Introduction
Platelet transfusion is associated with the risk of sepsis from bacterial contamination due to the need to store units at room temperature in oxygen-permeable bags. This risk associated with platelet transfusion is highest among all transfusable blood components. Measures applied in the past several decades have significantly reduced the occurrence of patient morbidity and mortality but have not eliminated it totally. Sampling of processed platelets for bacterial culture after 24 hours post-collection lowered the rate of infection but still allowed contaminated transfusions to occur due to low levels of bacteria being missed during sampling. Bacteria entering log phase growth after 24 hours may not be detected.
Bacteria can enter platelet bags via the collection process where the donor’s skin flora can contaminate catheter connection sites. Bacteria can also be endogenous to the donor’s bloodstream. Environmental bacteria can also enter the platelet storage bag via undetected leaks and improper handling during the room temperature holding period.
Whereas many bacteria in platelets can immediately enter proliferative log phase growth, they can also remain in the non-proliferative lag phase for several days and initiate log phase growth only much later during storage. Bacterial growth rates are not predictable and will vary from unit to unit depending on the initial inoculum levels, nutritional factors available for growth, anti-bacterial factors such as endogenous antibodies in the sample, and environmental conditions [1]. Late growing bacteria can be missed by culture methods applied early in the storage period.
On 30 September 2019, the US Food and Drug Administration (FDA) issued a guidance entitled “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion”. The guidance was updated in December 2020 principally to extend the compliance date to October 01, 2021 [2]. In the guidance document, the FDA identified several testing strategies that would be acceptable to ensure safety of platelet units up to 5 days or to extend the use of platelets up to 7 days.
As the deadline approaches for implementation, it is worthwhile to consider the options provided.
The FDA makes no distinctions among the options based on safety, cost, convenience, or platelet availability. In these matters, the Guidance is silent. Furthermore, the options of five- and seven-day dating should be considered separately since seven-day dating has been proven to substantially reduce outdating and lower costs [3].
For apheresis and pre-storage pooled platelets (PSPP), the Guidance provides for four options for five-day dating and four options for seven-day dating.
The five-day outdate options are:
1. A primary culture no earlier than 24 hours after collection, followed by a rapid test within 24 hours of transfusion after day 3
2. A primary culture no earlier than 24 hours after collection followed by a secondary culture no earlier than day 3
3. Large volume delayed sampling (LVDS) no earlier than 36 hours after collection
4. Pathogen reduction (not indicated for PSPP)
The seven-day outdate options are:
1. LVDS no earlier than 48 hours after collection
2. LVDS no earlier than 36 hours after collection followed by a rapid test within 24 hours of transfusion after day 5 or a secondary culture no earlier than day 4.
3. A primary culture no earlier than 24 hours after collection followed by a secondary culture no earlier than day 4
4. A primary culture no earlier than 24 hours after collection followed by a rapid test within 24 hours of transfusion after day 3
Note: Bacterial testing to extend dating beyond day 5 and up to day 7 must be performed with devices labeled as a safety measure. Platelet storage containers must be cleared or approved by FDA for 7-day storage.
We examine the relative merits and vulnerabilities of the test strategies recommended by the FDA guidance as updated in 2020.
Testing Technologies for Detection of Bacteria in Platelets
Primary culture
Primary culture has been typically conducted in the US by aseptically sampling 8 mL from a platelet bag around 24 hours after collection and inoculating the sample into an aerobic bottle only. The process is automated to colorimetrically detect the generation of carbon dioxide as bacteria grow. The Guidance stipulates a sample must be taken of at least 16 mL divided evenly into aerobic and anaerobic bottles after a 24-hour hold. A 12-hour minimum incubation is recommended. Only the mother bag must be sampled, although splits may be cultured.
Large Volume Delayed Sampling (LVDS) after no less than 36 or 48 hours
LVDS attempts to address the limitation of sampling errors inherent in the 24-hour culture test where bacteria may not be recovered due to extremely low levels. LVDS endeavors to improve recovery of bacteria by allowing bacterial growth to occur over an additional 12-24 hours. In addition, the sampling volume is doubled from 8 mL to 16 mL inoculated evenly into aerobic and anaerobic culture media. A 12-hour minimum incubation is recommended. These two measures increase the chances of detection in that the bacterial population would have increased after a longer growth period and that bacterial recovery is more efficient with the larger volume.
