Abstract
For many decades (1945–2011) narrowed, uterine arterioles were thought to be the histological hallmark of preeclampsia after their original description by AT Hertig, Harvard, MA, in 1945. More recently Professors Brosens & Romero, described narrowed, uterine arterioles in many of the “great” obstetric syndromes including preterm labour, placental abruption, foetal growth restriction, pre-eclampsia, etc. In unrelated studies, our group have found narrowed arterioles surrounded by “halos” of injured nerves in the uterus, and other pelvic organs, in many of the gynaecological syndromes including chronic pelvic pain, endometriosis, “painful” adenomyosis, vulval pain, interstitial cystitis, etc.
Keywords: Autonomic nerves, Obstetric syndromes, Gynaecological syndromes, Difficult first labours, Straining on the toilet, Denervation, Reinnervation
Condensation: Many obstetric and gynaecological syndromes result from preventable injuries to pelvic autonomic nerves sustained during “difficult” first labours, minor gynaecological surgery, and, straining on the toilet.
Keywords
Autonomic nerves, Obstetric syndromes, Gynaecological syndromes, Difficult first labours, Straining on the toilet, Denervation, Reinnervation
Introduction
Reviewing historic reports of the obstetric syndromes there is clear evidence of partial or complete, single or multi-layered, “halos” of empty, nerve sheaths around narrowed arterioles in the placental bed though these histological features have not been previously discussed or analysed. Traumatic injuries to autonomic nerves release cytokines and growth factors with the dual effects of (i) regeneration of injured nerve fibres and, (ii) medial hyperplasia (“narrowing”) of the adjacent, denervated arterioles. Common causes of neuropathic injury in the female pelvis include [1] “difficult” primigravid labours (prolonged >12 hours, or, complex), [2] some forms of “neuropathic” gynaecologic surgery, and [3], coordinate, and, incoordinate, physical efforts during defaecation.
We propose that many obstetric and gynaecological syndromes originate from injuries to pelvic autonomic nerves leaving characteristic histological signatures of (i) narrowed arterioles surrounded by “halos” of injured nerves in the gynaecological syndromes, and, (ii) narrowed arterioles surrounded by “halos” of injured nerve sheaths in the obstetric syndromes.
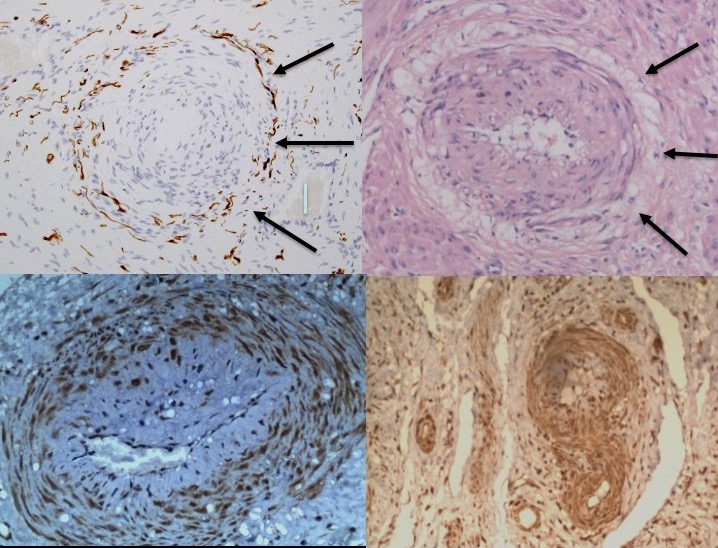
For many decades (1945–2011), narrowed uterine arterioles in the placental bed were held to be the characteristic injury in pre-eclampsia [1–5]. More recently, Professors Brosens and Romero found that narrowed, uterine arterioles were characteristic of many of the “great” obstetric syndromes (Figures 1A and 1B), and in their account, attributed them to “defective placentation” [6]. Independent, and unrelated, studies by our group found evidence of narrowed arterioles (Figures 1A and 1B), together with “halos” of injured autonomic nerves, in many of the gynaecological syndromes e.g. “endometriosis”, different patterns of adenomyosis, interstitial cystitis, vulval pain, etc. [7] (Figures 2 and 3). Reviewing historical papers in preeclampsia between 1945 and 1972 reveals many illustrations of narrowed, uterine arterioles in the placental bed with complete or incomplete, single or multi-layered, “halos” of empty nerve sheaths that have neither attracted comment, nor analysis [1–5], (Figures 1A and 1B). In AT Hertig’s original paper there is some evidence of a partial “halo” of empty cells around narrowed arterioles though accounts by later authors including Zeek & Assali, Dixon & Robertson, and, Brosens & Pijnenborg include many more illustrations of “halos” of injured nerve sheaths in their respective accounts [1–5].
