Abstract
Gastroesophageal Reflux Disease (GERD) is a chronic condition characterized by the retrograde flow of gastric contents into the esophagus, leading to symptoms such as heartburn, regurgitation, and potential esophageal injury. The pathophysiology of GERD primarily involves dysfunction of the lower esophageal sphincter (LES), impaired esophageal clearance, delayed gastric emptying, and transient LES relaxations. Additional contributing factors include hiatal hernia and obesity. The pharmacological management of GERD focuses on acid suppression and includes the use of proton pump inhibitors (PPIs), H2-receptor antagonists, antacids, and prokinetic agents. While PPIs remain the mainstay of treatment due to their superior efficacy, concerns about long-term use necessitate consideration of alternative strategies. Nonpharmacological interventions, such as lifestyle and dietary modifications: weight loss, head-of-bed elevation, and avoidance of trigger foods: play a critical role in symptom control and disease management. This review explores the underlying mechanisms of GERD, evaluates current pharmacologic therapies, and highlights the importance of holistic, nonpharmacologic approaches in the comprehensive management of the disease.
Keywords
GERD, Proton Pump Inhibitors, P-CABs, H2RAs, Antacids
Introduction
Gastroesophageal reflux disease (GERD) is a chronic condition defined by the reverse flow of gastric contents into the esophagus, causing unpleasant symptoms and potential complications.
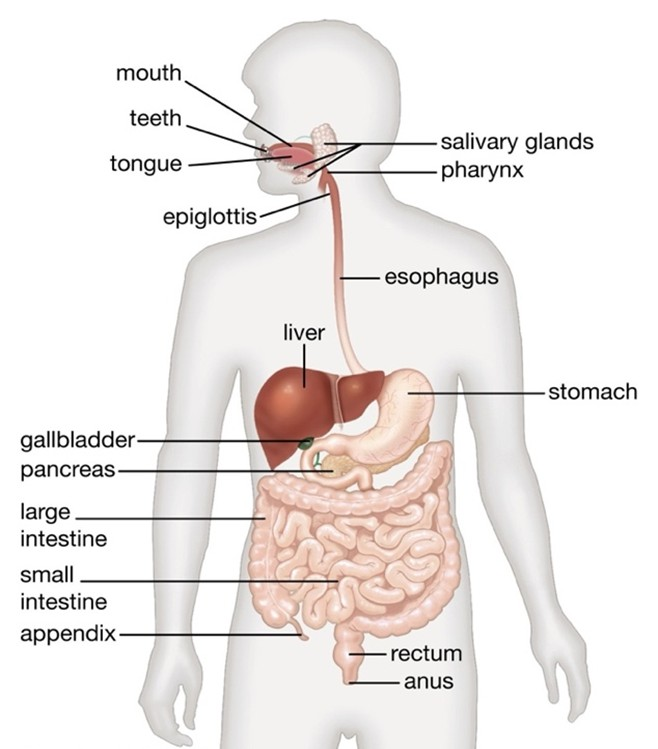
The human digestive system is an intricate assembly of organs and tissues that collaborate to decompose food, absorb nutrients, and release waste. The gastrointestinal (GI) tract: a long tube that runs from the mouth to the anus, including the esophagus, stomach, small intestine, and large intestine (Figure 1). Organs that assist in digestion but are not part of the GI tract, including the liver, pancreas, and gallbladder. Gastrointestinal disorders encompass a broad range of conditions affecting the digestive tract, which includes the esophagus, stomach, intestines, liver, pancreas, and gallbladder. These disorders can be categorized into functional and structural types, with functional disorders involving normal-looking structures but impaired motility, and structural disorders involving both abnormal structure and function [1].
Figure 1. In the mouth, the digestion process begins through mechanical breakdown (chewing) and chemical breakdown (salivary enzymes). Saliva moistens food and contains amylase to begin carbohydrate digestion. Esophagus: Muscular tube that connects the throat to the stomach, uses peristalsis (wave-like muscle contractions) to move food to the stomach. Stomach secretes gastric juices which contains hydrochloric acid and pepsin. Churns and mixes food to produce a thick paste known as chyme. Small intestine where 90% of food absorption involves duodenum (receives chyme from the stomach and digestive enzymes from the liver and pancreas), jejunum (chemical digestion and nutrient absorption occur) and ileum (Contains the ileocecal valve leading to the large intestine). Large intestine or Colon absorbs water and electrolytes, it hosts beneficial bacteria that aid in digestion, forms and stores feces until elimination. Accessories organs i.e. Liver: Produces bile for fat digestion and removes toxins. Gallbladder: Stores and concentrates bile, Pancreas: Secretes digestive enzymes and hormones that regulate blood sugar [2].
