Abstract
Apoptosis is a physiological response in development and homeostasis of metazoans. Apoptosis is triggered during pathological events as a means to renew affected tissues and eliminate cancer cells. The immune system regulates the extrinsic pathway of apoptosis, where signals such as TNFα or displayed ligands on the surface of immune cells trigger signal cascades by death receptors present on targeted cells. Therapeutics, like Doxorubicin, lead to apoptosis successfully. In this article, we present a design for a constitutively active death receptor (CADRE) based on a single mutation within the transmembrane domain of Fas (CD95, Apo-1, TNFRSF6), a prototypical death receptor in the tumor necrosis factor receptor superfamily. Overexpression of CADRE in HEK-293 cells initially results in decreased proliferation. Upon closer inspection, CADRE overexpression in HEK-293 caused apoptosis, similar to the effects of Doxorubicin. Finally, using 10 different cancer cell lines, we report that CADRE induced apoptosis in all of them. CADRE is a new tool in the search for a single solution to fight cancer.
Introduction
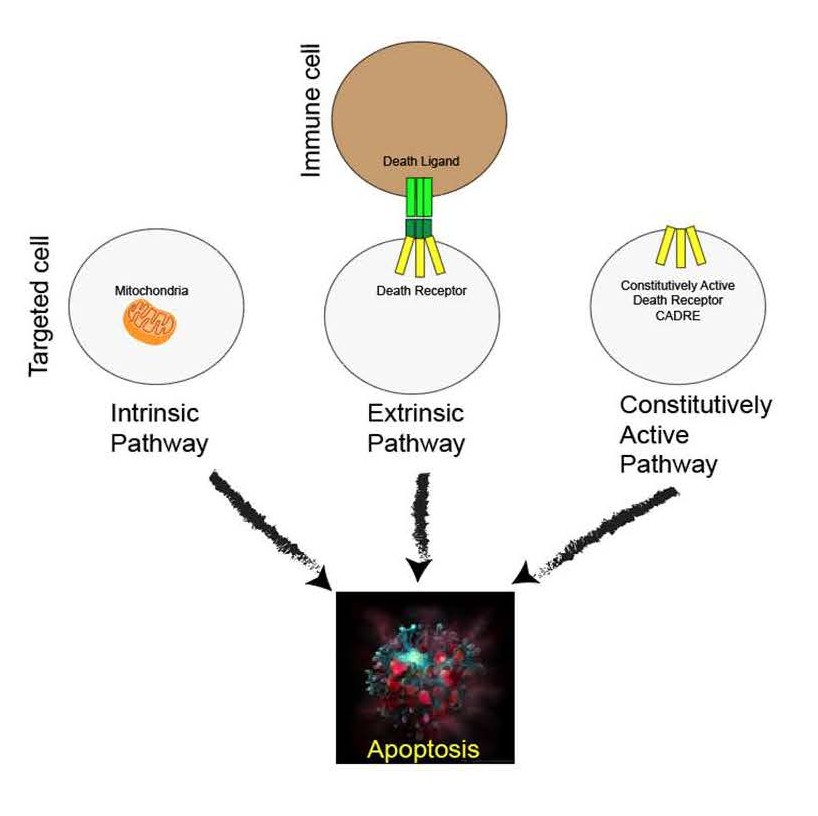
Cell death plays an important role in eukaryotic development and maintenance by allowing newer cells to replace those that are fatigued, damaged, or unwanted. Apoptosis is a cell’s natural mechanism for programmed cell death (PCD) that includes the organization and packaging of cellular components to be released by membrane breakdown [1,2]. Eukaryotic cells undergoing extreme environmental stressors are favored to undergo necrosis, or accidental cell death (ACD) [3]. Via the necrotic pathway, injured cells swell and burst causing damage to nearby cells. This results in an inflammatory response by the immune system, making this route unideal in medical treatment [4]. The mechanism taken by cells in apoptosis results in programmed death by way of phagocytosis and allows cells to be devoured without causing inflammation [5]. By mechanism of both intrinsic and extrinsic pathways, apoptosis signals a caspase cascade which prepares cell components for degradation [1,3].
Intrinsic apoptosis is signaled by destabilization of internal cellular homeostasis caused by DNA damage, replication abnormalities or high reactive oxidant species levels and is stimulated in the outer mitochondrial membrane [6]. In the intrinsic pathway, the practical consequences of pro-apoptotic signaling include mitochondrial membrane perturbation and release of cytochrome c in the cytoplasm [1]. In this position, cytochrome c forms an apoptosome complex with apoptotic protease activating factor 1 (APAF1) and also to the inactive form of caspase-9 [7]. This structure hydrolyzes adenosine triphosphate to cleave and activate caspase-9. The initiator caspase-9 then cleaves and activates the executioner caspases-3/6/7. This results in cell apoptosis [8].