Figure 1. Common histological origins of the obstetric and gynaecological syndromes. A-B. Narrowed, uterine arterioles in gynaecological (A) (x100, anti-S100) and obstetrics (B) (x100, haematoxylin and eosin) syndromes, result from injuries to “mixed”, autonomic nerve bundles. In painful gynaecological syndromes there are “halos of injured nerves around narrowed arterioles” (A, arrowed) whereas in the obstetric syndromes there are “halos of empty, injured, nerve sheaths”. “Halos” of empty, injured nerve sheaths (B, arrowed) are illustrated in many historical reports of pregnant uteri (B,C,E,F) though have not attracted comment, discussion or analysis [1–5]. C. Narrowed uterine arteriole (x100, anti-P2X3, brown) in preeclampsia expressing specialized, purinergic, P2X3, “stretch” receptors that may represent the afferent mechanism in the early-onset (<34 weeks) preeclampsia syndromes. P2X3 receptor proteins are expressed in the maternal chorion during normal pregnancy and may play a role in the onset of labour [53,54]. D. Expression of specialized, purinergic, P2X3, “stretch” receptors in myometrium (x100, anti-P2X3, brown) in the “late-onset” (>34 weeks) preeclampsia syndromes.
Injuries to autonomic nerves release cytokines and growth factors that have the dual effects of (i) relentless, regeneration of injured nerves, (ii) hyperplasia of the denervated tunica media leading to narrowing of adjacent arterioles [8]. Narrowed uterine arterioles (9, vessel:lumen diameter > 1.4) are frequently surrounded by “halos of injured nerves” in gynaecological syndromes (Figures 1A and 2d) and undiscussed, “halos” of injured nerve sheaths in the pregnant uterus [1–5], (Figure 1A). Any injury to an autonomic nerve (denervation) result in regeneration of injured nerves (reinnervation), with largely-unknown biological characteristics, from the proximal stump [8]. They have unpredictable, clinical consequences that produce wide-ranging, gynaecological symptoms and presentations over varying periods of time (3-10 years, (Table 1)). Variable, latent periods between initial neurological injuries and subsequent, diverse, clinical presentations vary with the nature of the injury, tissue of origin, the neurapraxis effects of intervening pregnancies, etc., and, may have obscured their etiological origins. These unstudied, regenerating nerves continue to proliferate causing recurrent, painful symptoms that may become apparent in a woman’s postmenopausal years despite prior hysterectomy and her menopause. The obstetric syndromes largely result from the consequences of pre-pregnancy injuries to uterine nerves rather than an early pregnancy process (8-18 weeks) such as “defective placentation” as suggested by Brosens and Romero [6]. These common origins may explain some of the many, clinical associations between the obstetric and gynaecological syndromes e.g. endometriosis and intrauterine growth retardation [10], or, adenomyosis and preterm labour [11].
Pelvic Autonomic Nerves (Figures 2 and 3)
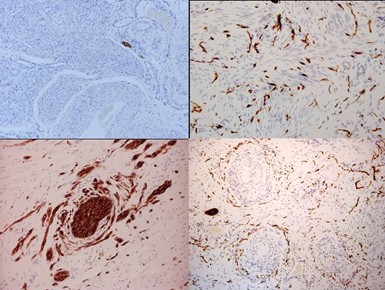
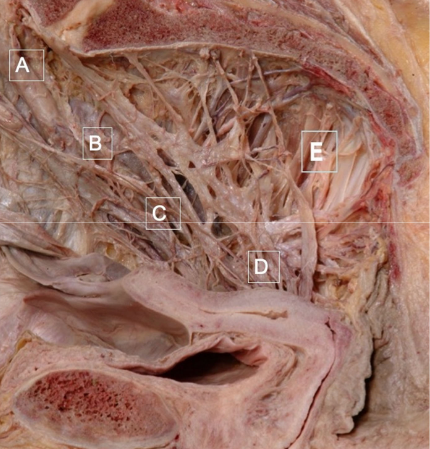
In the post-1945 era, many medical schools changed their embalming agents from alcohol to formalin to prolong the teaching life of cadavers; formalin selectively destroys autonomic nerves [12], (Figure 3). As a consequence, many contemporary physicians have not seen the full morphology of the cardiac, coeliac and pelvic autonomic plexi, and, are unfamiliar with their pelvic neuroanatomy. In the female pelvis, two bundles of uterine and tubal nerves from sympathetic segments T10-L2 and parasympathetic segments S2-4, pass through (i) the central neurovascular bundle of the uterosacral ligaments, and, (ii) with the uterine vessels to supply the uterus, cervix and Fallopian tubes [13,14], (Figure 3). Most viscera carry similar submucosal and subserosal plexi that may be important during embryogenesis. The nerves in the uterosacral ligaments supply the inner layers of the three target organs (uterus, cervix and fallopian tube) including the endometrial-myometrial nerve plexus [14], inner concentric layer of nerves in the cervix [14], and, the inner layer of the proximal four-fifths of the fallopian tubes [15,16]. Injuries to the uterosacral ligaments may be visible as avulsion, scarring, thinning or thickening, with direct consequences for their contained nerves that supply the submucosal layers of the uterus and fallopian tubes (Figures 4A–4F). Reinnervation in the insertions of the uterosacral ligament may be a frequent contributory source of chronic pelvic pain [15], (Figure 2B). Nerve bundles passing with the uterine vessels supply the uterine subserosal, outer, concentric layer of the cervical plexi, and, outer layers of the proximal four fifths of the fallopian tube [13]. Injuries to these nerves often result in ampullary ectopic pregnancy or hydrosalpinx [16]; the fimbriae receive a dense innervation from the ovarian plexus via the mesosalpinx [17].