GI disorders can affect the human body's digestion process in various ways. Gastrointestinal disorders are ailments of the digestive system some common GI symptom includes foul breath, heartburn, lack of appetite, belching, diarrhea, constipation, indigestion, and nausea. GI disorders are classified as functional and structural disorders. Functional disorders have no physical damage but cause complications or pain in the working of the digestive system. Structural disorders have damage to the digestive tract or damage to its tissues.
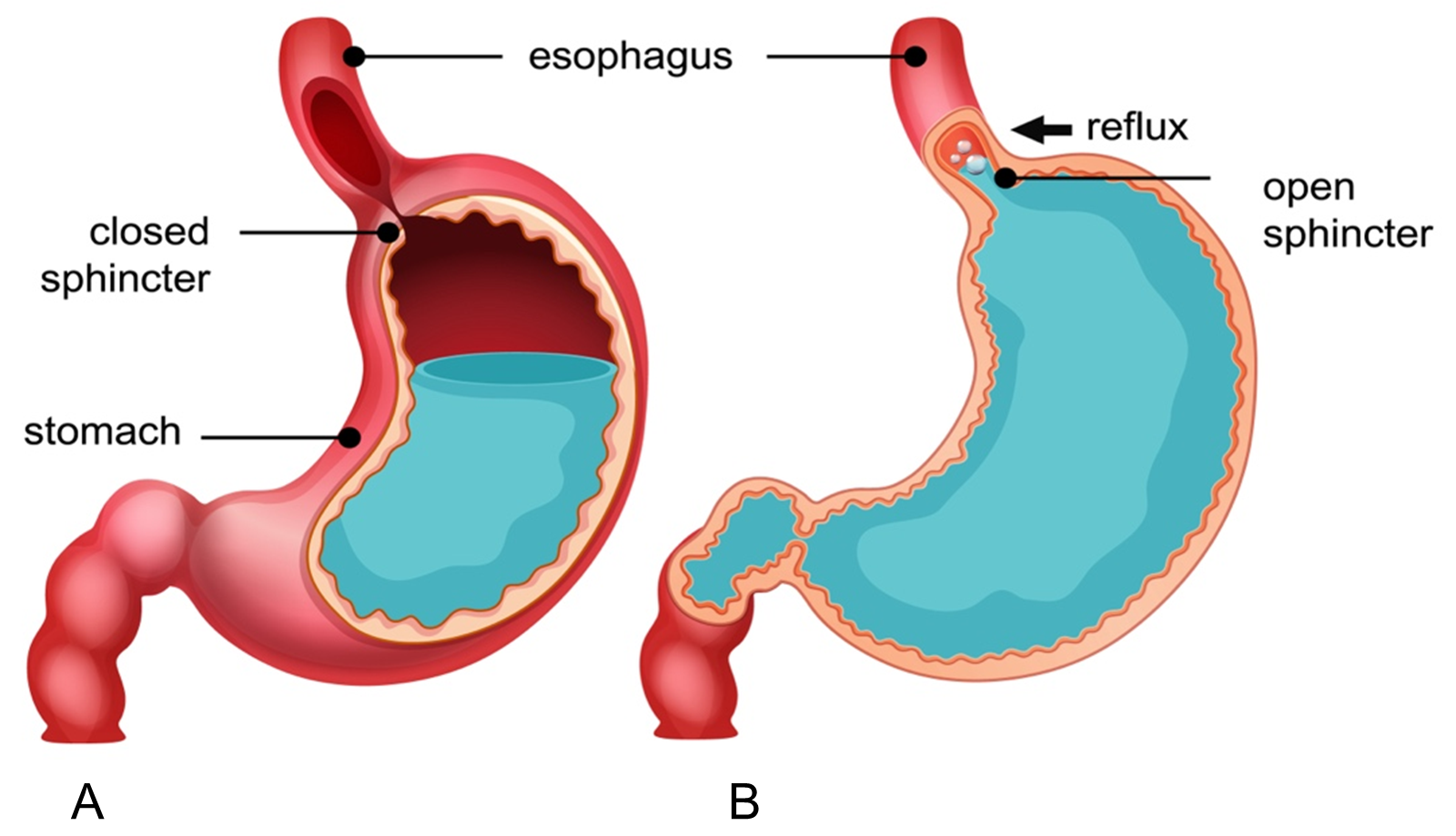
GERD is a common gastrointestinal disorder characterized by the backward flow of gastric content into the esophagus, resulting in uncomfortable symptoms and/or complications (Figure 2). The stomach produces the refluxate material, which is where GERD begins. As reflux progresses, the anti-reflux barrier—the lower esophageal sphincter and the crural diaphragm in particular—fails, allowing refluxate to enter the esophageal lumen and causing symptoms that are either extra or esophageal [3]. In Western culture, about 20% of adults suffer from GERD, one of the most prevalent gastrointestinal disorders. El-Serag et al.'s systematic review estimated that 18.1% to 27.8% of Americans had GERD. However, since more people have access to over-the-counter acid, fewer people may need medication, meaning that the true prevalence of this disorder may be higher [4].