Extrinsic apoptosis is signaled by intramembrane death receptors or dependence receptors [3]. Dependence receptors initiate PCD when microenvironmental ligand concentrations are subthreshold. A surface receptor, tumor necrosis factor receptor-1 (TNF-R1) will interact with tumor necrosis factor (TNF) to induce the involvement of the adaptor proteins FASassociated protein with death domain (FADD) and Tumor necrosis factor receptor type 1-associated death domain protein (TRADD) [9]. These proteins activate a series of downstream factors, including Caspase-8, which results in cell apoptosis [3].
TNF is the principal member of the TNF superfamily (TNFSF) of cytokines, and many of the receptors for TNFSF ligands are capable of initiating cell death [10]. FAS (CD95, Apo- 1, TNFRSF6) is a prototypical death receptor in the tumor necrosis factor receptor superfamily (TNFRSM) and contains an intracellular death domain which can trigger apoptosis [11,12]. FASL is naturally a trimeric ligand that promotes the trimerization of the FAS transmembrane region [11]. Triple helix bundle formation of the FAS transmembrane domain or the death inducing signal complex (DISC) is a prerequisite for activation of extrinsic Caspase pathway [11,12].
Cytokines control immune related events and are critically involved in a large number of pathophysiological events including autoimmunity and cancer development [13]. Mutations responsible for ligand-independent or constitutive activation of cytokine receptors have been observed [14]. The phenomenon of constitutive activation of receptors has been well documented in several protein families [15-19]. Many constitutively active cytokine receptor variants have been directly connected to disease [14]. Research has focused also in generating constitutive cytokine receptors with the goal to optimize immune therapeutics.
Cytokine signaling via receptors of the gp130 family are generally sub-classified into IL-6 and IL-12 family cytokines [20].Synthetic gp130 variants were generated. The first successful synthetic strategy replaced the entirety of the extracellular domain of gp130 by the 39-amino acid residue long leucine zipper of the transcription factor Jun (L-gp130), resulting in forced dimerization capable of intracellular signaling [21].
A second example is that of the IL-12 cytokine family, consisting of five members, involved in a very broad range of immune functions, including pro- and anti-inflammatory responses [22]. No naturally occurring constitutive-active receptors of the IL-12 cytokine family have been described to date. Synthetic constitutively active IL-23R variants by deletion of amino acids within the membrane-proximal stalk region of the receptor was successful [23]. The IL-23R lacks the three fibronectin-like domains typical of these family of receptors [22]. Instead of that the cytokine-binding domains (D1-D3) are connected with the transmembrane domain by a 37 amino acid long flexible linker peptide in the stalk region [24]. Extensive deletion of the stalk region (amino acid V319 to D353) and complete deletion of the extracellular domains (G24 to D353) both resulted in constitutive activation [23]. There have been no reports of constitutively active death receptors. Perhaps the idea that these receptors will lead to the cellular demise prevents us from observing them in nature.
Pharmacological interventions in cancer include Doxorubicin (DOX), a drug known to induce apoptosis in mammalian cells via the intrinsic pathway by triggering perturbations in metabolism [25]. Though effective in cancer treatment, Doxorubicin exhibits several limitations, including cardiotoxicity [26]. There are limits in DOX cumulative dose because of the damage it causes to the heart, therefore providing a restricted solution [26].
In this article, we developed FAS (TNFRSF6) as a constitutively active receptor. The transmembrane domain contains a mutation that was described to lead to a trimeric conformation [27]. To determine if this mutation was sufficient to activate the receptor, we also removed the extracellular domain, that naturally interacts with FASL. Here we present evidence that TNFRSF6, designed as a constitutively active death receptor (CADRE) induces apoptosis in mammalian cells.
Materials and Methods
CADRE plasmid
The CADRE plasmid was engineered to remove the extracellular domain and synthetically form the activated DISC trimmer of the human TNFRSF6 or TNFR6 (protein accession number P25445). The amino acid containing Cys178Ser mutation and devoid of the extracellular domain was codon optimized and synthesized by TWIST Biosciences (San Francisco CA, USA). The CADRE sequence was subcloned into pcDNA3.1 vector. DH5α cells were used for plasmid production. Plasmid purification was carried out with Monarch miniprep kit from New England Biolabs (Ipswich MA, USA). The complete DNA sequence of pcDNA3.1 CADRE is provided in the Supplementary File.
Reagents
Doxorubicin 2 mM was purchased from VWR (Randor PA, USA). Acridine orange, 10 mg/mL, (Biotium, San Francisco, CA, USA) and ethidium bromide, 10 mg/mL, (VWR, Radnor PA, USA) staining is used to visualize nuclear changes and apoptotic body formation that are characteristic of apoptosis [28]. Cells are viewed under a fluorescence microscope. The dye mix for the EB/AO staining contained 1 μL acridine orange and 1 μL ethidium bromide mixed in 1 mL PBS [28]. The transfection kit used, jetPEI Max, in these experiments was purchased from Polyplus Transfection (Illkirch-Graffenstaden, France). Mammalian cell lines transformation was carried out with 1 μg/mL of plasmid according to manufacturer’s specifications.