Krantz described important subserosal (lateral) and endometrial-myometrial nerve (medial) nerve plexi in the uterus; the latter being vulnerable to uterine curettage [14] whereas the cervix contains two concentric layers of “mixed” autonomic nerve bundles in 12-16 individual bundles that may be at risk during dilatation and curettage, and other minor surgical procedures [14]. Applying a multi-toothed vulsellum to the anterior lip of the cervix may injure both layers of concentric nerves creating the conditions for a dense field of regenerating, injured nerves with subsequent, “painful” hysteroscopy or difficult, IUCD insertion after a latent period of approximately three years [18]. Applying excessive traction to the cervix may avulse both the uterosacral bundle of nerves (“uterosacral nerves”), and, the second bundle of nerves running with the uterine vessels ([14], “uterine nerves”) with potentially-significant impacts on a woman’s fertility [19]. The endometrial-myometrial nerve plexus (of Krantz, [14]) is clearly vulnerable to over-vigorous, uterine curettage that may also contribute to the development of some forms of adenomyosis (Figure 1A). Clinicians should be aware of the detailed neuroanatomy of the lower genital tract and potential “neuropathic” consequences of intrapartum and gynaecological surgical procedures.
Figure 2. Different patterns of neuropathic injury in the uterus (A-D). A. The normal, endometrial-myometrial nerve plexus separates endometrium from myometrium. It is vulnerable during evacuation of the uterus. B. Aberrant reinnervation in uterine stroma associated with traumatic injury to “mixed” uterine nerve bundles [19]. C. “Collateral sprouting” of uterine nerve bundles that is pathognomonic for prior traumatic injury [19]. D. Perivascular nerve fibre proliferation (PVNFP) around narrowed arterioles associated with prolonged physical efforts during defaecation, or, prolonged maternal, voluntary efforts in the second stages of labour [19,23].
Figure 3. The full morphology of the pelvic autonomic nerves. (A) Superior hypogastric nerve plexus, (B) hypogastric nerve, (C) inferior hypogastric nerve plexus, (D) uterovaginal nerve plexus, (E) pelvic parasympathetic nerves (S2-4).
Consequences of Injuries to Pelvic Autonomic Nerves
Typically, there is a smooth, nerve plexus between the endometrium and the myometrium [13], (Figure 2A) though traumatic neural injuries result in collateral sprouting of nerve bundles (Figure 2B) and widespread stromal reinnervation (Figure 2C). Such injuries are, by definition, traumatic in origin and, may result from traumatic vaginal deliveries, or, gynaecological surgery, or, straining on the toilet [19]. “Longitudinal” injuries to the nerve bundles caused by straining on the toilet, or, prolonged maternal voluntary efforts in the second stage of labour, results in the typical histological appearance of perivascular nerve fibre proliferation ([19], PVNFP) that has been found in almost all pelvic organs in different, painful, gynaecological syndromes [23], (Figure 2D). Women have different shapes of pelvis and different patterns of straining that result in anatomically-diverse, clinical syndromes ranging from vulvodynia in the anterior pelvis to rectal hypersensitivity in the posterior pelvis [24]. The range of pathophysiological consequences of neurological injury are shown in (Table 1).
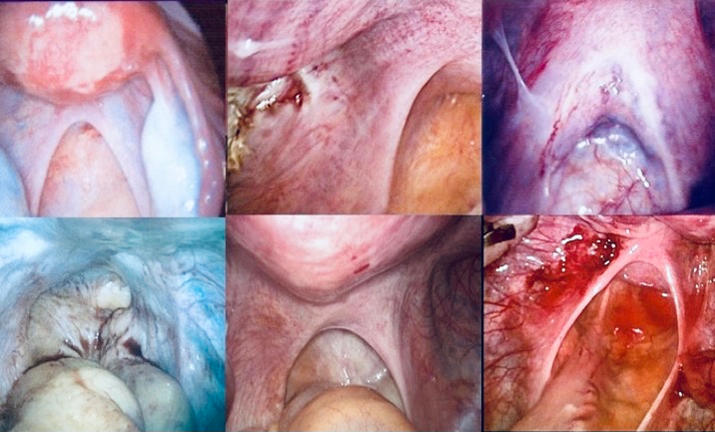
Figure 4. Injuries to the uterosacral ligaments. A. Normal uterosacral ligaments (USL) conduct uterine and tubal nerves to the subserosal and endometrial-myometrial nerve plexi. B. Minor scarring to the left USL illuminated by a deposit of ectopic endometrium. These minor injuries during evacuation of the uterus may contribute to remote development of myomas [27,28]. C. Thickened USL caused by prolonged and persistent, coordinate straining at stool may result in aberrant reinnervation, chronic pelvic pain and retrograde menstruation [23]. D. Absent USL caused by recurrent medical and surgical abortion procedures contribute to development of “painless” adenomyosis. Markedly, enlarged uteri (250-1500 g) contain little, or no, detectable neurological material [26]. E. Marked scarring of USL leads to aberrant reinnervation in the distal insertions and bilateral, “painful” adenomyosis [25,26]. F. Attenuation of USL associated with administration of oxytocin, prostaglandins and misoprostol results in aberrant reinnervation and unilateral or bilateral chronic pelvic pain [25,26]. Division of both USL at hysterectomy relieves pain promptly.