Pathogen reduction
Pathogen reduction is not an analytical method but a bactericidal treatment process wherein bacterial DNA are crosslinked irreversibly with psoralen (INTERCEPT™, Cerus Corporation) or riboflavin (MIRASOL®, Terumo BCT) activated by UV radiation in specialized treatment bags. After the crosslinking process is complete, the residual treatment reagents are removed (in the case of psoralen) and the PR-treated platelets transferred to a new storage bag. In current practice, there are no QC checks on the success and efficiency of the bactericidal process. In addition, PR-treated platelets are released and stored without further testing for post-treatment bacterial contamination and proliferation, on the assumption that the reduction by 5-7 logs of viable bacteria in laboratory studies is enough to ensure safety in clinical practice. Bacterial spores are not susceptible to PR treatment.
Rapid tests
The Verax Pan Genera Detection (PGD) rapid assay technology has been used as a day of transfusion “safety measure” since 2007. It is a single-use lateral flow immunoassay for the detection of Gram-positive (GP) and Gram-negative (GN) bacteria. It has recently been updated to PGDprime (Figure 1), simplifying the test procedure, improving specificity and sensitivity, and widening the breadth of bacterial detection [4]. It requires ~200 μL of sample.
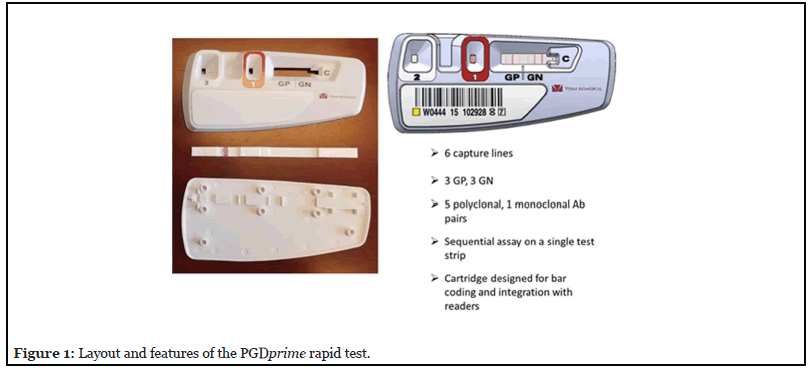
The test detects bacteria at or below their relevant concentrations in platelets associated with incipient patient sepsis (threshold of toxicity). Jacobs et al. [5] and Kundrapu et al. [6] published retrospective studies correlating CFU/mL levels of specific bacteria found in platelets and the morbidity observed in transfused patients. Minor toxicity effects were observed at 104 CFU/ mL for a few bacteria while the great majority of cases with moderate to severe toxicity were correlated with bacterial loads of 105 - 106 CFU/mL or greater.
The use of the Pan Genera rapid test on the day of transfusion has enabled interdiction of many of those platelets which primary culture at 24 hours had missed [7-9]. The rapid test can detect bacteria that may have been missed by culture at 24 hours, had contaminated the bag during storage and handling, and bacteria that initiated its log phase growth days after collection.
Review of FDA-Recommended Testing Strategies
The FDA guidance recommended four testing strategies that enable 5-day outdating for platelets and four testing strategies to allow 7-day outdating. One-step and two-step strategies are included. In the following discussion, unless otherwise noted, Bacteria A are bacteria that enter log phase growth within the first 24 hours after collection. Bacteria B are bacteria that start log phase growth sometime on Day 3. Bacteria C are bacteria that initiate log phase growth sometime on Day 5. The variability in the length of bacterial lag phase in platelets is well known [1] and is the biggest challenge for the timing of any testing strategy. For the same bacterial species, the time of initiation of log phase growth is highly dependent on the unique donor sample properties, the presence of inhibitory factors such as endogenous antibodies, the level of nutrients favorable to bacterial growth and the initial bacterial load. In addition, inter-species growth rates are also highly variable with certain species likely to initiate log phase earlier than others. In our experience, many GN bacteria would initiate log phase growth within 48 hours or less while some GP bacteria such as Staphylococcus epidermidis would often begin log phase growth well after 84-96 hours. These observations are by no means predictive for any contaminated platelets.
Five-Day Outdate Options
1. Primary Culture no earlier than 24 h after collection followed by a rapid test within 24 hours of transfusion after Day 3
Sampling volume of 16 mL divided equally in aerobic and anaerobic bottles is performed no earlier than 24 h after platelet collection. Release for transfusion up to Day 3 is allowed with a negative result after at least 12 h of incubation in culture. After Day 3, a rapid test is required 24 h or less before transfusion up to Day 5. Figure 2 shows the timeline for this testing strategy. Bacteria C may be undetected by the rapid test if they have not reached their threshold of toxicity. However, the level of bacteria should not cause sepsis in the patient [7].