Cell culture
Eleven mammalian cell lines were used in analysis of CADRE. Two lung cell types, two colorectal, two prostate, two mammary gland, one placental and one adrenal gland cell line were used. Tissue culture procedure was followed to grow and maintain the cells. The following includes all cell lines used: human Embryonic Kidney cells HEK-293 (ATCC CRL-1573); human epithelial lung carcinoma A549 (ATCC CRM-CCL-185); large human pleural effusion lung carcinoma NCI-H460 (ATCC HTB-177); human colorectal adenocarcinoma HT-29 (ATCC HTB-38); human colorectal adenocarcinoma SW620 (ATCC CCL- 227); human prostate adenocarcinoma PC-3 (ATCC CRL-1435); human prostate carcinoma DU 145 (ATCC HTB-81) - human mammary gland epithelial adenocarcinoma MCF7 (ATCC HTB- 22); human mammary gland epithelial adenocarcinoma MDAMB- 231 (ATCC HTB-26); human trophoblast carcinoma hTERT SW.71 (CAT.NO T0532); and Adrenal gland pheochromocytoma hPheo1(RRID:CVCL_IY88)[29]. Excluding the control HEK cells, all cell lines were carcinoma or adenocarcinoma lines. Cell lines were grown in 8-well chambered cell culture slides on a single glass microscope slide (MatTek, Ashlan MA, USA), through standard tissue culture procedures. Each cell line was grown in two adjacent wells and transfected at 60% confluency. Negative control consisted of empty pcDNA3.1 plasmid.
Microscope and imaging
In this article, a confocal microscope was used to observe the transfected cells. The microscope used was the Olympus (Tokyo, Japan) FluoView FV1000 confocal laser scanning microscope designed for research applications in biology. The microscope is equipped with an argon laser with excitation light at wavelengths of 405 nm, 458 nm, 488 nm, and 515 nm. In addition, it provides green Helium-Neon and red Helium- Neon laser sources with respective excitation wavelengths of 543 nm and 633 nm. The acridine orange emitted light at 488 nm and the ethidium bromide emitted light at 534 nm. The cells were then observed and imaged at 20x magnification using standard procedure. The Olympus FluoView FV1000 software was used to obtain images of the samples. The program provides a set of tools for acquiring the highest quality image and allows adjustment of the plane being captured. Images can then be saved directly to the desktop being used.
Cell proliferation assay
The first day of the proliferation assay, HEK cells consisted of 50,000 cells seeded on 48-well plates. On the second day (at approximately 16 hours), cells were transfected with pcDNA3.1 empty or CADRE plasmid. After 24 and 48 hours, proliferation assays were performed using ATPlite Luminescence Assay System (PerkinElmer, American Fork, Utah) following the manufacturer’s instructions.
Results and Discussion
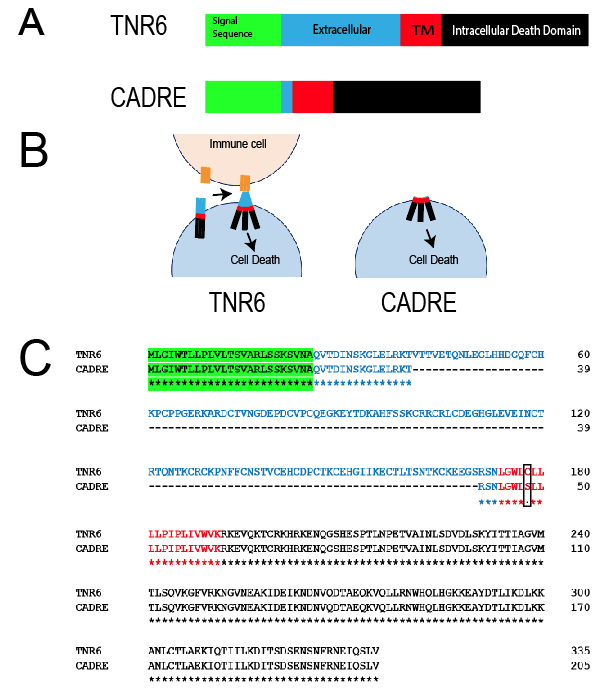
The initial design of a constitutively active death receptor was inspired by Fu and colleagues’ report [27]. These authors used NMR to study the structural basis of intramembrane trimerization of the FAS receptor (TNFRSF6). Their data identified Cys178 as part of the interface of the active trimeric transmembrane region [27]. The data collected by this group suggest that the structures represent the signaling-active conformation of FAS receptor transmembrane, which appears to be different from the pre-ligand conformation [27]. Endowed with these facts we attempted to construct and examine the phenotype of a constitutively active FAS receptor (TNFRSF6). We named this reagent CADRE (constitutively active death receptor). We demonstrate CADRE’s design in Figure 1. Another influence in the strategy was the reports of constitutively active IL-23, where the extracellular domain was omitted [23]. In the case of TNR6, FASL is required to stimulate apoptosis in the target cell expressing the receptor. We hypothesized that the extracellular region would not be necessary for the activation of CADRE. We also retained the original signal sequence in order to foster the proper expression and cellular localization od CADRE. Additionally, to enable the cleavage of the signal sequence after processing we preserved the first 15 amino acids following the cleavage site. Finally, to enable the proper insertion of the transmembrane domain of CADRE into the plasma membrane we preserved the three preceding amino acids according to known rules of this process [30]. We also added a Myc tag at the C-terminal of the protein. Western blot analysis was insufficient to determine the expression of CADRE due to events related to cell death. Thus, we resourced to cellular phenotypes like proliferation and Acridine orange/ Ethidium bromide stains.
