Commentary
The physiology of coagulation routes and paths is a cascade of several molecular phenomena and biological events which was classified into two categories based on their phenomena i.e., intrinsic and extrinsic (Figure 1), originated separately, consisting of various factors and features such as fibrinogen, prothrombin, plasma thromboplastin, Hageman factor, Christmas factor, and Stuart-Prower factor, participate in its physiology [1].
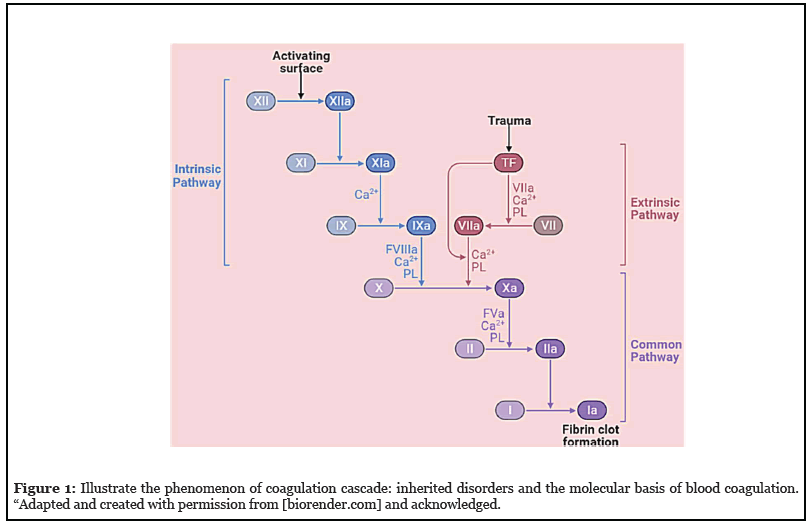
Several questions related to the coagulation mechanism come up here. However, the perfect interpretation and elucidation of the molecular phenomenon of coagulation routes and paths can answer back these aforesaid queries. The author summarizes the responses of a few scientific responses and queries out of many questions and reasons that existed in routes and paths of the coagulation pathway. A few of them are mentioned here, such as; how do platelets and factors of clotting cascade play a part in hemostasis? What is a factor specifically responsible for a potential bleeding disorder? How do laboratory tests do to differentiate coagulation disorders? What methods can be useful to do vascular changes that were observed in the abnormalities, occurred during hemostasis? What are the reasons answerable for the origin of many blood complications i.e., platelet deficiencies, extremes, and dysfunction cooperatively? and in the last, what are the why’s and wherefores inheriting in common disorders of coagulation?
By bearing in mind, the researchers so far conferred a lot about the coagulation cascade, and the above-mentioned inquiries and probes. The author envisions key steps for an accurate revision of inherited disorders and the underlying molecular phenomenon of alterations in hemostasis. These findings will be ready to lend a hand in re-countering probes that are specified during the elucidation and interpretation of the phenomenon of hemostasis [2]. In the intrinsic pathway, various dynamics opened by a variety of enzymes via various routes, events, and paths of the cellular process, and in the end, the endothelial damages happened via constituents of collagen. Every route, event, and path catalyze itself for initiating the interrelated proceedings [3]. These biochemical transformations are defined as cascades and signified at the time of partial thromboplastin. Alternatively, an extrinsic pathway is initiated when endothelial cells release tissue factors and are dignified by prothrombin time [4]. Based on physiological responses originating in the hemostasis process, paths and routes of the coagulation cascade identified by their useful physicochemical features that were transformed chemically via versatile biochemical reactions and proceedings according to the needs. Therefore, the learning of primary and secondary components of hemostasis offers numerous alternatives and possibilities about the routes, events, and paths for exploring interrelated connections for improving understanding of numerous phenomena of blood vessel injuries, bleeding, blood clotting processes, and coagulation factors.
At a glance, the foremost roots of disorders of coagulation cascade are as follows: (i) vitamin K deficiency, (ii) liver disease, and (iii) disseminated intravascular coagulation. A few other causes are Willebr and hemophilia, which are responsible for the initiation of earlier discoursed reasons, are defined as hereditary disorders of hemostasis. Moreover, the elucidation of the concept of “molecular phenomenon of alterations in hemostasis” is also helpful for elaborating the other causes and searching the facts that can be applied for making strategies for boosting the practice of endowing strategies to resolve underlined causes [5]. The above-mentioned reasons are responsible for initiating underlined disorders in the physiology of coagulation cascade, either by stimulating or regenerating the cellular responses. These findings would be applicable in the context of therapy development suggested for treating disorders of coagulation cascade as well as for defying the hereditary disorders of hemostasis, respectively [6]. Current research practices on the development of medical devices and searching remedies for dealing with the disorders of the coagulation cascade, is inadequate and even though, insufficient to alter hereditary disorders and as well impotent to diminish the root causes. Nonetheless, only a few specific therapeutics might be useful and can be painstaking for implementation.