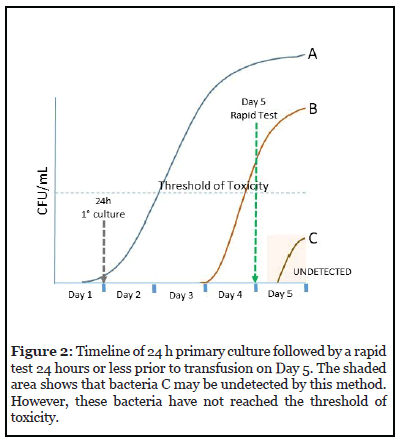
There is no need to perform a PGD Test every day. Just one test within 24 hours of transfusion suffices.
2. Primary Culture no earlier than 24 hours after collection followed by a secondary culture no earlier than Day 3.
The day 3 culture can be performed in an aerobic bottle only. If the platelet collection has been split into multiple units, each unit or pool must be tested individually.
The timeline for this testing strategy is presented in Figure 3. Even when sampling for the secondary culture occurs on Day 3, bacteria B and C may not be detected. Bacteria B may exceed the threshold of toxicity and pose a threat of sepsis to the patient.
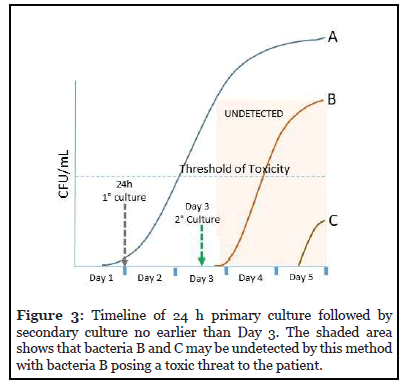
3. Large Volume Delayed Sampling (LVDS) at ≥ 36 hours.
Sampling of the platelet unit is performed no earlier than 36 hours from the time of collection. A minimum of 16 mL is sampled and inoculated evenly into aerobic and anaerobic culture media. A minimum 12-hour incubation for growth in culture is recommended. If the platelet collection has been split into multiple unit or pools, each unit must be tested individually.
Figure 4 shows the timeline for this strategy against the 3 types of bacteria. As depicted, any bacteria that initiate log phase growth after sampling (B and C) may be undetected if the sample obtained did not recover these bacteria due to their very low levels at the time of sampling. Bacteria C may not pose a threat to the patient, but Bacteria B will.
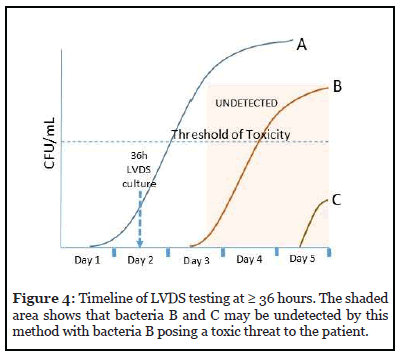
4. Pathogen reduction
This method requires pathogen reduction treatment of the entire platelet unit, typically within the first 24 hours after collection. This allows the unit to be released for distribution and used within 5 days of collection.
Figure 5 depicts this timeline and the possible effects of any post-PR treatment contamination of the PR platelet. Since no sampling or testing is required for a PR platelet, any post-treatment contamination will remain undetected. Bacteria A and B can pose a toxic threat to the patient.
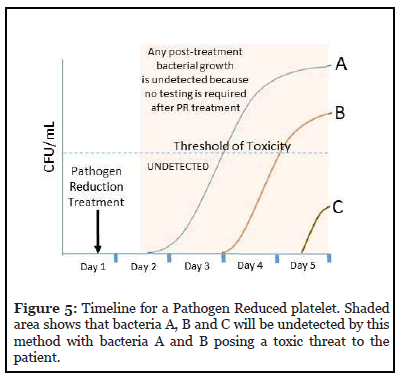
This scenario has occurred recently. One patient morbidity event [10,11] (2018) and one mortality event [12] (2020) have been recently reported due to transfused PR platelets that were contaminated by Acinetobacter and other bacteria during the holding period after PR treatment.