Figure 1. CADRE Strategy and design. A) Schematic design of the transformation of TNR6. The design of CADRE preserved the original signal sequence (green), the transmembrane domain (red) and the DISC domain (black). B) Hypothesized assembly and effect of CADRE in mammalian cells. The comparison is made with the wild-type receptor and FASL. C) Amino acid sequence of CADRE. Colors match those of panel A. Cys178Ser mutation is boxed for clarity.
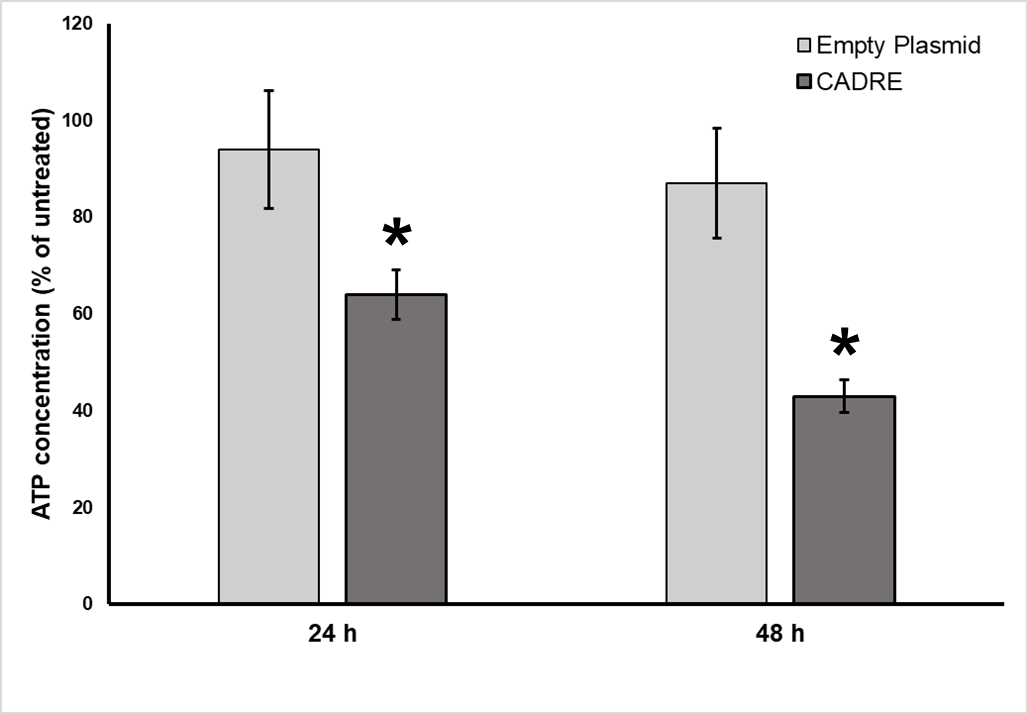
Following the careful design of CADRE, we synthesized the gene and clone it in pcDNA3.1. Knowing that apoptosis affects cell proliferation [31] we proceeded to examine the effects of transforming cells with pcDNA3.1 CADRE or empty vector as control (Figure 2). Cell proliferation and cell death are opposing cellular processes. A balance between these two processes promotes homeostasis, and limiting the growth and survival of cells with oncogenic mutations. A proliferation assay was performed in order to quickly identify potential phenotypes driven by CADRE overexpression. Overexpression of tumor necrosis factor receptors have been reported in HEK 293 cells to lead successfully to apoptosis [32]. HEK 293 cells provided an excellent model due to the fact that it contained all signaling elements to trigger apoptotic phenotypes.
Figure 2. HEK 293 cells Proliferation assay (ATPlite). Cells were prepared as described in Materials and Methods. ATP concentration of HEK-293 cells are normalized to that of untreated cells (pcDNA3.1 empty plasmid) after 24 and 48 h. Results were expressed as mean value ± standard deviation (SD). Student’s t-test and nonparametric t-test (Mann–Whitney) were used where appropriate. All statistical tests were two sided and unpaired, and p < 0.05 was considered statistically significant (*p<0.01). Statistical analyses were performed with GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Data analysis performed with six (n=6) independent experiments.
