Abstract
Introduction and objective: Growing multidisciplinary field of tissue engineering aims to regenerate, enhance, or replace predictably damaged or missing tissues. We carried out a prospective study with equine blood for the evaluation of specific scientific techniques of second-generation platelet concentrates in terms of Weight, Length, Width, Thickness, Surface in clots and membranes of L-PRF and A-PRF so that they are readily available and relatively easy to use in the daily clinical routine.
Methods: Horse blood, which was collected in anticoagulant-free PET tubes with silica for clot production and L-PRF membranes and in glass tubes for clots and A-PRF membranes in six healthy horses. Membranes and the clots produced were examined.
Results: Horse L-PRF/A-PRF membranes produced at the time Ø after centrifugation are made up of 66% red blood cells, 1.56% white blood cells and 32% platelets. Average morphological characteristics (± D.S.) detected among the various types of clots and membranes are: Weight of the clot gr. 3.52 (± 0.64); Exudate weight gr. 2.54 (± 0.48); Membrane weight gr. 0.84 (± 0.15); Coagulum length 40.8 mm (± 4.40); Clot width mm 13.89 (± 1.18); Coagulum thickness mm 6.48 (± 0.92); Coagulum surface mm2 48.64 (± 5.65); Membrane length 34.37 mm (± 3.79); Clot width mm 11.55 (± 1.58); Coagulum thickness mm 2.78 (± 0.38); Coagulum surface mm2 35.78 (± 5.67); Clot/Blood Sample weight ratio 10ml% 22.15 (± 2.20). Most useful values in clinical terms are those obtained in the A-PRF of the clot surface 53.94 (± 14.39) mm2 p=0.001 <0.05 and of the membrane surface 43.52 (± 8.68) mm2 p=0.560> 0.05.
Conclusion: Our studio has tried to standardize the L-PRF/A-PRF preparation procedure, which while remaining easy to perform and low-cost technique, does not require specialized equipment and has a certain consistency in the production of an L-PRF membrane/A-PRF in terms of macroscopic and microscopic characteristics.
Keywords
Blood derivatives; Growth factors; Leucocyte and platelets-rich fibrin; Advanced platelets-rich fibrin; L-PRF wound box; Stem cells
Introduction
Platelet preparations (PDPs) have gained success, mainly due to their high concentrations of biologically active molecules, such as growth factors and cytokines, which play an important role in tissue repair and reconstruction. Recent knowledge shows that platelets can play a new role in tissue reproduction and vascular restoration, as well as being the protagonists of inflammatory processes and immune system responses. They release bio-active proteins and other active ingredients that can affect a number of phenomena that promote cell consumption, growth and transformation (growth factors). These substances are released or presented on the surface of activated platelets. The capability of platelets to release agents from within a coagulum makes the latter a natural autologous source of growth factors and cytokines that can be used therapeutically to accelerate physiological healing. Many of these substances are accumulated in the α-granules easily identifiable with the Electronic Microscope (SEM) and with immunofluorescence staining. The fine fibrin fibers contained in the Human Platelet Concentrate (HPC) could be related to the initial high platelet concentration in the HPC (3-5×1011 platelets/l), in which local procoagulant activity can also be enhanced with the initiation of ‘amplification of the prothrombotic stimulus, it leads to an almost explosive production of thrombin with a consequent increase in fibrogenesis on the surface of the platelets with consequent formation of fibrin and its polymerization [1].
The introduction of blood concentrates such as Platelet Rich Plasma and Fibrin Rich Plasma (PRP/PRF) was the first step towards achieving clinical (Figure 1) and biotechnological criteria [2]. This blood concentrate (PRF) is obtained from the patient’s peripheral blood after a single centrifugation without anticoagulants to obtain a matrix containing platelets and leukocytes. The availability of platelets, leukocytes and fibrin has been shown in the past where the need to heal the wound has been shown to be critical [3,4]. In supplement to the power of leukocytes to affect angiogenesis and lymphogenesis, this flexible 3D fibrin grid, including leukocytes and platelets, is a resource of cytokines and growth factors [5]. The use of specific A-P (A-PRF) glass tubes promotes the non-coagulation of PRF and has led to the subsequent development of a PRF-based liquid matrix (i-PRF). In the past, methodological studies have analyzed the influence of relative centrifugal force exerted (RCF) on the constitution and bioactivity of PRF matrices [6]. This fibrin tower, which has no cytotoxic value, is obtained from 9 ml of blood of subjects examined after 1 stage of centrifugation and includes a variety of blood cells - including platelets, B and T lymphocytes, monocytes, stem cells, and granulocytes neutrophils - as well as various growth factors. L-PRF (also called leukocytes-PRF) is the progenitor of the second generation platelet concentrates; it also contains leukocytes, cells that are important during the wound-healing procedure [7]. Considering also that leukocytes, including neutrophils and macrophages, are among the main types of cells found in wound sites, their contribution also concerns the removal of phagocytic fragments, bacteria, and necrotic tissue, thus preventing infection. Macrophages are also key cells derived from the myeloid lineage and are considered to be one of the key cells involved in the secretion of growth factors during injury cicatrization, comprising the transformative beta growth factor (TGF-β), PDGF (platelet growth factor) and vascular endothelial growth factor (VEGF). Such cells, in combination with neutrophils and platelets, and their secreted/cytokine growth factors, can stimulate tissue reconstruction, the formation of new blood vessels (angiogenesis), and infection prophylaxis.