Figure 2. A. Normal Physiological Condition, B. Gastroesophageal Reflux Disease (GERD): stomach acid flows back into esophagus.
The pathophysiology of GERD is multifactorial and involves several key mechanisms:
Anti-reflux barrier dysfunction
The esophagogastric junction normally functions as an anti-reflux barrier. This barrier is compromised in GERD due to: Increased frequency of Transient lower esophageal sphincter relaxations (TLESRs), which are the most common mechanism for reflux in both healthy individuals and GERD patients [5]. Presence of hiatal hernia, which impairs the structural integrity of the esophagogastric junction, is frequently linked with GERD and can exist independently without causing any symptoms [6].
Refluxate characteristics
The refluxate in GERD is a mixture of gastric and biliopancreatic secretions. Acid reflux is associated with heartburn and mucosal damage. Bile reflux can lead to more severe esophagitis or Barrett's esophagus. Non-acid reflux is mainly associated with symptoms but typically does not cause mucosal damage [7].
Esophageal clearance and mucosal integrity
Reduced salivary bicarbonate production, Ineffective primary, secondary peristalsis and impaired esophageal clearance mechanisms contribute to GERD by prolonging contact time between refluxate and esophageal mucosa. This review article covers the available pharmacological and non-pharmacological treatment for GERD.
Biomarkers for GERD
The research suggests that no single biomarker is likely to be sufficient for diagnosing GERD due to the heterogeneity of the condition. A combination of biomarkers from different biological compartments (saliva, serum, breath, tissue) may provide the most accurate diagnostic approach [8].
Salivary pepsin is the most studied noninvasive biomarker for GERD, showing significant sensitivity and specificity in multiple studies [8].
Detection of pepsin in saliva is associated with both GERD and laryngopharyngeal reflux (LPR), with sensitivity ranging from 50% to 85% and specificity from 60% to 100% depending on study design and population [9]. Changes in the oral microbiome, specifically increased abundance of Lautropia, Streptococcus, and Bacteroidetes, have shown high discrimination between BE and controls, suggesting potential for risk stratification in GERD-related complications [10,11].
Serum biomarkers
Elevated levels of tumor necrosis factor-alpha (TNF-α) seen in both GERD and Barrett's esophagus. C-peptide levels ≥360 pg/mL predicted GERD in a study of WTC-exposed firefighters. Matrix metalloproteinase-9 (MMP-9) levels were significantly different between GERD and control groups.
Inflammatory markers
IP-10 and fractalkine predicted Barrett's esophagus in one study. TNF-α predicted both GERD and Barrett's esophagus.
Esophageal mucosal integrity
Dilated intercellular spaces and reduced trans-epithelial resistance in esophageal mucosa are promising biomarkers. Baseline impedance measurements of esophageal mucosa show potential [12].