In order to efficiently characterize both antiproliferative potency and mechanism of action of CADRE, we employed a Cell Proliferation assay based on the content of ATP in cells in culture [33,34]. Increased ATP content have been associated with increased cell size proliferation [34]. Conversely, ATP reduction acts as a proxy for cell number and antiproliferative activity of cell lines for drug sensitivity [33], and apoptosis [34]. Considering CADRE’s overexpression decreased proliferation both at 24 and 48 post transformation when compared to empty vector we decided to compare apoptotic phenotypes of CADRE with those induced by DOX. An example in which DOX was used in HEK 293 cells to determine the biocidal activity of surfactants was used as part of the experimental design [35].
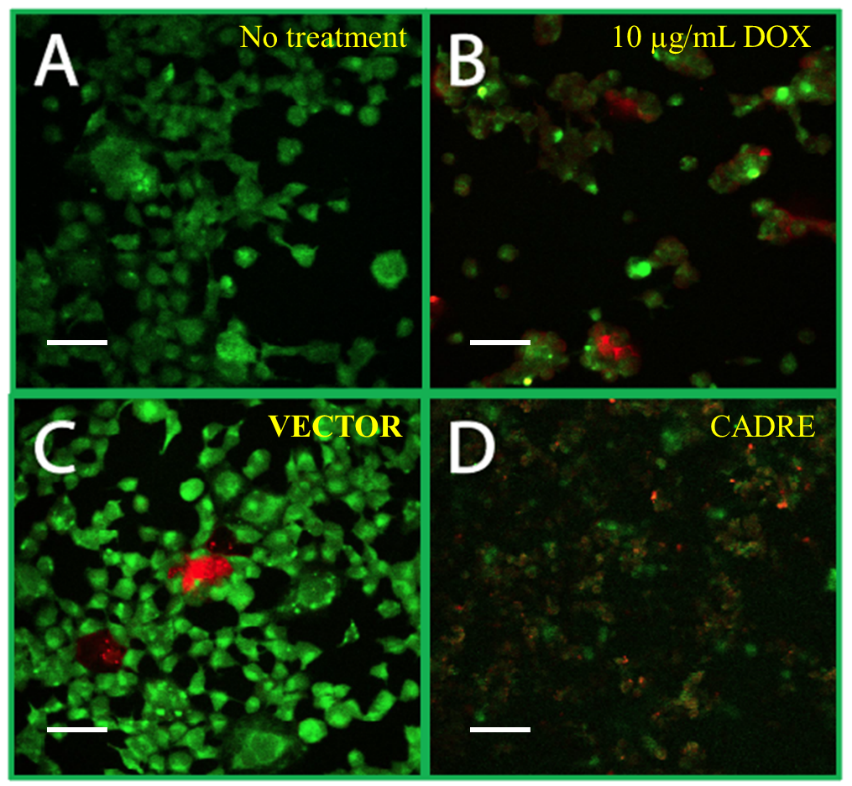
Acridine orange/ethidium bromide (AO/EB) staining is used to visualize nuclear changes and apoptotic body formation that are characteristic of apoptosis [28,36]. AO stains green the membrane of intact cells, apoptotic nonviable cells are stained bright orange because of the entry of EB into these cells. In Figure 3 we present the results of confocal microscopy imaging using AO/EB staining to determine apoptotic phenotypes [28]. Our observations indicate that both DOX and CADRE have similar effect and can result in changes in the AO/EB staining of HEK 293 cells that can be associated with apoptosis. Acridine orange is a dye that stains cells with intact cellular components, while EB will only stain the cells with decreased membrane integrity. Cells stained by AO will appear green and cells stained by EB will appear red. The color of the stain is an indicator of the cells’ progress toward apoptosis. Green cells represent those that are living or early apoptotic while red cells represent those that are in the late stages of apoptosis. Apoptotic cells will often show condensed and fragmented chromatin. This is opposed to necrotic cells, which resemble viable cells and contain no condensed chromatin [6,28].
Figure 3. Comparison of induction of apoptosis by CADRE and DOX. A) HEK 293 cells no treatment. B) HEK 293 cells exposed to 10 µg/mL DOX. C) HEK 293 cells transfected with Empty plasmid, pcDNA3.1 (VECTOR). D) HEK 293 cells transfected with pcDNA3.1 CADRE (CADRE). All treatments were imaged after 48 hours post transfection or treatment. All panels stained with AO/EB. All images obtained with 40X magnification (Materials and Methods). Scale bar equals 50 µm. Green color corresponds to intact cells. Red color corresponds to DNA stain with Ethidium bromide as a result of the formation of apoptotic bodies.