Seven-Day Outdate Options
1. Large Volume Delayed Sampling no earlier than 48 hours after collection in a storage container cleared or approved for 7-day storage
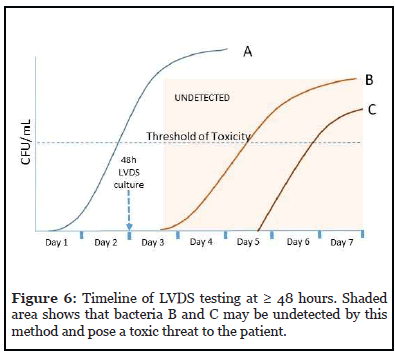
Sampling of the platelet unit is performed no sooner than 48 hours from the time of collection. A minimum of 16 mL is sampled and inoculated evenly into aerobic and anaerobic culture media. A minimum 12-hour incubation for growth in culture is recommended. If the platelet collection has been split into multiple unit or pools, each unit or pool must be tested individually.
Figure 6 shows the timeline for this strategy against the 3 types of bacteria. Any bacteria that initiate log phase growth after sampling (B and C) may be undetected if the sample obtained did not recover these bacteria due to their very low levels at the time of sampling. These can pose a toxic threat to the patient.
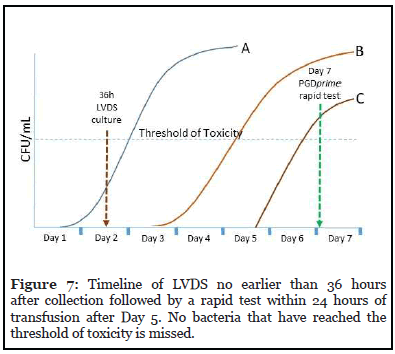
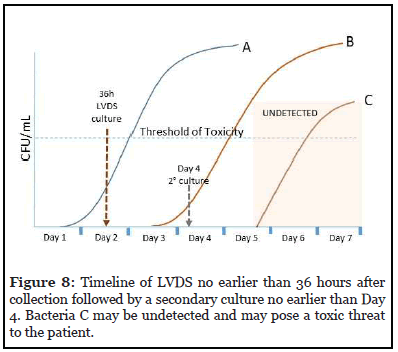
2. LVDS no earlier than 36 hours after collection followed by a rapid test within 24 hours of transfusion after Day 5 or a secondary culture no earlier than Day 4 in a storage container cleared or approved for 7-day storage.
The timelines of each option are shown in Figures 7 and 8. When a rapid test is used on the day of transfusion as shown in Figure 7, no bacteria that have reached their threshold of toxicity are undetected. However, if secondary culture is performed on Day 4 or later (Figure 8), lategrowing bacteria C may be undetected and pose a toxic threat to the patient.
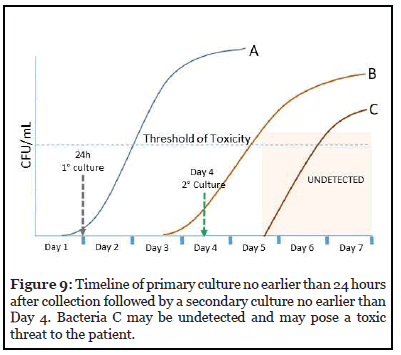
3. Primary Culture no earlier than 24 hours after collection followed by a secondary culture no earlier than Day 4 in a storage container cleared or approved for 7-day storage.
The timeline for this option is presented in Figure 9. Bacteria C which enters log phase growth on Day 5 may go undetected in this testing strategy and pose a toxic threat to the patient.
4. Primary Culture no earlier than 24 h after collection followed by a rapid test within 24 hours of transfusion after Day 3.
The use of the rapid test very close to transfusion enables detection of any bacteria that have entered log phase growth before transfusion, provided they have reached the threshold where sepsis is incipient. Figure 10 shows the timeline of this test strategy. No septic bacterial growth is missed in this test scenario. A rapid test does not need to be performed every day, only one time within 24 hours of transfusion is necessary.
Discussion
The testing strategies recommended by the FDA possess varying degrees of improvement to patient safety. As shown in the preceding review of these options, each culture procedure can miss populations of late growing bacteria. No post-storage bacterial testing is performed for pathogen-reduced platelets meaning that any bacteria that enter the storage bag after processing will likely not be detected. The use of LVDS culture at 36 hours for 5-day platelets and 48 hours for 7-day platelets shows minimal improvement at best since the potential to miss late growing bacteria still exists. FDA has not provided evidence of statistically significant enhanced bacterial detection by these methods.
The best option available is that which uses a rapid test on the day of transfusion regardless of sampling and testing performed before. Whether transfusion is performed on Day 4, Day 5, Day 6, or Day 7, any bacteria that would have grown to septic levels will be detected.