The Gynaecological Syndromes
Many painful, gynaecological syndromes are associated with different patterns of neurological injury e.g. vulvodynia, dyspareunia, chronic pelvic pain, irritative bladder and bowel syndromes, interstitial cystitis, etc. [2,7,19], (Figure 2). All of these syndromes have been shown to be associated with neurological injury by different authors in different clinical contexts. These may best be interpreted as “pain or discomfort in response to light touch” (technically these are different patterns of visceral allodynia) where direct touch, or, dilating blood vessels in the second half of the menstrual cycle, create different, “premenstrual” pain syndromes [19,23], (Figure 2D). Reducing pelvic blood flow with oral contraceptive preparations, levonorgestrel-IUS, GnRH agonists, oophorectomy, menopause etc. may be helpful.
Endometriosis, Adenomyosis, and Leiomyoma
These three, specific, gynaecological pathologies frequently coexist because they result from injuries to pelvic nerves at different anatomical sites en route, or within, the uterus [27], (Figures 2A–2D, Figures 4A–4D) Savitskii [28] described myometrial denervation in many forms of myoma in the early 1980’s. The clinical and histological differences between “painful” and “painless” adenomyosis may be helpful in understanding their pathoanatomy [26]. In a series of 31 nulliparous women with one- to three prior abortions, undergoing hysterectomy in their 40’s for uteri weighing 250-1160 g, the uterosacral ligaments were completely avulsed (Figure 4D), and, there were few, if any, nerves in the uterus and fallopian tubes [26]. In a second series of 16 women undergoing hysterectomy for unilateral, or bilateral, chronic pelvic pain; there was unilateral, or bilateral, scarring of the ipsilateral uterosacral ligament with aberrant reinnervation within the insertion of the ligament or adjacent myometrium [26], (Figures 4B and 4D). Division of the uterosacral ligaments relieves the pain immediately. So, complete avulsion of the uterine nerve supply, including the endometrial-myometrial nerve plexus, produces hyperplasia of both endometrium and myometrium producing “painless” adenomyosis (Figure 4B). Partial injury to the uterosacral ligaments and their contained nerves, also clearly impairs the endometrial-myometrial nerve plexus enabling endometrium to invade the myometrium (Figure 4E).
Several groups have drawn attention to injuries to pelvic nerves in “endometriosis” [8,29–31], (Figure 5) though many clinicians continue to manage women with chronic pelvic pain according to the 1922 observations by John Sampson that attribute symptoms to deposits of ectopic endometrium ([32], ‘the 1922 narrative”) rather than injuries to pelvic autonomic nerves (“the 2022 narrative” [33–35]). More recently our group has demonstrated that denervation of the interstitial myometrium adjacent to the Fallopian tube, is the likely source of retrograde menstruation [36], (Figures 4C–4F). It follows from the pathoanatomy of this injury that fertility will be impaired, and, chronic pelvic pain may result from reinnervation of the uterus [36]. The distinctive pathological feature and clinical presentations of “endometriosis” may result from a single denervatory pathophysiology with deposits of ectopic endometrium simply adhering to anatomic sites of tissue injury (“the 2022 narrative”, [35]). Excision or ablation of the overlying ectopic endometrium may not alter the underlying injured nerves (“the 1922 narrative” [32]).
The Obstetric Syndromes
In an important collation of clinical evidence, Brosens & Romero [6] found that many of the “great” obstetric syndromes were associated with the histological finding of narrowed uterine arterioles, without articulating that these may result from injuries to adjacent uterine nerves [7,38]. Such neurovascular injuries have many diverse and varied, clinical consequences to produce the clinical obstetric syndromes (Table 1). Injured uterine nerves may induce purinergic, P2X3, “stretch” receptors in arterioles and myometrium in preeclampsia, may weaken arterial integrity (placental abruption), increase susceptibility to “opportunist” infection (preterm labour, preterm PROM), etc. Parous, obstetric pain syndromes, often labelled as “symphysis pubis dysfunction”, are frequently associated with injuries to uterosacral ligaments and levator avulsion syndromes, that may also be associated with denervation-reinnervation sequences following “difficult” first labours [39]. The pain is largely intractable during the pregnancy and may lead to chronic pelvic pain in the postpartum period [39].