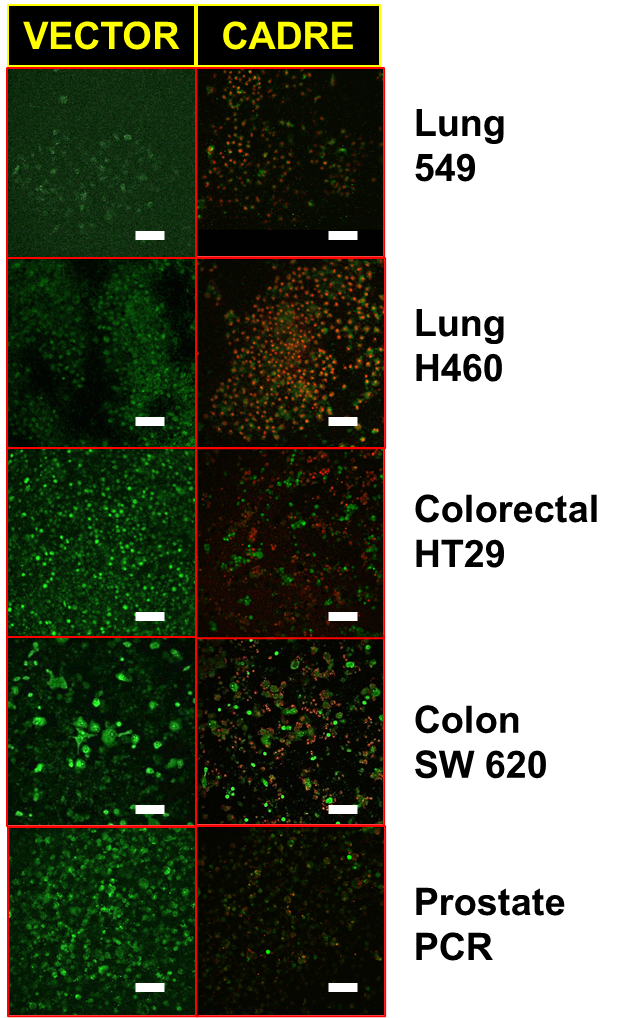
Our final experiment was to observe the apoptotic phenotypes triggered by the overexpression of CADRE in a variety of cancer-derived cells in vitro. Figure 4 contains the results of these experiments in which we compared cancer cells transformed with empty pcDNA3.1 and pcDNA3.1- CADRE. By examining CADRE’s effects over a variety of cancerderived cells we desired to distinguish between possible cancer idiopathy or tissue of origin. We examined 10 cell lines: Lung 549 and Lung H460; Colorectal HT29; Colon SW 620;Prostate PC3 and Prostate DU145; Placenta SW71; Breast MCF7 and Breast MDA-MB-231; Adrenal hPHEO1.
Figure 4. Effects of CADRE on cancer-derived cell lines. Cells were transfected with empty vector, pcDNA3.1 (VECTOR) or pcDNA3.1 CADRE (CADRE). Cells were imaged after 48 hours post transfection or treatment. All panels stained with AO/EB (Materials and Methods). All images obtained with 20X magnification. Scale bar equals100 µm. Green color corresponds to intact cells. Red color corresponds to DNA stain with Ethidium bromide as a result of the formation of apoptotic bodies.
Our results suggest that transfecting mammalian cells with CADRE leads to apoptosis. In the case of Non-small-cell lung cancer cell lines, H460 cells are a faster growing cell line, with a growth rate almost double that of A549 cells, as a result it was found that certain plasma membrane markers may directly correspond to tumor proliferation [37]. This and other features may distinguish tumors at the molecular level. Considering molecular differences in tumors identified through immortalized cell lines, we suggest that CADRE may cause apoptotic phenotypes in a wide range of cancers. Our work in this article is more qualitative. We manipulated all cells equally, seeding the same number of cells prior to transfection. It is possible that cell populations might be at different stages of proliferation. This might be expected of such a heterogeneous group of cells.
In its current state, a plasmid for transfection of mammalian cells (pcDNA3.1 CADRE) it cannot distinguish between normal and cancer cells. Thus, in its present form CADRE’s administration will result in all cells of a model organism to be affected. To mitigate this bottleneck, the use of gene delivery must be explored. CRISPR techniques can be used to identify cancer-driving mutations [38] and specifically target tumor cells containing these malignant information, perhaps replacing it with CADRE. Additionally, using viral particles to deliver CADRE to tumor cells is a more attractive perspective [39]. If the viral particles can be delivered specifically to the tumor cells [40], CADRE may represent a safe therapeutic for a broad range of malignancies. Convection-enhanced delivery (CED) is a promising technique that generates a pressure gradient to deliver therapeutics directly through the interstitial spaces of the central nervous system [41]. Perhaps, CED can also be used to deliver CADRE to neoplasms, with the advantage that as a viral particle CADRE will be a reagent that can’t regenerate since it will cause apoptosis in hosting cells. Thus, titrating or careful dosing of CADRE combined with CED can be a successful anti-cancer therapy.
Remaining work, ahead of these results, will include determining which intracellular machinery is activated through CADRE. Does CADRE compare to the FASL/TNR6 activation? Such research can identify other targets that can be cancer specific and can replace CADRE. Our reagent seems non-specific apoptosis triggering for mammalian cells. This scenario could be a negative aspect of CADRE as a cancer therapeutic. On the other hand, a specific delivery system of the CADRE gene can result in an umbrella-like therapy for all cancers as long as it can be dosed and delivered specifically to the tumor. Current developments in virus-like particles for gene therapy seem promising [42]. The dosing of CADRE or CADRE derived reagents could be a key factor to avoid side effects. Nevertheless, a virus-like particle carrying the CADRE gene may not survive the death of the host, making CADRE a reagent that can be controlled because it will be consumed but not regenerated within the body.