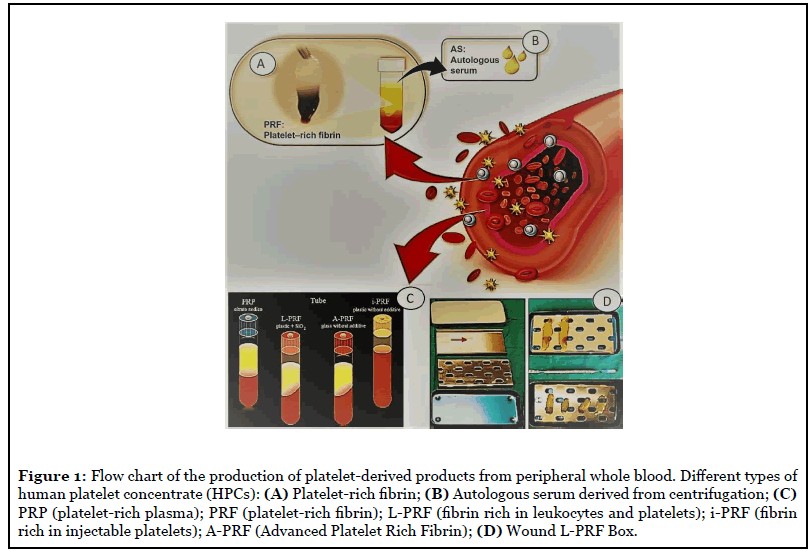
Various types of second generation concentrates have been produced:
-L-PRF: In the longitudinal section of the L-PRF clot, produced according to the standard centrifugation protocol (30” acceleration, 2’ at 2700 rpm, 4’ at 2400 rpm, 3’ at 3000 rpm, and 36” slowdown and stop) [8], is in the presence of a compact fibrin thrombus with reduced inter-fiber space. Cells are observed throughout the clot, although decreasing towards the more distal parts of the PRF clot (Figures 1 and 2);
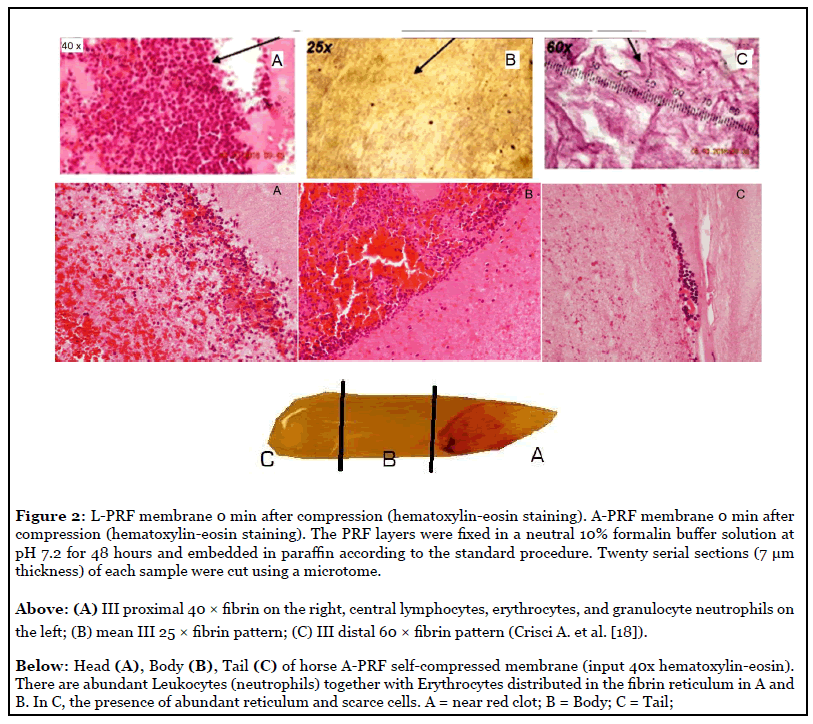
-Advanced-PRF: PRF clots formed with the Advanced- PRF (A-PRF) centrifugation protocol (1300 rpm, 8 minutes) (189 g-forces) [9] showed a looser structure with more interfiber space and more cells could be counted in the fibrin-rich thrombus. In addition, the cells are more evenly distributed in the clot. than L-PRF, and some cells can also be found in the more distal parts of the clot. Figure 3 shows a representation of the distribution of cells within the A-PRF;
- PRF injectable preparation: the development of the PRF injectable formulation (called i-PRF) [10,11] (centrifuged at 700 rpm [60 g-forces] for 3 minutes) was pursued to deliver an easy platelet concentrate to healthcare professionals to be used in a liquid formulation, which can be used alone or easily combined with various biomaterials. Benefiting from the lower and shorter centrifugation speed, more cells can be regenerated with higher concentrations of growth factors than other types of PRFs using higher centrifugation speeds.
Ghanaati et al. [9] reported that rate and time do not affect monocyte and stem cell concentrations, but do affect platelet and neutrophil concentrations. As a consequence, A-PRF has more platelets, a higher amount was found in the final part of the PRF membrane, and more neutrophils are present. This typology of compound has the advantage of increasing angiogenesis using the enzymatic structure of metalloproteinase-9. Consequently, the presence of neutrophils in PRF could be considered if angiogenesis is of particular interest.
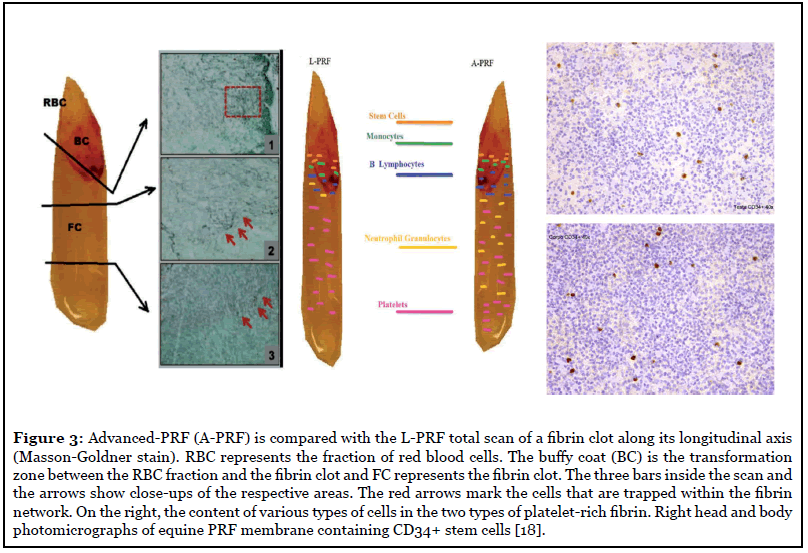
Analyzing the development of the study in Ghanaati et al., it also appeared that platelets were the only ones present in each area of thrombus up to 87 ± 13% in the L-PRF group and up to 84 ± 16% in the A-PRF group. In addition, tests indicated that T lymphocytes (L-PRF: 12 ± 5%, A-PRF: 17 ± 9%), B lymphocytes (L-PRF: 14 ± 7%, A-PRF: 12 ± 9%), CD34-positive stem cells (L-PRF: 17 ± 6%, A-PRF: 21 ± 11%) and monocytes (L-PRF: 19 ± 9%, A-PRF: 22 ± 8%) were not detected beyond a certain distance, at most 30% of the total area of the clot, since they are distributed in proximity to the BC generated by the centrifugation process.