It must be noted that pathogen reduction is the only method that has not been required to perform a process efficacy check. Additionally, stored PR platelets are not sampled for bacterial contamination and growth during post-treatment storage, which can last up to the 5th day post collection (more than 4 days after PR treatment). As previously noted, this oversight has led to one morbidity event in 2018 and one mortality event in 2020. In both cases, Acinetobacter contamination was implicated.
The lone rapid test approved for use as a safety measure with platelets failed to detect Acinetobacter in a separate morbidity event in 2018. (Primary culture also did not detect the contamination in the same event.) Acinetobacter had not been included among the targets detected by the rapid test since at the time of its development, Acinetobacter had not been identified as a platelet contaminant. PGDprime has now been updated to include Acinetobacter detection [13,14]. This updated test has also been shown to detect these bacteria in PR platelets [14].
Another aspect of platelet testing is the reduction in volume of available platelets for transfusion after testing or the loss of platelet efficacy after PR treatment. Table 1 summarizes the effect on platelet quality associated with each testing method recommended in the Guidance. A relative cost comparison is also included. Table 2 lists the consumption of platelets associated with each testing strategy. For example, if primary culture or LVDS at 36 hours of three split components is followed by culture of these split products on or after day 4 then at least 96 mL will be needed for this testing.
Owing to the volume losses associated with the LVDS procedures and the guard bands imposed by the FDA in its approval of the INTERCEPT process, split rates of platelet collections that use these pathways are expected to decrease.
Effects of pathogen reduction on platelet activity
The INTERCEPT Blood System for Platelets was approved by the FDA on 18 December 2014. The Agency issued a “SUMMARY OF SAFETY AND EFFECTIVENESS DATA” to accompany this action [15]. This document reviews in detail the quantitative and qualitative degradation of platelets processed by the use of the INTERCEPT system in pivotal studies. Platelet dose at the end of storage was 7.5% lower in the INTERCEPT-treated platelets than controls. Regarding in vitro studies, FDA concluded that “in general” more INTERCEPT-treated platelet sample results were less favorable than untreated platelets. However, the mean values for all parameters tested were similar between the treated and untreated platelets. These results were considered “adequate”. Human radiolabeling studies showed significantly lower platelet recovery and survival for INTERCEPT-treated platelets compared to untreated platelets. FDA noted, “The results of the radiolabeling studies of IBS treated platelets did not meet the FDA criterion of a difference no greater than 20% of the value of the control. However, for this particular platelet product that involves a pathogen reduction process, these results were regarded as acceptable because of the potential benefit of reducing risk of transfusiontransmitted infection.” The pivotal clinical trial of treated platelets met the primary endpoint of the proportion of subjects with Grade 2 bleeding: 58.5% in the Test group compared to 57.5% in the Reference group (upper bound of the one-sided 95% confidence interval of difference 7.3%, which was within the prespecified non-inferiority margin of 12.5%) [15]. However, the outcome of many secondary endpoints in this trial demonstrated statistically significant differences against INTERCEPT-treated platelets. These included mean days between transfusions, mean days of grade 2 bleeding, mean number of platelet transfusions, and corrected count increments (and count increments) at 1- and 24-hours post-transfusion, Further, the incidence of platelet refractoriness was 21.0% versus 7.0%.
Data related to Day 4 secondary culture.
The data in the Package Inserts for clearing day 4 or later culture showed a rate of culture positivity at storage days 3 and 4 of 0.03% and at greater than day 6 of 0.14% [16,17]. This suggests that a culture on day 4 will likely miss about 80% of bacterial contaminants present on day 7.
Large volume delayed sampling
No data have been presented that demonstrate that LVDS results is a statistically significant reduction of bacterial contamination in platelets. In a simulation study, Walker et al found that LVDS at 48 hours with seven-day storage “performed relatively poorly” compared to other strategies permitted by the guidance [18].
Conclusion
The recommended test strategies for mitigating the risk of bacterial contamination of platelets and subsequent patient sepsis are not equal in efficacy, cost, product availability, and outcomes. Each approach has limitations that the blood collection establishments and transfusion services will need to consider carefully. In our analysis, any culture method within the first 4 days will still expose the patient to risk of sepsis due to late growing bacteria. Pathogen reduction, in addition to deleterious (but deemed acceptable) effects on platelet activity, is vulnerable to post-treatment contamination for which no testing is required. Bacterial risks are best mitigated by performing an FDA-cleared rapid test on the day of transfusion. It is the ultimate delayed sampling strategy.