Conclusions
In this brief communication we present evidence that CADRE, a synthetic design of a constitutively active death receptor, demonstrates apoptotic phenotypes. The constitutive activity arises from a single mutation in the transmembrane domain of TNR6, and it was introduced in our design due to a literature report in which transmembrane domain carrying this mutation resulted in the formation of a trimer, a well-known feature of members of the TNFRSF. We consider CADRE to be a novel reagent with therapeutic applications. We propose that our design and findings can be further developed into a new anti-cancer therapeutic. In CADRE’s case it can result in a single therapy to any cancer.
Acknowledgements
All funding was derived from intramural awards at Brigham Young University.
Patent resulted from this work, 2021. Provisional patent application number 63/217,322. Patent sponsored by Brigham Young University, Tech Transfer Office. Director Mr. Michael Alder, mike_alder@byu.edu. All inquiries must be directed to Mr. Alder.
Conflicts of Interest
The authors declare no conflicts of interest.
References
2. Studzinski G, editor. Apoptosis: a practical approach. OUP Oxford; 1999 Dec 9;pp. xxii, 244 p.
3. Elmore S. Apoptosis: a review of programmed cell death. Toxicologic Pathology. 2007 Jun;35(4):495-516.
4. Tait SW, Ichim G, Green DR. Die another way–non-apoptotic mechanisms of cell death. Journal of Cell Science. 2014 May 15;127(10):2135-44.
5. Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nature Immunology. 2015 Sep;16(9):907-17.
6. Negroni A, Cucchiara S, Stronati L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediators of Inflammation. 2015 Sep 21;2015,250762
7. Byskov K, Le Gall SM, Thiede B, Camerer E, Kanse SM. Protease activated receptors (PAR)-1 and -2 mediate cellular effects of factor VII activating protease (FSAP). The FASEB Journal. 2020 Jan;34(1):1079-90.
8. Conde-Rubio MD, Mylonas R, Widmann C. The proteolytic landscape of cells exposed to non-lethal stresses is shaped by executioner caspases. Cell Death Discovery. 2021 Jun 19;7(1):164.
9. Xu H, Sethi JK, Hotamisligil GS. Transmembrane tumor necrosis factor (TNF)-α inhibits adipocyte differentiation by selectively activating TNF receptor 1. Journal of Biological Chemistry. 1999 Sep 10;274(37):26287-95.
10. Wallach D. The tumor necrosis factor family: family conventions and private idiosyncrasies. Cold Spring Harbor Perspectives in Biology. 2018 Oct 1;10(10):a028431.
11. Levoin N, Jean M, Legembre P. CD95 structure, aggregation and cell signaling. Frontiers in Cell and Developmental Biology. 2020 May 5;8:314.
12. Tauzin S, Debure L, Moreau JF, Legembre P. CD95-mediated cell signaling in cancer: mutations and post-translational modulations.Cellular and Molecular Life Sciences. 2012 Apr;69(8):1261-77.
13. Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. Journal of Interferon & Cytokine Research. 2019 Jan 1;39(1):6-21.
14. Floss DM, Scheller J. Naturally occurring and synthetic constitutive-active cytokine receptors in disease and therapy. Cytokine & Growth Factor Reviews. 2019 Jun 1;47:1-20.
15. Connor M, Traynor J. Constitutively active μ-opioid receptors. InMethods in Enzymology. Academic Press. 2010 Jan 1; pp. 445-469.
16. de Voux A, Chan MC, Folefoc AT, Madziva MT, Flanagan CA. Constitutively active CCR5 chemokine receptors differ in mediating HIV envelope-dependent fusion. PLoS One. 2013 Jan 23;8(1):e54532.
17. Neumann S, Raaka BM, Gershengorn MC. Constitutively active thyrotropin and thyrotropin-releasing hormone receptors and their inverse agonists. InMethods in Enzymology. Academic Press. 2010; pp. 147-160.
18. Searl TJ, Silinsky EM. Evidence for constitutively-active adenosine receptors at mammalian motor nerve endings. European Journal of Pharmacology. 2012 Jun 15;685(1-3):38-41.
19. Sharma A, Toy W, Guillen VS, Sharma N, Min J, Carlson KE, et al. Antagonists for Constitutively Active Mutant Estrogen Receptors: Insights into the Roles of Antiestrogen-Core and Side-Chain. ACS Chemical Biology. 2018 Nov 7;13(12):3374-84.
20. Rose-John S, Scheller J, Schaper F. “Family reunion”–A structured view on the composition of the receptor complexes of interleukin-6- type and interleukin-12-type cytokines. Cytokine and Growth Factor Reviews. 2015;5(26):471-4.