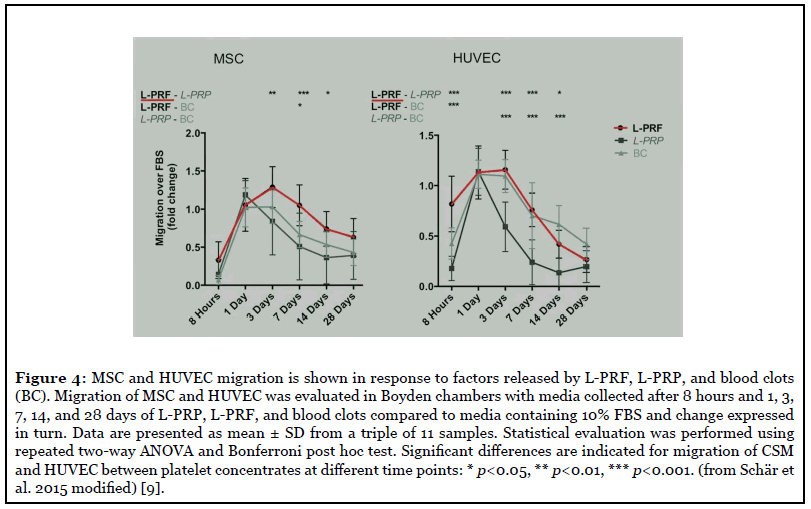
Stamina cells: Recently, an L-PRF has been analyzed by the authors, which appears to contain hematopoietic stem cells (HSC) [12]. The presence of these HSC cells is mainly determined by immunohistochemical analysis for the detection of specific CD34 cell markers. Hematopoietic stem cells were obtained without mobilization from equine peripheral blood (Figure 4), CPE endothelial progenitor cells were observed in the generation of new vessels in the endometrium after ovulation, in the vessels of colon tumors, and skin wounds. In this model, they induced significant vasculogenesis . [13] Cellular mobility plays a crucial role in the successful wound healing procedure. Furthermore, MSCs represent a pool of stem cells, able to reconstruct damaged tissue, and endothelial cells contribute to angiogenesis. The migration phenomena induced by the supernatant of platelet concentrates are not different between the two cell types. The increased migration of MSC and HUVEC has been recorded as a consequence of L-PRF (Figure 5). This entire means that L-PRF could be useful as a curative biomaterial and as a natural antihemorrhagic agent for use in surgical sites [12-13].
Materials and Methods
Interpretation of platelets in the horse
Platelets release multiple growth factors capable of regulating growth and endothelial division cells and fibroblasts. In this way, among other things, angiogenesis and tissue regeneration are favored. In equines, platelets are round, oval, or elongated, measuring 2.5-3.5 μm in diameter, and have light blue cytoplasm with fine bluegreen granules. Under normal conditions the survival time of equine blood platelets is 5-9 days. Horse platelets are lightly colored with Wright-Giemsa and are therefore difficult to identify in blood smears. However, the platelets are well stained with Diff-Quick® dye. Giant platelets are morphologically larger than the diameter of an RBC and are associated with accelerated thrombocytopoiesis. In automated platelet counts, they artificially decrease if platelet aggregates are formed. Pseudopodia can be observed on activated platelets [11]. Equine platelet concentrations are among the lowest reported. The platelet concentration in a normal horse averaged about 6-10 platelets per field in oil immersion (100 × objectives). Normal values for equine platelets vary between 100 and 350,000/μL. The increase in MPV alone usually coincides with a greater release of large or giant platelets and is indicative of a platelet regenerative response. PCT represents better the total number of functional platelets than the platelet count. Repeated venipuncture, changes in blood flow, or delay in performing the analysis alters the platelet count. Processing temperature and storage conditions also have an important correlation with significant effects in platelet aggregation. It is advisable to perform the analysis within the first 2 hours after collection because the MPV can be modified if the EDTA samples are kept refrigerated. According to age, the numbers of platelets in foals do not change during the first year of life, but age determines their progressive decrease. Both short and maximum exercise produces a significant increase in platelet counts. However, moderate exercise does not seem to alter it. Furthermore, in response to high-intensity exercise, studies have reported a reduction in the aggregation capacity of platelets [11].
Preparation of L-PRF
The L-PRF® preparation protocol in humans and animals is very simple: the blood is immediately centrifuged within 2 minutes of collection following the following programming: 30” acceleration, 8 min at 2700 rpm (816 g-forces) and 36” of deceleration and stop. The resulting product consists of three layers: PPP (Platelet-free Plasma at the top), PRF (Clot at the center), Red Blood Cells (RBC) at the bottom, with a Duo centrifuge (Process for PRF, France) which has an off the rotor by 41.3° (Figures 1 and 3). The resulting PRF clots are collected and part of the red clot is removed with scissors without any macroscopic damage to the PRF [7,13].
When the preparation protocols for the preparation of the PRF are described, very often there are no indications of important parameters (for example, the volume of anticoagulant used, the volume of blood collected and final PDP reached, working temperature, the time between collection/processing/sample analysis), which leads to questionable and not comparable analyzes.
We carried out a prospective study with equine blood for the evaluation of specific scientific techniques of the second generation platelet concentrates in terms of Weight, Length, Width, Thickness, Surface in clots and membranes of L-PRF and A-PRF so that they are readily available and relatively easy to use in the daily clinical routine.