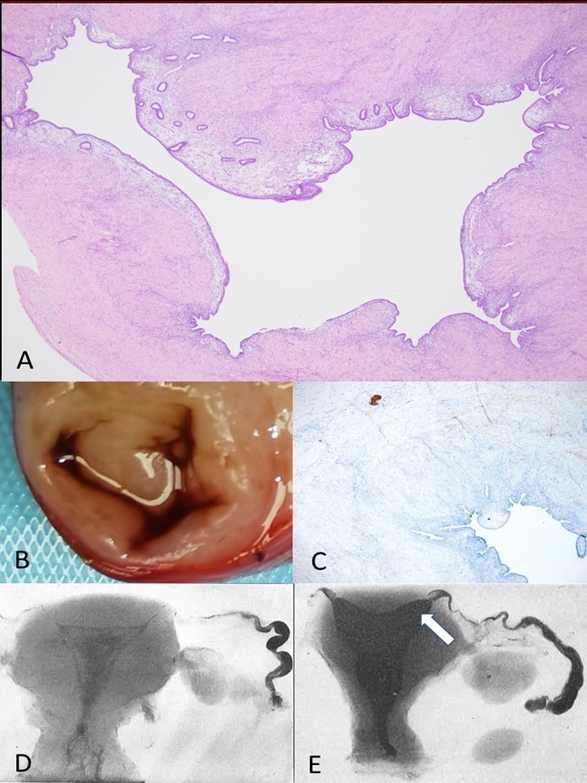
Figure 5. Retrograde menstruation, infertility and chronic pelvic pain in “endometriosis”. A. Dilated interstitial fallopian tube (stained with haematoxylin and eosin, x10). B. Dilated, interstitial fallopian tube. C. Loss of nerve bundles (brown) in interstitial myometrium [36]. D. Normal barium hysterogram [32]. E. Dilated, interstitial fallopian tube [32].
Other Chronic Diseases
Two, data-driven hypotheses emerged in the second half of the twentieth century to explain chronic diseases; DP Burkitt’s “dietary-fibre” hypothesis and DP Barkers “foetal origins of adult diseases” hypothesis [40,41]). Both have novel, though simple, explanations based on autonomic injury. Burkitt’s observations of reduced oro-anal transit times and increased stool weights in African (v European) populations simply reflect differences in unrefined fibre intake, which taken together with the sitting (v squatting) position for defaecation leads to marked increases in physical efforts during defaecation that result in characteristic injuries to autonomic nerves [40], (Figure 2D). It is not easy for humans to strain effectively in the squatting position. If the physical efforts are coordinate then they tend to injure pelvic sympathetic segments (T10-L2) and branches of parasympathetic segments with characteristic histological lesions; if they are, hypothetically, “incoordinate” (dyssynergic) then they may injure higher sympathetic segments and branches of the vagus nerves in the thorax and abdomen [24] to produce chronic diseases with known neuropathic lesions e.g. appendicitis [42], Crohn’s disease [43], ulcerative colitis [44], diverticulosis [45] etc. Similar injuries may result from prolonged physical efforts in the second stage of labour and result in vulvodynia, dyspareunia, chronic pelvic pain, irritative bladder and bowel syndromes, etc. after varying latent periods in the respective tissues [23].
DP Barker’s “foetal origins of adult diseases” may have been uncovered in a small series of poorly-grown (<1stcentile), mid-trimester stillbirths where examination of the viscera show widespread autonomic injury with narrowed arterioles, similar to that seen in essential hypertension [46,47]. The prevailing hypothesis for this pattern of “foetal hypertension” is that involuntary transmission of mediators of stress may cross the placenta to cause these visceral injuries in the foetus [48]. Previously labelled as “congenital” conditions, these autonomic injuries may further progress if the child has difficulties with establishing a smooth, regular, bowel habit.
Hypertension
Hypertension is a lifelong condition that may originate in utero (foetal hypertension), adopt different mechanisms during pregnancy (pregnancy hypertension, or, pre-eclampsia), or develop in later life (essential hypertension) owing to mechanical disruption of sympathetic vasomotor nerves by high intravascular pressures resulting from excessive stress, sugar, salt, smoking, etc. [49,50]. Narrowing of renal, splenic, pancreatic and adrenal arterioles was originally described in 1937 by Moritz & Oldt, Case Western University, in a detailed histological analysis of 100 hypertensive postmortems where they found narrowed arterioles in kidney, spleen, pancreas, and adrenals [9] though not the adjacent injured vasomotor nerves [49,50].
Many hypotheses have been advanced to explain pre-eclampsia. The “uterine stretch” hypothesis was first articulated by Otto Speigelburg in 1878 [51]; it requires an afferent intrauterine mechanism with neural connections between the uterus and kidneys. GJ Sophian’s animal experiments in the 1950’s provided direct evidence for uterorenal “reflexes” in rabbits [52]. The finding of “new”, P2X3, “stretch” receptors in uterine arterioles (“early-onset” preeclampsia) and myometrium (“late-onset” preeclampsia), may be helpful in promoting this view of the condition (Figures 1C and 1D), [53,54]. Most clinicians will agree that delivery of the baby (“release of stretch”) relieves a woman of the clinical syndrome. Absolute proof of these findings in preeclamptic uteri may not be possible with contemporary technology, though the “uterine stretch” hypothesis looks like a reasonable explanation of most features of the clinical syndrome. Sustained hypertension during pregnancy is clearly unhelpful to an individual woman at high blood pressures will lead to injuries to sympathetic vasomotor nerves, narrowing of adjacent arterioles, and, increases her chances of developing chronic hypertension in later life [55].