Pharmacotherapy
Proton pump inhibitors (PPIs)
PPIs were first introduced in the late 1980s, with omeprazole being the first to market in 1989. This marked a significant advancement in GERD treatment, as PPIs offered more potent and longer-lasting acid suppression compared to previous therapies like H2 receptor antagonists. PPIs remain the most effective and widely used medication for GERD treatment. They work by blocking acid production in the stomach and are more potent than other acid-suppressing medications. They are the first-choice therapy for most GERD patients [13]. Standard dosages are typically recommended for an 8-week course (Table 1). PPIs are most effective when taken 30-60 minutes before meals, usually breakfast.
|
Proton Pump Inhibitors (PPIs) |
Dose (mg) |
Over-the-Counter (OTC) option |
|
Omeprazole |
10, 20, 40 |
Yes |
|
Esomperazole |
20, 40 |
Yes |
|
Lansoprazole |
15, 30 |
Yes |
|
Rabeprazole |
10, 20 |
No |
|
Pantoprazole |
20, 40 |
No |
|
Dexlansoprazole |
30, 60 |
No |
|
Omeprazole with bicarbonate |
20, 40 |
Yes |
Mechanism of action: PPIs are administered as inactive prodrugs. They are weak bases that can freely cross cell membranes. Once in the acidic environment of the stomach, specifically in the secretory canaliculi of parietal cells, the prodrug becomes protonated and rearranges into its active form. The active form of the PPI then covalently and irreversibly binds to the hydrogen/potassium adenosine triphosphatase enzyme (H+/K+ ATPase), also known as the gastric proton pump. This enzyme is responsible for the final step of acid secretion in the stomach, pumping hydrogen ions into the gastric lumen in exchange for potassium ions. By inhibiting the H+/K+ ATPase, PPIs block this final step of acid production, resulting in a profound and long-lasting reduction of gastric acid secretion [14]. The irreversible nature of this binding means that the inhibitory effect lasts much longer than the plasma half-life of the drug.
Acid secretion only returns to normal after new proton pumps are synthesized and inserted into the parietal cell membrane. PPIs can reduce gastric acid secretion by up to 99%, making them more effective than other acid-reducing medications like H2 receptor antagonists.
Potassium-competitive acid blockers (P-CABs)
Potassium-competitive acid blockers (P-CABs) are a newer class of acid-suppressing medications that offer several advantages over traditional proton pump inhibitors (PPIs) in the treatment of GERD and other acid-related disorders (Table 2).
Unlike PPIs, P-CABs are not prodrugs do not require activation. Bind to both active and inactive forms of the proton pump. P-CABs are acid-stable and do not need enteric coating [15].
|
Proton Pump Inhibitors (PPIs) |
Potassium Competitive Acid Blockers (P-CABs) |
|
Direct action on H+-K+ ATP-ase |
Pro-drugs that need to be transformed to the active form |
|
Binding covalently to H+-K+ ATP-ase |
Binding to K+ site of H+-K+ ATP–ase |
|
Irreversible binding to the proton pump |
Reversible binding to the proton pump |
|
Full effect after 3–5 days |
Full effect after the first dose |
|
Affected by genetic polymorphism |
Not affected by genetic polymorphism |
|
Pharmacodynamic effect greater during the daytime |
Pharmacodynamic effect lasting for both the daytime and nocturnal hours |
Mechanism of action: P-CABs target the H+/K+ ATPase transporter on the luminal membrane of gastric parietal cells, which is the same proton pump targeted by PPIs. To prevent acidifying proton secretion, P-CABs concentrate in parietal cell canaliculi and ionically bond to H+/K+ ATPase transporters following systemic absorption. Once bound, the P-CAB prevents K+ ion access to the proton pump. P-CABs, unlike PPIs, are acid-stable, so they do not require enteric coating or optimal dosing 30 minutes before meals. Furthermore, P-CABs are not prodrugs and act directly at the proton pump. These P-CAB mechanistic differences allow for more rapid attainment of peak plasma levels and onset of action [16].
Vonoprazan is the most studied drug, which was first approved in Japan in 2015 (Takecab, Takeda) to be used in the management of acid-related conditions (reflux esophagitis, duodenal ulcer, gastric ulcer, and prevention of occurrences of gastric or duodenal ulcer, as well as an adjuvant to the eradication of Helicobacter pylori [HP]). Subsequent approvals were extended to other nations [17].
The US Food and Drug Administration (FDA) accepted the filing of a New Drug Application for vonoprazan for the treatment of erosive esophagitis (EE) in May 2022 and approved two vonoprazan-containing HP treatment regimens vonoprazan plus amoxicillin and clarithromycin.
The developing P-CAB class currently includes fexuprazan, keverprazan, revaprazan, tegoprazan, and vonoprazan, with others under development (such as linaprazan and zastaprazan) [16].