The blood was collected in tubes without anticoagulant in PET with silica, nor a separator gel (tubes Vacuaptaca clot activator code 30023 for serum 9.0 ml) and glass tubes without anticoagulant, nor separator gel (AP tubes for A-PRF Vacutainer for Serum 9.0 ml), for the formation of thrombus and L-PRF membranes in six healthy horses of various ages (average ± SD, 10 ± 4.1 years, range: 4 - 17 years), gender and breed.,
The use of the Vacutainer is determined by the fact that the use of a syringe does not produce a usable clot, as constant suction of the venous blood is necessary [18]. Written owner consent was required for all horses and the blood collection procedure was performed in accordance with current ARRIVE (Animal Research: Reporting of in Vivo Experiments) guidelines.
Blood was quickly collected with a 19 G needle into the 9cc Vacutainer tubes with jacket system (average withdrawal rate of 22”, less than 25” per tube) and immediately (within 1 minute) centrifuged according to the above description at a temperature above 21°C. Tubes for producing clots and L-PRF membranes and tubes for producing clots and A-PRF membranes at an ambient temperature greater than 21°C (21-30°C) were used. The processing temperature adopted 21.3/22.5°C.
Fibrinogen is initially concentrated in the middle and top of the tube, right between the red blood cells at the bottom (RBC) and the acellulated plasma at the top (PPP). Thrombus compression with a metal compression system (L-PRF box) stimulates cell proliferation and the production of new vessels more effectively [14].
In addition to standard compositions, PRFs can also be obtained in an inoculable form (i-PRF). The i-PRF is obtained by producing a PRF, which is not subsequently pressed. Fortunately, this inoculable material can coagulate quickly (within 10-12 minutes) after injection to form a biocompound and can be combined with any biological substance of choice for non-covalent incorporation [15].
The L-PRF and A-PRF in this experiment were divided into 3 regions of equal length (Figures 2 and 3) and the presence of platelets in each region was observed in the horse under the Optical Microscope at various magnifications [16-19].
Zone 1 is the one closest to the red thrombus (head) and has numerous attached platelets; there are some lymphocytes and other white blood cells. The number of platelets decreases with increasing distance from the red clot. In region 2, (body) there are fibrin fibers (primary and secondary) and some platelets. In region 3 (tail) the fibrin reticulum is very evident, while platelets are scarce (Figure 2).
From a scientific point of view, L-PRF and its derived products (A-PRF, A-PRF+) have excellent handling properties: the individual L-PRF clots are converted into films of suitable size and thickness, thanks to the “L-PRF Wound Box”; several membranes can be joined together (even sutured) and will serve to create a larger bioactive membrane to cover and build larger grafts. The PRF membrane can be cut to size and being flexible it adapts well to different anatomical areas.
The L-PRF/A-PRF family, therefore, adapts to the needs of various surgical interventions. Like thrombi and membranes, PRF has a size and shape that is easy to combine with most surgical techniques, such as filling and insertion of repair biomaterials or as protective membranes for wound healing. Finally, it is easy to prepare in large quantities and inexpensive, making it particularly suitable for daily clinical practice. It has been used successfully in humans by the AA. in particular in the treatment of diabetic skin ulcers, even with osteomyelitis [20]. The cold storage of clots and membranes has led to the impoverishment of the biological and physical characteristics of the product.
The ideal minimum temperature for storage for a few hours is +4°C.
In this experimental design, the number of samples may seem a bit low, but the study objectives emphasized a natural mechanism, so maximizing statistical significance was not necessary.
Macroscopic analysis
After spinning, the L-PRF and A-PRF thrombi were extracted from the tube with sterile forceps and a smooth paddle or equally sterile scissors to softly release the red clot from the buffy coat (BC). Each obtained L-PRF/APRF fibrin clot was placed in a tray to measure weight and size with a goldsmith’s digital scale and digital caliper three times by three different operators and averaging the three measurements. The compression of the clot was performed with the L-PRF Wound Box we designed with a constant pressure of 142.437 Pa/cm2 for two minutes (compressions of longer duration were not used as they did not prove useful in our previous study compared to the two-minute one) [19]. The L-PRF/A-PRF membranes were photographed with a digital camera (Nikon D5000 12 megapixel) to calculate the surface in cm2 according to the method we have reported in the literature [13]. The surface in cm2 of clots and membranes were measured with the “Calcderm” area measurement software designed by us [13], but any software for measuring the surface of an image can be used (for example IC Measure 2.0 .0.133 or other).
Procedure for light microscopy
The membranes obtained at Ø’ from centrifugation were fixed in 10% neutral buffered formalin for 24 h at room temperature for inclusion in paraffin. Subsequent 4 μm sections were performed along the center along the longitudinal axis of the membranes and stained with Hematoxylin-Eosin. Each section was divided into three areas of equal size: Proximal (head), Center (body), Distal (tail). Each section was examined under the light microscope (Kern OBN-148) and evaluated by the visible cell body count (shown in dark purple, mostly leukocytes) in the center of each area observed at a magnification of 25×, 40×, 60×, 100× (immersion). The total number of cell bodies counted was used to correlate their distribution between the three areas of the membrane (head, body, and tail). We suppose that, unlike L-PRF where the majority of the cells are grouped in the area adjacent to the head, closer to the red thrombus, in A-PRF the cellular breakdown is more homogeneous, to the point of being able to use the membrane in all its length in the clinical setting.
The haematological smears prepared from the blood left in the tubes were also examined for morphological verification, after the removal of the thrombus of PRF, with a paddle (two for each tube) and fixed with alcohol at 90° for the May-Grunwald-Giemsa dye to identify the various corpuscular components, in particular the platelets and neutrophils, and relate them to the blood count.
A blood sample was also taken from each horse to perform a blood count using K3E 5.4 mg EDTA tubes (VacuMed).