Besides, the most difficult aspect of medical research is learning in the area of the physiology of the coagulation cascade. Overall, these discoveries help in developing novel tools for diagnosis and therapy to cure bleeding disorders when dysregulation of the coagulation cascade happened. In more or fewer circumstances, when any exposure of the interface of blood, the drugs or toxins materialized, only the clinical examinations can be proficient to determine the impact of platelet or coagulation factor on molecular phenomenon of emerged interface and can be noticed by diagnostics tools as an inherited bleeding disorder [7]. These same were noticed earlier during the clot formation, and when the activation of plasminogen initiated the breaking of fibrin. As wound healing is a complex phenomenon, and so, a need is there to balance its theory in both ways; biologically and chemically. The rate of formation of a clot or breaking down should always be well-adjusted [8]. The molecular machinery of a cell has the phenomenon of the coagulation cascade, which was associated with the molecular phenomenon of it, and always control/alterations in hemostasis and the formation of fibrin to form a clot. Any deficiency of enzyme or dysregulation in the phenomenon of coagulation cascade introduces abnormalities in its natural proceedings. As a result, it increased the rate of bleeding, and therefore, a severe deficiency occurred. Later on, these complications of clinical bleeding are noticed in clinical settings as an example [9]. The route of proteolysis manages enzyme events, for example, serine proteases activate proenzymes, and promo factors can be listed. Various other similar transformations (tissue factor and contact pathway) ultimately activate fibrin and platelets initiate the process of hemostasis for the formation of a blood clot.
Chemical conversion of prothrombin into thrombin via chemical routes initiates coagulation in the presence of a glycoprotein. The deficiency of vitamin K promotes the insufficiency of prothrombin, and finally initiates hypoprothrombinemia [10]. Endothelium secretes plasminogen to affect Willebrand factor naturally, which acts as a catalyst while activating fibrinolysis, which governs the biological process of platelet adhesion and shear-stressinduced aggregation, respectively. Collectively, various endogenous coagulation factors influence the activation of hemostasis in cerebral tissues. It will be interesting to know which biological conversion is it and how many biological routes are involved in the process when the brain answers back to minute hemostasis proceedings. The proper investigation of tangled biological routes will open new avenues for discovering newer research prospects. On the other side, inflammatory pathways participate in the routes of biochemical happenings for modulating the features of coagulation and platelet activation. These transformations and routes of biological conversion directly influence the pathology of various routes and paths such as immune responses, tissue factor activation, adhesion molecule expression, and inflammatory mediator.
Principally, these innovative insights into routes and paths applied for monitoring the physiological changes occurred in the coagulation cascade and the molecular phenomenon of alterations in hemostasis for engendering a more authentic description [11]. Based on the aforesaid justifications, the investigations of blood systems biology elucidate the diverse phenomena, for example, the inclusion of contact, fibrinolytic pathways, and activation proceedings of neutrophils and endothelial. Therefore, it was defined as omics of molecular events, which can predict the multifactorial biological responses, explore the knowledge of bleeding and clotting scenarios for a better therapeutic outcome. The author is signifying the impact of a perfect scientific interpretation on blood functioning and physiology of the coagulation cascade that can help improve various clinical practices i.e., drug target selection, biomedical device design, assessment of patientspecific disease risk, patient-specific dosing, clinical trial design, and severity.
Acknowledgments
Author, Dr. Rajiv Kumar gratefully acknowledges his elder brother Shri Shivinder Tomar for inspiring and motivating him for this research work.
References
2. Periayah MH, Halim AS, Saad AZ. Mechanism action of platelets and crucial blood coagulation pathways in hemostasis. International Journal of Hematology- Oncology and Stem Cell Research. Tehran University of Medical Sciences (TUMS); 2017 Oct 1;11(4):319-27.
3. Manzoor S, Ganie MA, Majid S, Shabir I, Kawa IA, Fatima Q, et al. Analysis of Intrinsic and Extrinsic Coagulation Pathway Factors in OCP Treated PCOS Women. Indian Journal of Clinical Biochemistry. 2020 Jun 17:1-0.
4. Vojacek JF. Should we replace the terms intrinsic and extrinsic coagulation pathways with tissue factor pathway?. Clinical and Applied Thrombosis/Hemostasis. 2017 Nov;23(8):922-7.
5. Ulrichts H, Harsfalvi J, Bene L, Matko J, Vermylen J, Ajzenberg N, et al. A monoclonal antibody directed against human von Willebrand factor induces type 2B-like alterations. Journal of Thrombosis and Haemostasis. 2004 Sep;2(9):1622-8.
6. Zhang J, Chen M, Zhao Y, Xiong H, Sneh T, Fan Y, et al. Complement and coagulation cascades pathway correlates with chemosensitivity and overall survival in patients with soft tissue sarcoma. European Journal of Pharmacology. 2020 Jul 15;879.
7. Reap L, McDonald K, Balakrishnan A, Vakhariya C. Kasabach-Merritt-like phenomenon in a massive uterine leiomyoma presenting with chronic disseminated intravascular coagulation: A case report. Case Reports in Women’s Health. 2020 Oct 1;28:e00262.
8. Shin HJ, Na HS, Jeon YT, Park HP, Nam SW, Hwang JW. The impact of irrigating fluid absorption on blood coagulation in patients undergoing transurethral resection of the prostate: A prospective observational study using rotational thromboelastometry. Medicine. 2017 Jan;96(2).
9. Asero R. Severe CSU and activation of the coagulation/ fibrinolysis system: clinical aspects. European Annals of Allergy and Clinical Immunology. 2019 Oct 8;52(1):15-7.
10. Intagliata NM, Davis JP, Caldwell SH. Coagulation pathways, hemostasis, and thrombosis in liver failure. InSeminars in Respiratory and Critical Care Medicine 2018 Oct (Vol. 39, No. 05, pp. 598-608).
11. Lippi G, Favaloro EJ, Franchini M, Guidi GC. Milestones and perspectives in coagulation and hemostasis. InSeminars in Thrombosis and Hemostasis 2009 Feb;35(1):9-22.
