereby, show positive effects in various chronic metabolic conditions, including type 2 diabetes and fatty liver disease conditions [25,26]. As with other fatty acid amides, PEA is also an endogenous ligand that increases the activity of GPR-119, and hence it is plausible that the use of PEA might show improvement in diabetes, metabolic syndrome, and fatty liver disease conditions, which is an interesting matter of research [25,26].
TRPV-1, also known as capsaicin receptor, is a voltage-gated ion channel belonging to a subfamily of TRP channels. TRPV-1 is a non-selective ion channel that gets stimulated by both physical and mechanical stimuli as well as both exogenous and endogenous ligands [27]. As the TRPV-1 channels are predominantly found in the dorsal root ganglia and sensory nerve fibers of the Aδ and C-type, the modulation of TRPV-1 is extensively studied as a target for pain transmission, inflammation, and neurotoxic activity [27]. The activation of TRPV-1 is associated with a series of phosphorylation reactions that open the calcium channels and increase the intracellular calcium level. The high intracellular calcium conversely increases the level of calmodulin or calcineurin, a calcium-dependent phosphatase, which in turn dephosphorylates the TRPV-1 channel (a process known as desensitization) and causes the inactivation of the TRPV-1 channel and thus shows anti-nociceptive and anti-inflammatory actions [27]. While PEA directly targets the PPAR-α, GPR-55, and GPR-119, it activates and desensitizes the TRPV-1 channel via an indirect mechanism known as the “entourage effect”. In simple terms, PEA being conformational similar to other fatty acid amides, PEA saturates the endocannabinoid-degrading enzymes (majorly FAAH and NAAA); and thereby, increases the level of other fatty acid amides. Among the fatty acids, the AEA and 2-AG are direct activators of TRPV-1 channels, and in this way, PEA is indirectly able to activate the TRPV-1 activity [27]. Additionally, the activation of PPAR-α by PEA increases the level of PPAR-α-mediated protein transcription, which further causes TRPV-1 activation and desensitization [28]. The entourage effect of PEA is also responsible for the activation of CB1 and CB2 receptors [27].
PEA: Role in Diabetic Nephropathy
As fibrates are a class of medications that have proven renal protective effect by stimulating the activity of PPAR-α and previously knowing the fact that the EC system is widely present in renal tissues, it can be postulated that the administration of PEA can positively modulate the renal physiological state and help in improving the overall renal health as well. While the number of clinical evidences to support this hypothesis is less, numerous experimental studies have supported the protective and therapeutic efficacy of PEA in renal pathological conditions. In an ischemia/reperfusion (I/R)-induced renal damage experimental model, mice were treated with PEA and/or silymarin before the induction of injury [29]. The combination of PEA with silymarin significantly prevented the I/R-mediated neutrophil infiltration and associated renal damage while reducing the level of pro-inflammatory cytokines. Similarly, the combination significantly improved the endogenous antioxidant potential and reduced the level of oxidative stress and associated lipid peroxidation level, demonstrating the anti-inflammatory and antioxidant potential of PEA. Immunohistological analysis revealed that PEA significantly attenuated the NF-κB signaling pathway, reduced the expression of pro-apoptotic proteins, and improved the expression of anti-apoptotic proteins, thereby leading to the anti-inflammatory and renal protective efficacy [29]. These beneficial effects might be due to the potent PPAR-α agonistic activity of PEA [8]. To support the hypothesis that PPAR-α activation is responsible for the PEA’s renal protective effect, another set of I/R-induced renal damage experimental studies were conducted by using wild-type and PPAR-α knockout (PPAR-αKO) mice [30]. Supplementation of PEA before inducing renal damage significantly prevented the I/R-induced renal damage, reduced the level of inflammatory cytokines and oxidative stress, and improved the antioxidant biomarkers. This renal protective effect of PEA was observed to be significantly attenuated in PPAR-αKO mice, which supports the hypothesis that PPAR-α activation by PEA is one of the major pathways responsible for its renal protective effect [30]. In another set of experiments involving spontaneously hypertensive rats (SHR), PEA therapy was associated with a significant reduction in blood pressure [31]. It was observed that PEA supplementation was associated with a significant reduction in renal oxidative stress and inflammatory markers, which in turn improved the overall angiotensin-1/angiotensin-2 balance, leading to improvement in the renin-angiotensin-aldosterone system activity, the system responsible for maintaining blood volume and blood pressure, and hence improved overall renal hemodynamics [31]. Such results underscore that PEA is able to positively modulate renal health by mechanisms other than PPAR-α activation as well. Similarly, in a contrast-induced nephropathy experimental study, PEA administration significantly prevented the glomerular dysfunction and renal parenchymal damage, improved the serum sodium and potassium levels, reduced inflammatory biomarkers, and thus prevented contrast-induced renal damage [32].