The supernatant was derived from the compression of the L-PRF and A-PRF membranes with the PRF-Box and was stored in a tube with K3E 5.4 mg EDTA for blood analysis and a blood count was performed on it for the evaluation of the corpuscular elements. It was comparative with the basic and corpuscular line of counts carried out on smears from red thrombus as an indirect measure of the amount of leukocytes and platelets of L-PRF and A-PRF. The supernatants derived from the pressing of L-PRF and A-PRF at Ø’ were analyzed with a blood count in standard housings.
Since the direct measurement of the quantity of PRF platelets is not yet possible, we have calculated the residual platelet concentration using the reduction method according to the following equation from Watanabe et al. 2017 [21]:
PLT / WBC in A-PRF and L-PRF = PLT / WBC in whole blood - [(PLT / WBC in red clot) - (PLT / WBC in serum above PRF clot) - (PLT / WBC in supernatant after compression of the PRF clot)]
Also in a study, the AA [22] obtained that, by reducing the platelet count value from the blood count by 15.12% and the leukocyte count value by 34.12%, the value obtained with the t-PA digestion method is obtained with a much simpler system. In this study, they validated a simple and inexpensive system for calculating the precise number of platelets and leukocytes present in self-compressed solid platelet concentrates. This study has shown that it is possible to quantify the number of cells in biomaterials, a complex cell system for the presence of platelets, leukocytes, stem cells, etc., using a clinical method that can be applied quickly (max 15 minutes) and safely (Statistical Method).
The blood chromatometric examination was conducted with a Cell Dyn 3500 R (ABBOTT) cell counter.
Statistical analysis
Statistical significance for differences between groups was calculated with Student’s t-test for repeated measures for parametric variables and with χ2 for non-parametric variables. Each p-value <0.005 was considered statistically significant.
Data were analyzed using version 6.0 of the Santon- Glantz 2007 Statistics for Biomedical Disciplines package.
Results
The procedure was well tolerated in all animals tested.
No significant differences were found in the baseline hematology comparison which had a mean RBC concentration of 7.6 × 106/mL (± 1.1 95% CI) (p = 0.34) (range: 7-13 × 106/mL), of WBC of 5.1 × 103/mL (± 0.37 95% CI) (p = 0.24) (range: 5-13 × 103/mL) and a mean platelet count of 106.8 × 103/mL (± 15.3 95% CI) (p = 0.15) (range: 100-350 × 103/mL). As it is not yet possible to directly quantify the platelet and WBC concentration trapped inside the L-PRF/A-PRF clot, it has been derived indirectly by making a comparison between the mean values of whole blood, the average values on the supernatant obtained after compression of the clot at 2’ and the average values obtained by counting on red clot smear after removal of the L-PRF clot. The values were also compared with the Statistical Method of Count [22].
Table 1 reports the number of leukocytes, red blood cells, and platelets in human whole blood (control group) and in the red clot after collection of the PRF membrane (both L-PRF and A-PRF) (test group) responsible for therapeutic enhancement [23].
| Leukocytes/µl | RBC/µl | Platelets/µl | ||||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |
| Control | 6.900 | 6.100-7.800 | 5.19 (106) | 5.01-5.52 (106) | 2.66 (105) | 2.18-3.09 (105) |
| Group 1 | 3.500 | 3.000-3.800 | 5.89 (106) | 5.75-6.08 (106) | 6.0 (103) | 4.0-80(103) |
| Group 2 | 3.600 | 3.300-4.000 | 5.84 (106) | 5.78-5.91 (106) | 7.0 (103) | 6.0-90(103) |
Table 1: Leukocytes, RBCs, and platelets are present in human whole blood (control group) and red clots after collection of the PRF membrane (test group).
Table 2 and Figure 4 compare the characteristics of the clots and L-PRF membranes obtained in humans (Intraspin centrifuge) reported by Pinto and coll., [24] and those observed by us in the horse (DUO centrifuge) with the use of various types of test tubes (A-P in glass, Vacuaptaca the coated PET). In this comparison, it was first verified that there are significant differences in the characteristics of the clot (weight), but these differences cancel out when the membranes derived after compression are examined. In our opinion, this difference should be attributed to different exudate content (weight of exudate =1.47 ± 0.13 g in humans, 3.05 ± 0.11 g in horses, p=0.000).
| Man(n=8) L-PRF |
Horse(n=6) L-PRF/A-PRF |
Significance test | ||
|---|---|---|---|---|
| Variable | Mean (± SD) | t-test Student | Significance | |
| Final T° of the tube (°C) | 27.5 (± 0.66) | |||
| Coagulum weight (g)* | 2.09 (± 0.19) | 4.23 (± 0.55) | P=0.000<0.005 | S |
| Membrane Weight (g) | 0.62 (± 0.15) | 0.78 (± 0.08) | P=0.036>0.005 | NS |
| Exudate Weight (g) | 1.47 (± 0.13) | 3.05 (± 0.11) | P=0.000<0.005 | S |
| Clot Length (mm) | 35.69 (± 3.43) | 44.38 (± 3.83) | P=0.000<0.005 | S |
| Clot Width (mm) | 12.81 (± 0.75) | 14.74 (± 1.23) | P=0.003<0.005 | S |
| Clot Height (mm) | 7.02 (± 1.09) | |||
| Clot Surface (mm2) | 4.10 (± 0.86) | |||
| Membrane Length (mm) | 34.81 (± 2.95) | 36.81 (± 3.18) X | P=0.248>0.005 | NS |
| Membrane Width (mm) | 12.25 (± 0.71) | 13.02 (± 1.01) X | P=0.119>0.005 | NS |
| Membrane Height (mm) | 3.02 (± 0.51) X | |||
| Membrane Surface (mm2) | 3.08 (± 0.5) X | |||
| Weight ratio Coagulo/ blood sample (%) 10ml | 20.94 (± 2.4) | 32.53 (± 0.54) | P=0.000<0.005 | S |
*The difference in Coagulum Weight is due to a difference in exudate content; Average values (± D.S.) after 2' of compression at 30°C.; N.B.: values are not related to Hb and Erythrocytes content in whole blood.
Table 2: Comparison between membranes and PRF clots obtained from human blood [24] and equine blood [12].