References
2. Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion. December 2020. Guidance for Industry. Center for Biologics Evaluation and Research. Food and Drug Administration. Available at: https://www.fda.gov/ regulatory-information/search-fda-guidance-documents/ bacterial-risk-control-strategies-blood-collectionestablishments- and-transfusion-services-enhance
3. Mintz PD, Sanders JR. Outdate reduction and cost savings with rapid testing for seven-day platelet storage. Annals of Clinical & Laboratory Science. 2020 May 1;50(3):404-7.
4. Vallejo RP, Shinefeld L, LaVerda D, Best N, Lawrence G, Lousararian A, et al. Performance profile of an updated safety measure rapid assay for bacteria in platelets. Transfusion. 2020 Nov;60(11):2622-32.
5. Jacobs MR, Good CE, Lazarus HM, Yomtovian RA. Relationship between bacterial load, species virulence, and transfusion reaction with transfusion of bacterially contaminated platelets. Clinical Infectious Diseases. 2008 Apr 15;46(8):1214-20.
6. Kundrapu S, Srivastava S, Good CE, Lazarus HM, Maitta RW, Jacobs MR. Bacterial contamination and septic transfusion reaction rates associated with platelet components before and after introduction of primary culture: experience at a US Academic Medical Center 1991 through 2017. Transfusion. 2020 May;60(5):974-85.
7. Jacobs MR, Smith D, Heaton WA, Zantek ND, Good CE, PGD Study Group. Detection of bacterial contamination in prestorage culture-negative apheresis platelets on day of issue with the Pan Genera Detection test. Transfusion. 2011 Dec;51(12):2573-82.
8. Platelet PGD Test Product Insert. Population study of Leukocyte Reduced Apheresis Platelets tested as negative by growth-based methods. Rev J:6-8.
9. Mintz PD, Sanders J, Blair J, Rasmussen P. Confirmed Positive Bacterial Detection in Platelet Concentrates by a Rapid Test after Negative Primary Culture. Transfusion 2019;59:66A. AABB Annual Meeting. October 2019
10. Jones SA, Jones JM, Leung V, Nakashima AK, Oakeson KF, Smith AR, et al. Sepsis attributed to bacterial contamination of platelets associated with a potential common source—multiple states, 2018. Morbidity and Mortality Weekly Report. 2019 Jun 14;68(23):519-23.
11. Fridey JL, Stramer SL, Nambiar A, Moayeri M, Bakkour S, Langelier C, et al. Sepsis from an apheresis platelet contaminated with Acinetobacter calcoaceticus/ baumannii complex bacteria and Staphylococcus saprophyticus after pathogen reduction. Transfusion. 2020 Sep;60(9):1960-9.
12. Fadeyi EA, Wagner SJ, Goldberg C, Lu T, Young P, Bringmann PW, et al. Fatal sepsis associated with a storage container leak permitting platelet contamination with environmental bacteria after pathogen reduction. Transfusion. 2021 Feb;61(2):641-8.
13. LaVerda D, Shinefeld L, Best N, Lisitu J, Tambolleo G, Vallejo R. Updating the Platelet PGDprime Rapid Test (R) for Bacteria in Platelets to Detect Acinetobacter Strains. In Transfusion 2020 Sep 1 (Vol. 60, pp. 73A-74A). 111 RIVER ST, HOBOKEN 07030-5774, NJ USA: WILEY.
14. LaVerda D, Shinefeld L, Best N, et al. Evaluation of an Improved Rapid Bacterial Assay with Untreated and Pathogen-Reduced Platelets: Detection of Acinetobacter Strains (submitted for publication, Transfusion, 2021).
15. Summary of Safety And Effectiveness Data (SSED). December 2014. Center for Biologics Evaluation and Research. Food and Drug Administration. Available at: https://wayback.archive-it.org/7993/20190425015306/ https://www.fda.gov/downloads/BiologicsBloodVaccines /BloodBloodProducts/ApprovedProducts/Premarket ApprovalsPMAs/UCM431243.pdf
16. BK170142 Package Insert BacT/ALERT BPA. Available at https://www.fda.gov/media/111052/download
17. BK170142 Package Insert BacT/ALERT BPN. Available at:https://www.fda.gov/media/111061/download
18. Walker BS, Schmidt RL, Fisher MA, White SK, Blaylock RC, Metcalf RA. The comparative safety of bacterial risk control strategies for platelet components: a simulation study. Transfusion. 2020 Aug;60(8):1723-31.