Histamine-2 receptor antagonists (H2RAs)
Histamine-2 receptor antagonists (H2RAs) play an important role in the treatment of GERD, though they have largely been superseded by PPIs as first-line therapy. H2RAs still have a role in managing milder cases, providing on-demand therapy, and as adjuncts to PPI therapy in certain situations. Their faster onset of action and ability to be used as needed make them a useful option for some patients with GERD.
H2RAs, also known as H2 receptor blockers, are commonly used to treat a variety of gastrointestinal disorders by decreasing stomach acid production. For individuals with mild to infrequent heartburn or indigestion, stomach or duodenal ulcers, gastric hypersecretion, and uncomplicated GERD, FDA has approved the short-term administration of H2RAs. Off-label uses of H2RAs include esophagitis, gastritis, gastrointestinal hemorrhage, urticaria, and stress ulcer prevention. Sometimes a multidrug regimen for the eradication of Helicobacter pylori includes H2RAs [18].
In the United States, three H2RAs—famotidine, cimetidine, and nizatidine are available as prescription or over-the-counter (OTC) drugs that have FDA approval. Prescriptions may be needed for cimetidine and famotidine, depending on the dosage. Nizatidine at higher doses needs a prescription.
H2RAs reversibly bind to histamine H2 receptors on gastric parietal cells and blocking the binding and function of the endogenous ligand histamine, H2RAs reduce the release of stomach acid. Thus, H2 blockers act as antagonists that compete with one other. After a meal, gastrin often induces the production of histamine from enterochromaffin-like cells. This histamine subsequently attaches to histamine H2 receptors on gastric parietal cells, prompting the release of stomach acid. The activation of adenylate cyclase, which increases intracellular cyclic adenosine monophosphate (cAMP) levels, causes this rise in gastric acid output. Protein kinase A (PKA), which phosphorylates proteins involved in transporting H+/K+ ATPase transporters to the plasma membrane, is subsequently activated by cyclic AMP. Increased plasma membrane H+/K+ ATPase transporters permit parietal cells to secrete more acid.
H2RAs inhibit gastric acid production that is both basal and triggered by histamine by inhibiting the histamine receptor and so preventing histamine from stimulating parietal cell acid secretion. Research indicates that the addition of an H2RA enhances the regulation of intragastric pH at night. According to the study, H2RAs have a relatively milder effect on gut microbiota than PPIs, with negligible alterations in microbial composition and oral-to-gut transmission. This suggests that PPIs might influence the alteration of gut microbiota more than H2RAs [19].
Adverse effects: Adverse effects from the H2RAs are usually well tolerated. Headache, tiredness, drowsiness, abdominal pain, constipation, or diarrhea are examples of mild side effects. Patients over 50, those with hepatic or renal impairment, or both may experience central nervous system adverse effects such delirium, disorientation, hallucinations, and slurred speech when H2RAs are administered. The H2 blocker that is usually thought to be most likely to elicit these symptoms is cimetidine, however famotidine treatment has also been known to have comparable effects. Regular use of H2 receptor antagonists may lead to tolerance or tachyphylaxis, which would restrict its usefulness as a maintenance treatment for GERD symptoms. After seven to fourteen days of continuous treatment, tolerance to these effects may develop. Tachyphylaxis development may be halted by administration that occurs sporadically or only when necessary. Compared to PPIs, H2RAs have a comparatively low risk of bacterial overgrowth and infections [20].
|
Name |
Dosage form |
Strength |
Daily dose for gastroesophageal reflux disease (GERD) |
|
Famotidine |
Injectable solution Chewable tablet Oral powder for suspension |
0.4 mg/ml, 10 mg/ml 10 mg, 20 mg, and 40 mg 40 mg/ml |
20 mg twice daily |
|
Cimetidine |
Injectable solution Oral solution Tablets |
150 mg/ml 150 mg/5ml 200 mg, 300 mg, 400 mg, 600 mg, and 800 mg |
1600 mg daily, divided into 800 mg twice or 400 mg 4 times daily |
|
Nizatidine |
Tablet Oral solution |
75 mg, 150 mg, and 300 mg |
150 mg twice daily (oral) |
|
Aluminum hydroxide |
Suspension |
- |
640 mg up to 5 to 6 times a day |
|
Calcium carbonate |
Tablets |
- |
8,000 mg per day (1 to 4 tablets daily) |
Antacid
A class of medications known as antacids has been available for a long time. They served as the primary line of defense against peptic ulcer disease at first, but the development of PPIs completely changed how the condition was treated. Nowadays, the only indication for taking antacids is to treat mild intermittent GERD and its related heartburn. This activity goes over the important information that members of an interprofessional team managing the care of patients with mild GERD and heartburn need to know about the indications, contraindications, pharmacological action, adverse events, and other important aspects of antacid therapy in the clinical setting [21].