In this study, the dimensions of the membranes were not found to be in relation to the hemoglobin content or the erythrocyte content found in the basal blood count.
In light microscopy (Figure 2), most of the cell bodies (colored dark purple for the nuclei) were concentrated in the proximal part (head) of each membrane, the last 1/4 was observed in the center; the distal part had only residual traces of cell bodies. However, optical microscopy did not allow us to observe the exact state of these cell bodies in greater detail.
The results of blood count on whole blood, on the supernatant obtained by compressing the clot at Ø’, compared with the count of erythrocytes, platelets, and WBC on a red clot smear at Ø’ are reported with the corresponding statistical tests in Table 3. Table 3 demonstrates a statistically significant difference between the content of RBC, WBC, and Platelets between the supernatant derived from the compression of the clot at Ø’ and the values obtained with the blood count. It also demonstrates a statistically significant difference between the content of RBC, WBC, and PLT in smears obtained from red clots at Ø’ shown in Figures 3A-3C at various magnifications.
| Type | Cell Counter blood | Supernatant Cell Counter to Ø min | Difference Count to Ø min | Between Blood Cell Counter and Blood Cell Counter supernatant to Ø min |
Membranes L-PRF/A-PRF Ø min |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± D.S. | Mean ± D.S. | Mean ± D.S. | t-test* | χ2 | No./μl | % | |||
| RBC | 7.648,000 ± 1.130,981 |
13.428 ± 2.1345 |
7.399,440 ± 27.039,76 |
p=0.411 > 0.05 | NS | p=0.000 < 0.005 | S | 216,012 | 0.0028% |
| WBC | 5150 ± 369 | 30 ± 27.99 | 8.5 ± 2.12 | p=0.255 > 0.05 | NS | p=0.000 < 0.005 | S | 5111.15 | 99.24% |
| PLT | 106,780 ± 153.51 |
479 ± 77.614 | 500 ± 707.11 | p=0.031 < 0.05 | S | p=0.000 < 0.005 | S | 105,801 | 99.00% |
| Neutrophils | 3046 ± 857 | 0.29 ± 0.76 | p=0.280 > 0.05 | NS | p=0.991 > 0.005 |
NS |
|||
| Basophiles | 4.2 ± 1.3 | 2.29 ± 2.14 | p = 0.785 > 0.05 | NS | p = 0.611 > 0.005 |
NS |
|||
| Lymphocytes | 1606 ± 668 | 19 ± 23.15 | p = 0.238 > 0.05 | NS | p = 0.000 < 0.005 |
S |
|||
| Monocytes | 490.2 ± 138.06 |
4.57 ± 7.68 | p = 0.631 > 0.05 | NS | p = 0.928 > 0.005 |
NS |
|||
| Eosinophils | 5.4 ± 5.37 | 4 ± 9.71 | p = 0.906 > 0.05 | NS | p = 0.316 > 0.005 |
NS |
|||
Table 3: Results of blood counts of whole blood, supernatant obtained by compression of the clot at Ø min versus the counts of erythrocytes, platelets, and WBC counts of the red clot smear at 0 min, with a test of significance. * Processing performed on two comparisons. Hypothetical content of RBC, WBC, PLT in L-PRF membranes at Ø min with significance test. p> 0.05 = + 0.5% difference not significant; p <0.01 = -1% significant difference. Method of counting by subtraction.
Table 3 also demonstrates the theoretical RBC, WBC, and PLT content in L-PRF/A-PRF membranes derived from the difference of these corpuscular elements between whole blood, supernatant at Ø’, and red clot smear at Ø’ obtained by the method of subtraction.
The Student’s t-test demonstrates significant differences between RBC and PLT at Ø’ between L-PRF/A-PRF membranes and the blood count. The RBC content in the membranes is 0.0028% compared to whole blood, that of WBC is 99.24%, that of PLT is 99.0% compared to the content in whole blood.
The horse L-PRF/A-PRF membranes produced at the time Ø after centrifugation is made up of 66% red blood cells, 1.56% white blood cells, and 32% platelets, the one produced after 60’ it is made up of 65.5% of GR, of 1.7% of GB, and 32.8% of PLT.
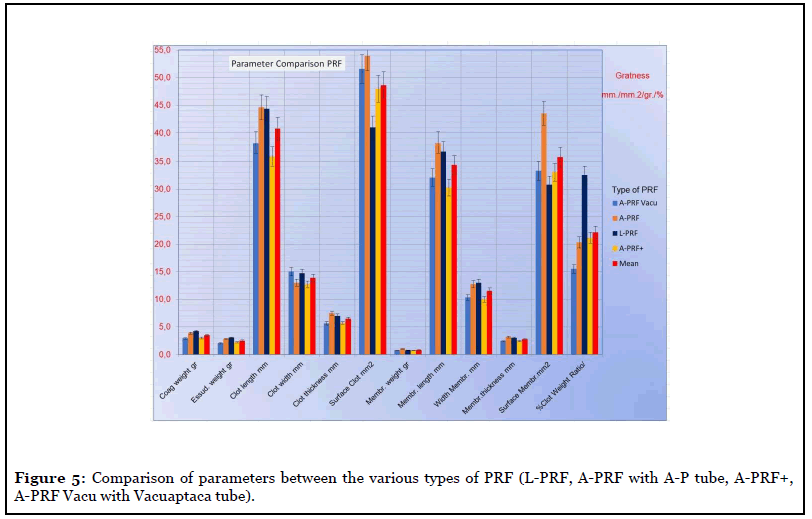
The average morphological characteristics (± D.S.) detected among the various types of clots and membranes are: Weight of the clot gr. 3.52 (± 0.64); Exudate weight gr. 2.54 (± 0.48); Membrane weight gr. 0.84 (± 0.15); Coagulum length 40.8 mm (± 4.40); Clot width mm 13.89 (± 1.18); Coagulum thickness mm 6.48 (± 0.92); Coagulum surface mm2 48.64 (± 5.65); Membrane length 34.37 mm (± 3.79); Clot width mm 11.55 (± 1.58); Coagulum thickness mm 2.78 (± 0.38); Coagulum surface mm2 35.78 (± 5.67); Weight Ratio Clot/Blood Sample 10ml% 22.15 (± 2.20) (Figure 5). The most useful values in clinical terms are those obtained in the A-PRF of the clot surface 53.94 (± 14.39) mm2 p=0.001 <0.05 and of the membrane surface 43.52 (± 8.68) mm2 p=0.560> 0.05.