|
1) Tissue hyperplasia. Endometrial hyperplasia (myoma), myometrial hyperplasia (adenomyosis), cervical hyperplasia are consequences of oestrogen-dependent hyperplasia [28]. |
|
2) Tissue hypoplasia. In non-oestrogen-dependent tissues there is atrophy of denervated tissues e.g. bladder, bowel, vagina, vulva, etc. (**) |
|
3) Loss of visceral function. Early pregnancy loss, loss of cervical function in labour (cervix will not dilate), or at hysteroscopy (cervix will not open easily) [16]. |
|
4) Loss of visceral motility. Dyspolar uterine and tubal motility leads to infertility, retrograde menstruation, and, dysfunctional labour [56]. |
|
5) Opportunist infection. E. coli in preterm labour, vulvovaginal Candidiasis, bacterial vaginosis in gynaecology [57]. |
|
6) Pain. Reinnervation leads to “pain in response to light touch” (hyperalgesia or allodynia) e.g. dysmenorrhea, vulval pain, uterosacral pain, “mesh” pain, etc. [58]. |
|
7) CNS sensitization. Interstitial cystitis, chronic pain syndromes, postmenopausal pain, chronic vulval pain, etc. or neurovascular reflexes in migraine and the menopause [58]. |
|
8) Induction of “new” receptors e.g. purinergic, P2X3, “stretch” receptors, vascular epithelial growth factor (VEGF), TRPV-1, etc. as secondary mechanisms of intercellular communication [53,54]. |
|
9) Viscero-visceral reflexes. The uterorenal reflex may lead to activation of a corticomedullary vascular “shunt” in pre-eclampsia. Wider activation of the autonomic nervous system including headache, migraines, nausea, vomiting, restlessness, fatigue, etc. may also take place in chronic pelvic pain with, or without, “endometriosis” [59]. |
|
10) Ischemia-thrombosis. Injuries to visceral vasomotor nerves cause narrowing of arterioles through secondary hyperplasia of the denervated arteriole. Pregnancy complications including placental abruption and placental infarction are associated with narrowing of visceral arterioles [60]. |
Conclusions
Many of the “great” obstetric and gynaecological syndromes show clear histological evidence of neurovascular injuries in the form of narrowed arterioles with associated injured nerves (gynaecological syndromes), or, injured nerve sheaths (obstetric syndromes). Injuries to uterine nerves result from a small number of preventable injuries including (i) coordinate and incoordinate, straining on the toilet, (ii) “difficult” first labours that are prolonged, or, “complex”, (iii) some forms of “neuropathic”, gynaecological surgery. They produce a wide range of diverse, and varied, clinical consequences that we recognize as different obstetric and gynaecological syndromes, and, chronic diseases. The denervation-reinnervation sequences takes place over a number of years in one or more adjacent viscera, with their timescales that vary in different tissues, and, may be further delayed by intervening vaginal deliveries. Selective injuries to autonomic nerves during embalming procedures in undergraduate medical education, and, variable latent periods for reinnervation in different tissues, may have obscured their origins.
Most obstetric and gynaecological syndromes are preventable. Preventive measures include neuroprotective intrapartum, and, surgical care with more liberal use of Caesarean section and thoughtful surgical techniques that avoid neuropathic injuries. Impaired bowel habits require specific advice to diet and bowel habits to prevent neuropathic injuries in the abdomen and pelvis. In 1993, DP Burkitt said “we will waste three or four decades in pursuing complex (genomic) explanations of disease before returning to simple, lifestyle, explanations”. Three decades after Burkitt’s admonition, there are few signs of significant changes in clinical, or research, policy. On this evidence, much of women’s reproductive ill-health may be preventable by simple, neuroprotective measures that avoid intrapartum, surgical and defecatory injuries.
Bilateral swathes of postganglionic sympathetic, autonomic nerves from T10-L2 pass through the uterosacral -cardinal ligament complex to converge on the vaginal vault. Sacral parasympathetic fibres (S2-4) emerge from sacral foramina to merge with them in the pelvic plexi. This specimen was prosected in alcohol, rather than formalin, since the latter selectively destroys these nerves [12]. They are vulnerable to injury in labour, during gynaecological surgery, and, through straining on the toilet [19,23].
Conflicts of Interest
There are no conflicts of interest associated with this manuscript. All clinical studies cited in this manuscript were carried out with the express permission of the local research ethics committees in UK and China.
References
2. Zeek PM, Assali NS. Vascular changes in the decidua associated with eclamptogenic toxemia of pregnancy. Am J Clin Pathol. 1950 Dec;20(12):1099–09.
3. Dixon HG, Robertson WB. A study of the vessels of the placental bed in normotensive and hypertensive women. J Obstet Gynaecol Br Emp. 1958 Oct;65(5):803–9.
4. Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of pre-eclampsia. J Pathol. 1970 Aug;101(4):vi.
5. Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002 Nov;187(5):1416–23.
6. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "Great Obstetrical Syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011 Mar;204(3):193–201.