Concluding Remarks
The presence of endocannabinoid system components in renal tissue supports the notion that the endocannabinoid system might directly influence the overall renal physiology, while they can also serve as potential targets in various renal disease conditions, including diabetic nephropathy as well. Palmitoylethanolamide is an endogenous lipid mediator that is proven to modulate the activity of the endocannabinoid system and supported by clinical evidence for its effectiveness in various inflammatory painful conditions as well. PEA is sought to have a protective effect on renal tissues, primarily due to its potent anti-inflammatory and antioxidant effects. The current review study supports the use of PEA in inflammatory conditions, while the need for well-designed clinical studies involving patients with renal impairment is also warranted.
Declarations
Conflict of interest
All authors are employees of Sundyota Numandis Probioceuticals Pvt. Ltd. The employer has no role either during conducting the study or in the manuscript publication process.
Funding
No funding was received.
References
2. Dubey V, Kansagra J, Sureja V, Kheni D. Efficacy evaluation of Berberis aristata and Silybum marianum fixed dose combination on glycaemic and insulin resistance parameters in adult population: a systematic review and meta-analysis of randomized controlled trials. Future Journal of Pharmaceutical Sciences. 2024 Feb 27;10(1):28.
3. Jeong D, Mok J, Jeon D, Kang HY, Kim HJ, Kim HS, et al. Prevalence and associated factors of diabetes mellitus among patients with tuberculosis in South Korea from 2011 to 2018: a nationwide cohort study. BMJ Open. 2023 Mar 8;13(3):e069642.
4. Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020 Sep 8;10(1):14790.
5. Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020 Apr;162:108086.
6. Samsu N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed Res Int. 2021 Jul 8;2021:1497449.
7. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond). 2013 Feb;124(3):139-52.
8. Impellizzeri D, Esposito E, Attley J, Cuzzocrea S. Targeting inflammation: New therapeutic approaches in chronic kidney disease (CKD). Pharmacol Res. 2014;81:91-102.
9. Lowe H, Toyang N, Steele B, Bryant J, Ngwa W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int J Mol Sci. 2021 Aug 31;22(17):9472.
10. Joshi N, Onaivi ES. Endocannabinoid System Components: Overview and Tissue Distribution. Adv Exp Med Biol. 2019;1162:1-12.
11. Chua JT, Argueta DA, DiPatrizio NV, Kovesdy CP, Vaziri ND, Kalantar-Zadeh K, et al. Endocannabinoid System and the Kidneys: From Renal Physiology to Injury and Disease. Cannabis Cannabinoid Res. 2019 Mar 13;4(1):10-20.
12. Tam J. The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J Basic Clin Physiol Pharmacol. 2016 May 1;27(3):267-76.
13. Ritter JK, Li G, Xia M, Boini K. Anandamide and its metabolites: what are their roles in the kidney? Front Biosci (Schol Ed). 2016 Jun 1;8(2):264-77.
14. Barutta F, Bruno G, Mastrocola R, Bellini S, Gruden G. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 2018 Aug;94(2):252-8.