Discussion
The study performed by McLellan et al. [25] demonstrated that equine PRF similar to human PRF provides an immediate and constant source of tissue growth factors. Our study tried to standardize the L-PRF/A-PRF preparation procedure, which while remaining easy to perform and low-cost technique that does not require specialized equipment but has a certain consistency in the production of an membrane L-PRF/A-PRF in terms of macroscopic and microscopic characteristics. Autologous platelet concentrates hold promise in the field of regenerative medicine and surgery due to the abundance of growth factors. L-PRF and its derivatives represent a huge critical advance in the evolution of platelet concentrates as it is essentially a fibrin membrane with platelets and leukocytes trapped along with stem cells. These solid membranes possess excellent handling characteristics and can be securely sutured to an anatomically desired position during open surgery.
However, the physical and biological properties are relatively unknown and have yet to be fully studied.
The L-PRF/A-PRF membrane will constantly form when the steps described above are strictly followed.
One of the important considerations in generating a good membrane is, in addition to the specific tube type used, the time delay between blood collection/centrifugation and the processing temperature. The success of the technique depends entirely on the speed of blood collection and the immediate transfer to the centrifuge, usually within one minute and from a centrifugation and compression temperature above 21°C (between 21 and 30°C) [18].
However, all physical and biological properties are relatively unknown and have yet to be fully studied.
A well-structured L-PRF/A-PRF clot (with its specific cell content, matrix architecture, and growth factor release profile) cannot be generated if blood collection is prolonged and non-homogeneous or centrifugation temperature is below 21°C or above 30°C; In these cases, instead, a small, incoherent, friable mass of fibrin with unknown content is formed. The functions of L-PRF/A-PRF as a provisional extracellular matrix, transformed into functional tissue during healing, being able to be subjected to mechanical forces with successful healing outcomes, depend on structural integrity and therefore it is important to clarify its physical properties.
L-PRF/A-PRF resembles dense connective tissue with superior handling characteristics. Therefore, it is assumed that as in the L-PRF also in the A-PRF there is a low stiffness (1-10 MPa) and a high tension (up to 150%) of the membrane before breaking.
Based on these results, it is clear that both L-PRF and A-PRF are a new biomaterial with unique characteristics: - a predictable preparation from autologous blood; - the simplicity of the protocol; -the defined architecture; -the impressive mechanical properties; - and the abundance of growth factors derived from activated platelets.
Our horse blood tests are undoubtedly capable of improving our understanding of the mechanisms of healing, as well as helping to advance the field of personalized medicine.
Limitations encountered in the clinical setting and use of these products includes:
1) Since PRF is an autologous product, it is difficult to achieve a greater need for the availability of biomaterials. Therefore, its use in surgical procedures must be strictly controlled and anticipated.
2) PRF contains circulating immune cells as well as antigenic molecules that prevent its use as an allogeneic material; an increased risk of transmission of infectious diseases must also be taken into account.
At this point, among the various parameters that have not been included in this type of classification, we recognize the concentration of platelets, the concentration of leukocytes, and the proportional quantity of the different types of leukocytes. Problems with platelet concentration are non-existent, as almost all platelets included in the blood sample (99%) are activated and integrated into the fibrin matrix of the clot.
Regarding the count and concentration of leukocytes, their influence should be studied with particular attention, as their presence or absence could explain the conflicting results we have observed.
In a recent study by Kitamura Y. et al. (2018) [4] a method for direct estimation of platelet count in PRF is shown. These authors used a commercially available recombinant t-PA, Alteplase (GRTPA®; Mitsubishi Tanabe Pharma Corp., Osaka, Japan) via a digestion method. Here, they proved that t-PA is powerful enough to be able to count dispersed platelets aggregated in platelet-enriched insoluble fibrin matrices. Recently, the AA collaborating with the Japanese group of Kawase T., have developed a statistically valid mathematical method which allows predicting the presence of cells in the PRF starting from the evaluation of a blood count. Thus, validating a simple and inexpensive system to calculate the precise number of platelets and leukocytes present in platelet concentrates.
Conclusions
Concluding this research, we can state that, to obtain a standard procedure for the preparation of PRF as graft material for tissue regeneration, we suggest the use of the PRF membrane region with the highest possible platelet enrichment and we suggest avoid squeezing all the plasma of the PRF clot. Therefore, it is advisable to compress the clot with a compression device (L-PRF Wound Box). It is therefore difficult to precisely control the quality of materials of human or equine origin, such as PRF preparations (L-PRF/A-PRF), but it is important to apply the highest possible quality control on PRF preparations before their application clinic.
The preparation of PDPs is extremely technically demanding due to the delicate nature of the platelets which are easily activated during processing. Therefore, special recommendations are needed to maximize the clinical benefits of these blood products.
Currently, their supply is based on a poorly controlled bulk release. Consequently, prolonged treatments require multiple treatments, such as numerous i-PRF injections.
This results in large fluctuations in growth factor concentrations, which compromise clinical predictability. Biomaterials can act as controlled-release devices, allowing for prolonged or even on-demand administration of these growth factor cocktails. Furthermore, it can be expected that biomaterials can covalently bind specific growth factors to locally maintain high levels of these molecules.
Further clinical, histological, and statistical studies are needed to understand the benefits of this new platelet concentration technique. However, we cannot overlook the fact that, when obtained from an autologous blood sample, the PRF produced is scarce and can only be used in a limited volume. This is a limitation to the systematic use of PRF in Veterinary and Human Regenerative Surgery. Although the potential applications of PRF are wide, a thorough understanding of the biomaterial, including information on its biology, efficacy, and limitations, is required to optimize its use in daily clinical practice.