Antacids work by neutralizing the stomach's acid, which lowers the amount of acid that reaches the duodenum. Some antacids also contain additional ingredients like alginates, which form a protective barrier on top of stomach contents, or simethicone, which reduces flatulence [22].
Antacids work best for reflux symptoms that occur intermittently and infrequently. They provide fast-acting relief, typically within minutes of ingestion, making them suitable for occasional use. However, they are not recommended for long-term management of GERD, as frequent use may worsen the problem by increasing acid production in the stomach [23].
It's important to note that while antacids can provide quick symptom relief, they do not prevent GERD symptoms or treat the underlying cause. For more severe or persistent GERD symptoms, other medications such as H2 blockers or PPIs may be more appropriate. As with any medication, antacids can have side effects, including diarrhea, constipation, and potential interactions with other medicine [24]. It's advisable to consult a healthcare professional if GERD symptoms persist or worsen despite the use of antacids [25].
Novel Therapeutic Approaches
Biologics
Biologics represent a class of advanced therapeutic agents derived from living organisms or containing components of living organisms. Unlike conventional small-molecule drugs, biologics are complex macromolecules designed to target specific pathways in disease pathogenesis. These innovative therapeutics include monoclonal antibodies, cytokines, and other immune-modulating agents that can precisely target inflammatory and immune-mediated mechanisms underlying various diseases. The emergence of biologics has revolutionized treatment approaches across multiple medical disciplines, providing targeted interventions for conditions previously managed with broader, less specific therapies. Their mechanism of action typically involves blocking specific cytokines, receptors, or other molecular targets implicated in disease pathophysiology, offering potential advantages in efficacy and safety profiles compared to conventional treatments.
While biologics have not yet established a role in mainstream GERD treatment, their success in related esophageal conditions like eosinophilic esophagitis suggests potential future applications. Dupilumab, as the first approved biologic for EoE, demonstrates the viability of targeting specific immune pathways in esophageal inflammatory conditions. However, the distinct pathophysiology of GERD compared to EoE means that direct translation of these therapies remains theoretical.
Dupilumab: pioneering biologic for eosinophilic esophagitis (EoE): Dupilumab (Dupixent®) has emerged as the first approved biologic for treating EoE in adults and children 12 years and older [26]. This monoclonal antibody targets the interleukin-4 receptor (IL-4Rα), which is involved in signaling for both IL-4 and IL-13, key cytokines in the inflammatory cascade of EoE [27].
While biologics have not yet established a role in mainstream GERD treatment, their success in related esophageal conditions like eosinophilic esophagitis suggests potential future applications. Dupilumab, as the first approved biologic for EoE, demonstrates the viability of targeting specific immune pathways in esophageal inflammatory conditions. However, the distinct pathophysiology of GERD compared to EoE means that direct translation of these therapies remains theoretical [28].
Mucosal protectants
In 2025, mucosal protectants for GERD focus on shielding the esophagus from acid, pepsin, and bile while promoting tissue repair. These agents are gaining traction as adjuncts or alternatives to acid suppressors, especially for refractory or non-erosive GERD.
- Vonoprazan + Alginate: P-CABs paired with alginate rafts provide dual action suppressing acid and shielding mucosa. Emerging as first-line for severe erosive esophagitis.
- PPI + Sucralfate-Plus: Enhances healing in Barrett’s esophagus patients by coupling acid suppression with active tissue repair.
- Deglycyrrhizinated Licorice (DGL): Chewable tablets with polyphenols that enhance mucus production and reduce oxidative stress. Widely used in Europe as adjunct therapy.
- Seaweed Polysaccharides: Carrageenan-based gels (e.g., GastroGard) show promise in reducing acid-induced epithelial damage.
- Sucralfate-Plus: A next-gen sucralfate derivative with rebamipide (a mucosal healing agent used in Asia). Binds to ulcers, stimulates prostaglandins, and reduces pepsin activity. Potential alternative for long-term PPI users.