15. Esposito E, Cuzzocrea S. Palmitoylethanolamide is a new possible pharmacological treatment for the inflammation associated with trauma. Mini Rev Med Chem. 2013 Feb;13(2):237-55.
16. LoVerme J, La Rana G, Russo R, Calignano A, Piomelli D. The search for the palmitoylethanolamide receptor. Life Sci. 2005 Aug 19;77(14):1685-98.
17. Davis MP, Behm B, Mehta Z, Fernandez C. The Potential Benefits of Palmitoylethanolamide in Palliation: A Qualitative Systematic Review. Am J Hosp Palliat Care. 2019 Dec;36(12):1134-54.
18. Clayton P, Hill M, Bogoda N, Subah S, Venkatesh R. Palmitoylethanolamide: A Natural Compound for Health Management. Int J Mol Sci. 2021 May 18;22(10):5305.
19. Rankin L, Fowler CJ. The Basal Pharmacology of Palmitoylethanolamide. Int J Mol Sci. 2020 Oct 26;21(21):7942.
20. Paterniti I, Impellizzeri D, Crupi R, Morabito R, Campolo M, Esposito E, et al. Molecular evidence for the involvement of PPAR-δ and PPAR-γ in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J Neuroinflammation. 2013 Feb 1;10:20.
21. Pawlak M, Lefebvre P, Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 2015 Mar;62(3):720-33.
22. Okine BN, Gaspar JC, Finn DP. PPARs and pain. Br J Pharmacol. 2019 May;176(10):1421-42.
23. Im DS. GPR119 and GPR55 as Receptors for Fatty Acid Ethanolamides, Oleoylethanolamide and Palmitoylethanolamide. Int J Mol Sci. 2021 Jan 21;22(3):1034.
24. Kramar C, Loureiro M, Renard J, Laviolette SR. Palmitoylethanolamide Modulates GPR55 Receptor Signaling in the Ventral Hippocampus to Regulate Mesolimbic Dopamine Activity, Social Interaction, and Memory Processing. Cannabis Cannabinoid Res. 2017 Jan 1;2(1):8-20.
25. Hansen HS, Rosenkilde MM, Holst JJ, Schwartz TW. GPR119 as a fat sensor. Trends Pharmacol Sci. 2012 Jul;33(7):374-81.
26. Zhao J, Zhao Y, Hu Y, Peng J. Targeting the GPR119/incretin axis: a promising new therapy for metabolic-associated fatty liver disease. Cell Mol Biol Lett. 2021 Jul 7;26(1):32.
27. Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. 2017 Jun;174(11):1349-65.
28. Ambrosino P, Soldovieri MV, Russo C, Taglialatela M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide. Br J Pharmacol. 2013 Mar;168(6):1430-44.
29. Impellizzeri D, Bruschetta G, Ahmad A, Crupi R, Siracusa R, Di Paola R, et al. Effects of palmitoylethanolamide and silymarin combination treatment in an animal model of kidney ischemia and reperfusion. Eur J Pharmacol. 2015 Sep 5;762:136-49.
30. Di Paola R, Impellizzeri D, Mondello P, Velardi E, Aloisi C, Cappellani A, et al. Palmitoylethanolamide reduces early renal dysfunction and injury caused by experimental ischemia and reperfusion in mice. Shock. 2012 Oct;38(4):356-66.
31. Mattace Raso G, Simeoli R, Russo R, Santoro A, Pirozzi C, d'Emmanuele di Villa Bianca R, et al. N-Palmitoylethanolamide protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress. Pharmacol Res. 2013 Oct;76:67-76.
32. Cordaro M, Impellizzeri D, Bruschetta G, Siracusa R, Crupi R, Di Paola R, et al. A novel protective formulation of Palmitoylethanolamide in experimental model of contrast agent induced nephropathy. Toxicol Lett. 2016 Jan 5;240(1):10-21.