7. Cui L, Quinn MJ, Zhang HJ. Origins of the "great" obstetric and gynecologic syndromes. Am J Obstet Gynecol. 2021 Sep;225(3):349.
8. Raivich G, Makwana M. The making of successful axonal regeneration: genes, molecules and signal transduction pathways. Brain Res Rev. 2007 Feb;53(2):287–311.
9. Moritz AR, Oldt MR. Arteriolar Sclerosis in Hypertensive and Non-Hypertensive Individuals. Am J Pathol. 1937 Sep;13(5):679–728.7.
10. Raivich G, Makwana M. The making of successful axonal regeneration: genes, molecules and signal transduction pathways. Brain Res Rev. 2007 Feb;53(2):287–311.
11. Horton J, Sterrenburg M, Lane S, Maheshwari A, Li TC, Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod Update. 2019 Sep 11;25(5):592–632.
12. Spackman R, Wrigley B, Roberts A, Quinn M. The inferior hypogastric plexus: a different view. J Obstet Gynaecol. 2007 Feb;27(2):130–3.
13. Krantz KE. Innervation of the human uterus. Ann N Y Acad Sci. 1959 Jan 9;75:770–84.
14. Krantz KE, Phillips WP. Anatomy of the human uterine cervix, gross and microscopic. Ann N Y Acad Sci. 1962 Sep 29;97:551–63.
15. Quinn M, Kirk N. Uterosacral nerve fibre proliferation in parous endometriosis. J Obstet Gynaecol. 2004 Feb;24(2):189–90.
16. Zhang XM, Huang X, Xu H, Quinn MJ. Altered innervation of the fallopian tube in ectopic pregnancy. J Obstet Gynaecol. 2014 Aug;34(6):531–2.
17. Eddy CA, Pauerstein CJ. Anatomy and physiology of the fallopian tube. Clin Obstet Gynecol. 1980 Dec;23(4):1177–93.
18. Guraslan H, Senturk MB, Dogan K, Yuksel B, Kaya C, Karacan T et al. Diagnostic office hysteroscopy; why is it still painful procedure despite the surgical experience and mini-hysteroscope? J Obstet Gynaecol Res. 2022 Jun;48(6):1418–25.
19. Atwal G, du Plessis D, Armstrong G, Slade R, Quinn M. Uterine innervation after hysterectomy for chronic pelvic pain with, and without, endometriosis. Am J Obstet Gynecol. 2005 Nov;193(5):1650–5.
20. Golding J. Children of the nineties. A longitudinal study of parents and children based on the (ALSPAC). West Engl Med J. 1990 Sep;105(3):80–2.
21. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997 Sep;32(9):920–4.
22. Probert CS, Emmett PM, Heaton KW. Some determinants of whole-gut transit time: a population-based study. QJM. 1995 May;88(5):311–5.
23. Quinn MJ. Perivascular nerve fibre proliferation: the consequence of prolonged straining. J Obstet Gynaecol. 2007 Feb;27(2):185–8.
24. Dimitriou N, Shah V, Stark D, Mathew R, Miller AS, Yeung JM. Defecating disorders: a common cause of constipation in women. Womens Health (Lond). 2015 Jul;11(4):485–500.
25. Vu D, Haylen BT, Tse K, Farnsworth A. Surgical anatomy of the uterosacral ligament. Int Urogynecol J. 2010 Sep;21(9):1123–8.
26. Xin YB, Hao GN, Lan JJ, Hong X, Juan ZH, MJ Q. Endometriosis”: a neuro-etiologic framework for its causes and consequences. Clin Obstet Gynecol. 2019;5:1–7.
27. Johnatty SE, Stewart CJR, Smith D, Nguyen A, O' Dwyer J, O'Mara TA, et al. Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci Rep. 2020 Feb 27;10(1):3621.
28. Savitskiĭ GA, Morozov VV, Svechnikova FA, Ivanova RD. Pathogenesis of uterine myoma development. Akusherstvo i ginekologiia. 1981 Apr(4):13–5.
29. Wang G, Tokushige N, Fraser IS. Nerve fibers and menstrual cycle in peritoneal endometriosis. Fertil Steril. 2011 Jun 30;95(8):2772–4.
30. Yan D, Liu X, Guo SW. Nerve fibers and endometriotic lesions: partners in crime in inflicting pains in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017 Feb;209:14–24.
31. Mechsner S, Kaiser A, Kopf A, Gericke C, Ebert A, Bartley J. A pilot study to evaluate the clinical relevance of endometriosis-associated nerve fibers in peritoneal endometriotic lesions. Fertil Steril. 2009 Dec;92(6):1856–61.
32. Sampson JA. The life history of ovarian hematomas (hemorrhagic cysts) of endometrial (Müllerian) type. Transactions of the American Gynecological Society. 1922;4(5):451–6.
33. Quinn MJ. Endometriosis: the consequence of uterine denervation-reinnervation. Arch Gynecol Obstet. 2011 Dec;284(6):1423–9.