21. Bieberich E. Sphingolipids and lipid rafts: Novel concepts and methods of analysis. Chemistry and Physics of Lipids. 2018 Nov 1;216:114-31.
22. Hasegawa H, Mizoguchi I, Chiba Y, Ohashi M, Xu M, Yoshimoto T. Expanding diversity in molecular structures and functions of the IL-6/ IL-12 heterodimeric cytokine family. Frontiers in Immunology. 2016 Nov 4;7:479.
23. Hummel TM, Ackfeld T, Schönberg M, Ciupka G, Schulz F, Oberdoerster A, et al. Synthetic deletion of the interleukin 23 receptor (IL-23R) stalk region led to autonomous IL-23R homodimerization and activation. Molecular and Cellular Biology. 2017 Aug 11;37(17):e00014-17.
24. Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL- 12Rβ1 and a novel cytokine receptor subunit, IL-23R. The Journal of Immunology. 2002 Jun 1;168(11):5699-708.
25. Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms: intermediacy of H2O2-and p53-dependent pathways. Journal of Biological Chemistry. 2004 Jun 11;279(24):25535-43.
26. Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenetics and Genomics. 2011 Jul;21(7):440-6.
27. Fu Q, Fu TM, Cruz AC, Sengupta P, Thomas SK, Wang S, et al. Structural basis and functional role of intramembrane trimerization of the Fas/CD95 death receptor. Molecular Cell. 2016 Feb 18;61(4):602- 13.
28. Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR. Acridine orange/ethidium bromide (AO/ EB) staining to detect apoptosis. Cold Spring Harbor Protocols. 2006 Aug 1;2006(3):pdb-rot4493.
29. Ghayee HK, Bhagwandin VJ, Stastny V, Click A, Ding LH, Mizrachi D, et al. Progenitor cell line (hPheo1) derived from a human pheochromocytoma tumor. PloS One. 2013 Jun 13;8(6):e65624.
30. Baker JA, Wong WC, Eisenhaber B, Warwicker J, Eisenhaber F. Charged residues next to transmembrane regions revisited:“Positiveinside rule” is complemented by the “negative inside depletion/ outside enrichment rule”. BMC Biology. 2017 Dec;15(1):1-29.
31. Guo M, Hay BA. Cell proliferation and apoptosis. Current opinion in Cell Biology. 1999 Dec 1;11(6):745-52.
32. Cusick JK, Mustian A, Goldberg K, Reyland ME. RELT induces cellular death in HEK 293 epithelial cells. Cellular Immunology. 2010 Jan 1;261(1):1-8.
33. Chan GK, Kleinheinz TL, Peterson D, Moffat JG. A simple highcontent cell cycle assay reveals frequent discrepancies between cell number and ATP and MTS proliferation assays. PloS One. 2013 May 17;8(5):e63583.
34. Simmons TD, Slater KJ, Crouch SP. Changes in intracellular adp: ATP ratios as a marker of apoptosis. The Scientific World Journal. 2001;1:58.
35. Akiyode O, George D, Getti G, Boateng J. Systematic comparison of the functional physico-chemical characteristics and biocidal activity of microbial derived biosurfactants on blood-derived and breast cancer cells. Journal of Colloid and Interface Science. 2016 Oct 1;479:221-33.
36. García-Rodríguez MD, Carvente-Juárez MM, Altamirano- Lozano MA. Antigenotoxic and apoptotic activity of green tea polyphenol extracts on hexavalent chromium-induced DNA damage in peripheral blood of CD-1 mice: analysis with differential acridine orange/ethidium bromide staining. Oxidative medicine and cellular longevity. 2013:486419.
37. Townsend MH, Anderson MD, Weagel EG, Velazquez EJ, Weber KS, Robison RA, et al. Non-small-cell lung cancer cell lines A549 and NCI-H460 express hypoxanthine guanine phosphoribosyltransferase on the plasma membrane. OncoTargets and Therapy. 2017;10:1921- 32.
38. Pal M, Herold MJ. CRISPR base editing applications for identifying cancer-driving mutations. Biochemical Society Transactions. 2021 Feb 26;49(1):269-80.
39. Zittersteijn HA, Gonçalves MA, Hoeben RC. A primer to gene therapy: Progress, prospects, and problems. Journal of Inherited Metabolic Disease. 2021 Jan;44(1):54-71.
40. Rubin JD, Nguyen TV, Allen KL, Ayasoufi K, Barry MA. Comparison of gene delivery to the kidney by adenovirus, adeno-associated virus, and lentiviral vectors after intravenous and direct kidney injections. Human Gene Therapy. 2019 Dec 1;30(12):1559-71.
41. Mehta AM, Sonabend AM, Bruce JN. Convection-enhanced delivery. Neurotherapeutics. 2017 Apr;14(2):358-71.
42. Bulcha JT, Wang Y, Ma H, Tai PW, Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduction and Targeted Therapy. 2021 Feb 8;6(1):1-24.