Esophageal Permeability Coatings: Heparosan-based sprays (in preclinical testing) form a temporary, acid-resistant layer on damaged esophageal tissue.
Non-Pharmacological Treatment
Non-pharmacological treatments for GERD focus on lifestyle and dietary modifications to alleviate symptoms and improve quality of life. Here are the key approaches:
Lifestyle modifications
Reducing body weight, especially in overweight or obese individuals, can decrease intra-abdominal pressure and alleviate GERD symptoms [4,5]. Raising the head of the bed by 10–20 cm (4–8 inches) can prevent acid reflux during sleep by utilizing gravity [29]. Quitting smoking improves lower esophageal sphincter (LES) function and reduces GERD symptoms [30]. Moderate exercise, such as post-dinner walking, can help manage symptoms, though vigorous activity immediately after meals should be avoided [4]. Avoid lying down or bending over immediately after eating to prevent acid from flowing back into the esophagus. Late-night meals should be avoided as they can exacerbate GERD symptoms.
Dietary adjustments
Eating smaller portions reduces stomach distension and minimizes reflux. Avoiding trigger foods such as acidic foods (e.g., citrus fruits, tomatoes), fatty or fried foods, caffeine (coffee, tea, cola), alcohol, chocolate, peppermint [31]. Avoid eating within 2–3 hours before bedtime to reduce nighttime reflux.
Conclusion
GERD is a multifactorial condition arising from a complex interplay of physiological dysfunctions, including lower esophageal sphincter incompetence, impaired gastric motility, and esophageal hypersensitivity. Understanding its pathophysiology is essential for guiding effective treatment strategies. The effective management of GERD requires a multifaceted approach tailored to the severity and frequency of symptoms. Lifestyle modifications, such as dietary changes, weight management, and avoiding trigger foods, play a foundational role in controlling mild symptoms. Pharmacologic therapies, particularly PPIs and H2 receptor blockers, remain the mainstay for more persistent or severe cases, offering substantial symptom relief and mucosal healing. Ultimately, the goal of GERD treatment is to improve symptoms, quality of life, and prevent complications, emphasizing the importance of individualized care by integrating both pharmacologic and nonpharmacologic strategies are key to improving outcomes and quality of life for patients with GERD.
References
2. McQuilken SA. The mouth, stomach and intestines. Anaesthesia & Intensive Care Medicine. 2021 May 1;22(5):330-5.
3. Argüero J, Sifrim D. Pathophysiology of gastro-oesophageal reflux disease: implications for diagnosis and management. Nature Reviews Gastroenterology & Hepatology. 2024 Apr;21(4):282-93.
4. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014 Jun 1;63(6):871-80.
5. Iwakiri K, Hayashi Y, Kotoyori M, Tanaka Y, Kawakami A, Sakamoto C, et al. Transient lower esophageal sphincter relaxations (TLESRs) are the major mechanism of gastroesophageal reflux but are not the cause of reflux disease. Digestive Diseases and Sciences. 2005 Jun;50:1072-7.
6. Antunes C, Aleem A, Curtis SA. Gastroesophageal Reflux Disease(Archived). 2023 Jul 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–.
7. De Giorgi F, Palmiero M, Esposito I, Mosca F, Cuomo R. Pathophysiology of gastro-oesophageal reflux disease. Acta Otorhinolaryngologica Italica. 2006 Oct;26(5):241-6.
8. Kia L, Pandolfino JE, Kahrilas PJ. Biomarkers of reflux disease. Clinical Gastroenterology and Hepatology. 2016 Jun 1;14(6):790-7.
9. Farooqi MS, Podury S, Crowley G, Javed U, Li Y, Liu M, et al. Noninvasive, MultiOmic, and multicompartmental biomarkers of reflux disease: a systematic review. Gastro Hep Advances. 2023 Jan 1;2(4):608-20.
10. Sasamori R, Sato Y, Nomura K, Wakita A, Nagaki Y, Kemuriyama K, et al. Original Article Lipopolysaccharide induces CCL2 through TLR4 signaling and promotes esophageal squamous cell carcinoma cell proliferation. Am J Cancer Res. 2024 Jul 15;14(7):3497-12.
11. Martins IJ. Bacterial Lipopolysaccharides and Neuron Toxicity in Neurodegenerative Diseases. Neurol Res Surg. 2018;1(1):1-3.