34. Quinn M. Endometriosis: the elusive epiphenomenon. J Obstet Gynaecol. 2009 Oct;29(7):590-3.
35. Xin YB, Hao GN, Lan JJ, Hong X, Juan ZH, MJ Q. Endometriosis”: a neuro-etiologic framework for its causes and consequences. Clin Obstet Gynecol. 2019;5:1–7.
36. Quinn MJ. Mechanisms of pain and retrograde menstruation in endometriosis. Am J Obstet Gynecol. 2024 Oct;231(4):e151-e152.
37. Xin YB, Hao GN, Lan JJ, Hong X, Juan ZH, MJ Q. Endometriosis”: a neuro-etiologic framework for its causes and consequences. Clin Obstet Gynecol. 2019;5:1-7.
38. Quinn MJ. The aetiology of narrowed uterine arterioles in obstetric and gynaecological syndromes. Placenta. 2016 Aug;44:114.
39. Leadbetter RE, Mawer D, Lindow SW. Symphysis pubis dysfunction: a review of the literature. J Matern Fetal Neonatal Med. 2004 Dec;16(6):349–54.
40. Burkitt DP. Some diseases characteristic of modern Western civilization. Br Med J. 1973 Feb 3;1(5848):274–8.
41. Barker DJ, Fall CH. Fetal and infant origins of cardiovascular disease. Arch Dis Child. 1993 Jun;68(6):797–9.
42. Di Sebastiano P, Fink T, di Mola FF, Weihe E, Innocenti P, Friess H, et al. Neuroimmune appendicitis. Lancet. 1999 Aug 7;354(9177):461–6.
43. Dvorak AM, Osage JE, Monahan RA, Dickersin GR. Crohn's disease: transmission electron microscopic studies. III. Target tissues. Proliferation of and injury to smooth muscle and the autonomic nervous system. Hum Pathol. 1980 Nov;11(6):620–34.
44. de Fontgalland D, Brookes SJ, Gibbins I, Sia TC, Wattchow DA. The neurochemical changes in the innervation of human colonic mesenteric and submucosal blood vessels in ulcerative colitis and Crohn's disease. Neurogastroenterol Motil. 2014 May;26(5):731–44.
45. Golder M, Burleigh DE, Belai A, Ghali L, Ashby D, Lunniss PJ, et al. Smooth muscle cholinergic denervation hypersensitivity in diverticular disease. Lancet. 2003 Jun 7;361(9373):1945–51.
46. Wang YQ, Zhang HJ, Quinn MJ. Fetal Hypertension. Am J Med. 2020 Feb;133(2):e72–3.
47. Wang YQ, Zhang HJ, Quinn MJ. Fetal hypertension and abnormal fetal cardiac morphology. Am J Obstet Gynecol. 2020 Feb;222(2):196–7.
48. Wang YQ, Zhang HJ, Quinn MJ. Fetal Hypertension and the "Barker Hypothesis". Angiology. 2020 Jan;71(1):92–3.
49. Quinn MJ. Uterine arteriolar injuries in chronic hypertension. Am J Obstet Gynecol. 2018 Nov;219(5):508–9.
50. Li YY, Gu QR, Chen GR, Quinn MJ. Arteriolar Injury in Hypertension. Am J Med. 2018 Mar;131(3):e133–4.
51. Spiegelburg O.The pathology and treatment of puerperal eclampsia. Trans Am. 1878;2:161–74.
52. Sophian GJ. The toxaemias of pregnancy. J Ir Med Assoc. 1957 Oct;41(244):101–11.
53. Wu XQ, Cai YY, Xia WT, Quinn MJ. The aetiology of pre-eclampsia, 1945-1953. BJOG. 2016 Dec;123(13):2130.
54. Quinn MJ. Preeclampsia-a disorder of uterine "stretch?". Am J Obstet Gynecol. 2020 Jul;223(1):135–7.
55. McNestry C, Killeen SL, Crowley RK, McAuliffe FM. Pregnancy complications and later life women's health. Acta Obstet Gynecol Scand. 2023 May;102(5):523–31.
56. Kissler S, Zangos S, Wiegratz I, Kohl J, Rody A, Gaetje R, et al. Utero-tubal sperm transport and its impairment in endometriosis and adenomyosis. Ann N Y Acad Sci. 2007 Apr;1101:38–48.
57. Alison WE Jr, Phillips LG, Linares HA, Hui PS, Hayward PG, Broemeling LD, et al. The effect of denervation on soft-tissue infection pathophysiology. Plast Reconstr Surg. 1992 Dec;90(6):1031–5.
58. Meacham K, Shepherd A, Mohapatra DP, Haroutounian S. Neuropathic Pain: Central vs. Peripheral Mechanisms. Curr Pain Headache Rep. 2017 Jun;21(6):28.
59. Morrison TC, Dmitrieva N, Winnard KP, Berkley KJ. Opposing viscerovisceral effects of surgically induced endometriosis and a control abdominal surgery on the rat bladder. Fertil Steril. 2006 Oct;86(4 Suppl):1067–73.
60. Koman LA, Smith BP, Pollock FE Jr, Smith TL, Pollock D, Russell GB. The microcirculatory effects of peripheral sympathectomy. J Hand Surg Am. 1995 Sep;20(5):709–17.