The effectiveness of autologous PRF as a biological promoter of tissue healing has been validated in human treatments. The minimalist PRF manufacturing procedure (a safe, easy and cost-effective strategy, without the need for advanced manufacturing capabilities), allied to low intrinsic variables in its preparation methodology, high level of reproducibility and consistent clinical performance have determined the success of this organic product.
References
2. Choukroun J, Adda F, Schoeffler C, Vervelle AP. Une opportunité en paro-implantologie: le PRF. Implantodontie. 2001;42(55): e62.
3. Litvinov RI, Weisel JW. What is the biological and clinical relevance of fibrin?. InSeminars in Thrombosis and Hemostasis 2016 Jun (Vol. 42, No. 4, p. 333-43). NIH Public Access.
4. Kitamura Y, Watanabe T, Nakamura M, Isobe K, Kawabata H, Uematsu K, et al. Platelet counts in insoluble platelet-rich fibrin clots: A direct method for accurate determination. Frontiers in Bioengineering and Biotechnology. 2018 Feb 1; 6:4.
5. Soloviev DA, Hazen SL, Szpak D, Bledzka KM, Ballantyne CM, Plow EF, et al. Dual role of the leukocyte integrin αMβ2 in angiogenesis. The Journal of Immunology. 2014 Nov 1;193(9):4712-21.
6. El Bagdadi K, Kubesch A, Yu X, Al-Maawi S, Orlowska A, Dias A, et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-richfibrin (PRF)-based matrices: a proof of concept of LSCC (low speed centrifugation concept). European Journal of Trauma and Emergency Surgery. 2019 Jun;45(3):467-79.
7. Choukroun J. Advanced PRF, & i-PRF: platelet concentrates or blood concentrates. J Periodontal Med Clin Pract. 2014;1(1):3.
8. Miron RJ, Fujioka-Kobayashi M, Hernandez M, Kandalam U, Zhang Y, Ghanaati S, et al. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry?. Clinical Oral Investigations. 2017 Nov 1;21(8):2619-27.
9. Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. Journal of Oral Implantology. 2014 Dec 1;40(6):679-89.
10. Schär MO, Diaz-Romero J, Kohl S, Zumstein MA, Nesic D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clinical Orthopaedics and Related Research®. 2015 May 1;473(5):1635-43.
11. Satué K, Gardón JC, Muñoz A. Interpretation of platelets in the horse. J. Hematol. Res. 2017;4:19-25.
12. Crisci A, Barillaro MC, Lepore G, Cardillo F. L-PRF Membrane (Fibrin Rich in Platelets and Leukocytes) and Its Derivatives (A-PRF, i-PRF) are Helpful as a Basis of Stem Cells in Regenerative Injury Treatment: Trial Work on the Horse. International Blood Research & Reviews. 2019:1-4.
13. Crisci A, Crisci M, Boccalone E. Final results of an experimental research about a technique of measurement of skin lesions. Esperienze Dermatologiche. 2014;16:147- 52.
14. Kobayashi M, Kawase T, Horimizu M, Okuda K, Wolff LF, Yoshie H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals. 2012 Sep 1;40(5):323-9.
15. Dohan Ehrenfest MD, Bielecki T, Jimbo R, Barbe G, Del Corso M, Inchingolo F, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte-and platelet-rich fibrin (L-PRF). Current Pharmaceutical Biotechnology. 2012 Jun 1;13(7):1145-52.
16. Crisci A, Benincasa G, Crisci M, Crisci F. Leukocyte Platelet-Rich Fibrin (L-PRF), a new biomembrane useful in tissue repair: basic science and literature review. Biointerface Research in Applied Chemistry. 2018 Oct 15;8(5):3635-43.
17. Crisci A, De Crescenzo U, Crisci M. Platelet-rich concentrates (L-PRF, PRP) in tissue regeneration: Control of apoptosis and interactions with regenerative cells. J Clin Mol Med. (2018)1:5-12.
18. Crisci A, Lombardi D, Serra E, Lombardi G, Cardillo F, Crisci M. Standardized protocol proposed for clinical use of L-PRF and the use of L-PRF Wound Box®. J Unexplored Med Data. 2017; 2: 77-87.
19. Crisci A, Manfredi S, Crisci M. Fibrin rich in Leukocyte- Platelets (L-PRF) and Injectable Fibrin Rich Platelets (I-PRF), two opportunity in regenerative surgery: Review of the sciences and literature. IOSR Journal of Dental and Medical Sciences (IOSR-JDMS). 2019;18:66-79.
20. Crisci A, Marotta G, Licito A, Serra E, Benincasa G, Crisci M. Use of leukocyte platelet (L-PRF) rich fibrin in diabetic foot ulcer with osteomyelitis (three clinical cases report). Diseases. 2018 Jun;6(2):30.
21. Watanabe T, Isobe K, Suzuki T, Kawabata H, Nakamura M, Tsukioka T, et al. An evaluation of the accuracy of the subtraction method used for determining platelet counts in advanced platelet-rich fibrin and concentrated growth factor preparations. Dentistry Journal. 2017 Mar;5(1):7.
22. Crisci A, Kawase T, D’Adamo R, Crisci M. Experimental research on a technique for quantification of platelets and leukocytes in second-generation platelet concentrates. International Journal of Current Medical and Pharmaceutical Research. 2019 Oct;5(12):4792-9.
23. Hasan FK. Characterization of leukocyte-platelet rich Fibrin. A Novel Biomaterial. 2015.
24. Pinto NR, Pereda A, Jiménez P, Del Corso M, Kang BS, Wang HL, et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors and fibrin architecture of a Leukocyteand Platelet-Rich Fibrin (L-PRF) clot and membrane. Part 2: macroscopic, photonic microscopy and Scanning Electron Microscopy analysis of 4 kinds of L-PRF clots and membranes POSEIDO. 2014;2(2):141-54.
25. McLellan J, Plevin S. Temporal release of growth factors from platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) in the horse: a comparative in vitro analysis. International Journal of Applied Research in Veterinary Medicine. 2014;12(1):48-57.