12. Haider SH, Kwon S, Lam R, Lee AK, Caraher EJ, Crowley G, et al. Predictive biomarkers of gastroesophageal reflux disease and Barrett’s esophagus in World Trade Center exposed firefighters: A 15 year longitudinal study. Scientific Reports. 2018 Feb 15;8(1):3106.
13. Sandhu DS, Fass R. Current Trends in the Management of Gastroesophageal Reflux Disease. Gut and Liver. 2018 Jan 1;12(1):7-16.
14. Lata T, Trautman J, Townend P, Wilson RB. Current management of gastro-oesophageal reflux disease—treatment costs, safety profile, and effectiveness: a narrative review. Gastroenterology Report. 2023 Jan 1;11:goad008.
15. Wong N, Reddy A, Patel A. Potassium-competitive acid blockers: present and potential utility in the armamentarium for acid peptic disorders. Gastroenterology & Hepatology. 2022 Dec;18(12):693-700.
16. Abdel‐Aziz Y, Metz DC, Howden CW. potassium‐competitive acid blockers for the treatment of acid‐related disorders. Alimentary Pharmacology & Therapeutics. 2021 Apr;53(7):794-809.
17. Oshima T, Miwa H. Potent potassium-competitive acid blockers: a new era for the treatment of acid-related diseases. Journal of Neurogastroenterology and Motility. 2018 Jul 1;24(3):334-44.
18. Zhu J, Sun C, Li M, Hu G, Zhao XM, Chen WH. Compared to histamine-2 receptor antagonist, proton pump inhibitor induces stronger oral-to-gut microbial transmission and gut microbiome alterations: a randomised controlled trial. Gut. 2024 Jul 1;73(7):1087-97.
19. Nugent CC, Falkson SR, Terrell JM. H2 Blockers. 2024 Aug 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–.
20. Habibi A, Riley ET. Antihistamines: H1-and H2-Blockers. In: Atlee JL, Editor. Complications in Anesthesia (Second Edition). Philadelphia: WB Saunders; 2007. P. 92-3.
21. Garg V, Narang P, Taneja R. Antacids revisited: review on contemporary facts and relevance for self-management. Journal of International Medical Research. 2022 Mar;50(3):03000605221086457.
22. Yuan YZ, Fang JY, Zou DW, Levinson N, Jenner B, Wilkinson J. Alginate antacid (Gaviscon DA) chewable tablets reduce esophageal acid exposure in Chinese patients with gastroesophageal reflux disease and heartburn symptoms. Journal of Digestive Diseases. 2016 Nov;17(11):725-34.
23. Salisbury BH, Terrell JM. Antacids. 2023 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–.
24. Walden DM, Khotimchenko M, Hou H, Chakravarty K, Varshney J. Effects of magnesium, calcium, and aluminum chelation on fluoroquinolone absorption rate and bioavailability: A computational study. Pharmaceutics. 2021 Apr 21;13(5):594.
25. “GERD Treatment: How Do Antacids Work?” Accessed: Apr. 09, 2025. [Online]. Available: https://www.healthline.com/health/gerd/antacids
26. Syabbalo N. The role of biologics in the pathogenesis and treatment of eosinophilic esophagitis. Gastroenterol Hepatol Open Access. 2023;14(1):27-9.
27. Rossi CM, Santacroce G, Lenti MV, di Sabatino A. Eosinophilic esophagitis in the era of biologics. Expert Review of Gastroenterology & Hepatology. 2024 Jun 2;18(6):271-81.
28. Sher ER, Ross JA, Weine DM, Arjun AC. Current and emerging therapies for eosinophilic esophagitis. Allergy & Asthma Proceedings. 2022 May 1;43(3):178-86.
29. Velanovich V. Nonmedical treatment of gastroesophageal reflux disease. Gastroenterology & Hepatology. 2015 May;11(5):343-5.
30. Heidelbaugh JJ, Nostrant TT, Kim C, Van Harrison R. Management of gastroesophageal reflux disease. American Family Physician. 2003 Oct 1;68(7):1311-9.
31. Mukhtar M, Alzubaidee MJ, Dwarampudi RS, Mathew S, Bichenapally S, Khachatryan V, et al. Role of non-pharmacological interventions and weight loss in the management of gastroesophageal reflux disease in obese individuals: A systematic review. Cureus. 2022 Aug 31;14(8):e28637