Abstract
The objective of this paper is to review what is known about intestinal mucus in neurologic health and disease states. To set the stage, we summarize the physiology of mucus within the gastrointestinal tract and its role in gastrointestinal homeostasis and disease states. There is documentation of barrier dysfunction as well as damage to epithelial or mucosal defense mechanisms in prototypical neurological diseases, such as multiple sclerosis and Parkinson disease. The evidence is tabulated to summarize the data, and the strength of that evidence is appraised. Although there is relatively less evidence available regarding the association of abnormal mucus in these diseases, it is noted that changes in diet (such as the Western diet, emulsifiers, and lack of fiber) and specific microbial alterations, best exemplified by Akkermansia muciniphilia, result in deleterious alterations in mucin molecules or mucus (as a whole) in neurological diseases. These data suggest that further appraisal of the role of intestinal mucus and barrier may be relevant to the study of neurological diseases.
Keywords
Mucus, Multiple sclerosis, Parkinson disease, Akkermansia muciniphilia
Introduction
The intestinal epithelial barrier is a dynamic structure comprising epithelial cells (including enterocytes, goblet cells, Paneth cells, M cells, and enterochromaffin cells), tight junctions, immune cells and structures (such as gut-associated lymphoid tissue), luminal contents (such as bile acids, digestive enzymes, antimicrobial peptides), microbiota, and mucus [1]. These components form a semipermeable structure facilitating passage of dietary nutrients and beneficial microbial-derived metabolites, while preventing passage of toxins, dietary antigens, and microbes (and their inflammatory components such as endotoxin or lipopolysaccharide) [2].
Disruption of the epithelial barrier and the resultant increase in intestinal permeability initiate an inflammatory cascade which has been implicated in numerous gastrointestinal conditions (e.g., inflammatory bowel disease, celiac disease) and conditions associated with other digestive tract organs, specifically the pancreas (e.g., facilitating a role of intestinal dysbiosis in autoimmune pancreatitis) [3] and chronic liver disease (e.g., in spontaneous bacterial peritonitis [4] or hepatic encephalopathy) [5]. However, disruption of the intestinal barrier is also documented in non-gastrointestinal conditions including metabolic diseases, immune-mediated inflammatory diseases, and neuropsychiatric diseases [1,2]. We have previously described the physiology of mucus within the intestinal tract as it relates to maintenance of the gut epithelial barrier, and how certain lifestyle and environmental factors either harm or support the mucus layer [6].
The aims of this review are to summarize the physiology of mucus within the gastrointestinal tract and its role in gastrointestinal homeostasis and disease states, and to review what is known about intestinal mucus in neurological disease states.
Physiology of Intestinal Mucus
Mucus is a gel-like substance composed primarily of water and mucins, and it forms a protective coating over the entire gastrointestinal tract. Mucus helps to preserve intestinal permeability by forming a physical barrier, protecting epithelial cells from luminal contents such as gastric acid, digestive enzymes, pancreaticobiliary secretions, bile acids, dietary components, allergenic antigens, environmental toxins, and microbes [6]. In the small intestine, a single thin mucus layer is enriched with antimicrobial peptides. The colon features two mucus layers: a densely adherent and microbially sterile inner mucus layer and a looser outer layer colonized by the commensal microbiota. The microbiota utilize mucins (particularly MUC2) as substrate, and result in products which support the growth and sustenance of other beneficial microbes [6]. One especially important class of such metabolites are the short-chain fatty acids (e.g., butyrate, acetate, propionate), which nourish intestinal epithelial cells, regulate luminal pH, reduce local and systemic inflammation, and stimulate mucus production [6]. Mucosal biofilms, which are distinct microbial communities residing within the mucus layer, respond to the diverse luminal contents and can benefit the host. On the other hand, the microbes within the biofilm may turn pathogenic and become enriched with pathobionts which colonize the inner mucus layer, disrupting the gut epithelial barrier and inducing persistent inflammation [7].
Factors Which Affect the Integrity of the Mucus Layer
Many of the factors which either damage or fortify the gut epithelial barrier do so via effects on mucus [6,8]; hence, it is important to understand control of mucus. Diet is a principal determinant of the health of intestinal mucus. Diets high in fiber preferentially promote the growth of beneficial gut bacteria, which in turn elaborate short-chain fatty acids that have direct stimulatory effects on mucus production [9,10]. Conversely, a lack of dietary fiber requires microbiota to utilize mucus glycoproteins as a source of nutrition, thereby degrading the barrier [11]. Western-style diets, characterized by insufficient fiber, high fats, simple sugars, and processed or ultra-processed foods, are associated with reduced mucus production and thinning of the mucus layer, leading to increased intestinal permeability and inflammation [6,9]. Consumption of meat is also associated with thinning of the mucus layer and impaired mucus barrier function [12].
In addition to typically lacking adequate fiber and containing excess refined sugars, ultra-processed foods are particularly deleterious to the mucus layer due to their emulsifier content. Emulsifiers are common food additives which aid in texturizing foods. However, many of these agents (e.g., carboxymethylcellulose, polysorbate 80, sodium stearoyl lactylate, etc.) act as detergents, depleting the mucus layer and allowing bacterial encroachment into the normally sterile inner layer [13,14]. Alcohol consumption also affects the mucus layer by acutely dissolving lipids from the mucus layer, rapidly reducing mucosal surface hydrophobicity, [15] and chronically altering the gene expression of MUC2 (a secreted protein produced by epithelial goblet cells and a main component of mucus) and goblet cell function [16].
In addition to fiber, other dietary components which support the mucus layer include polyphenols [17] and omega-3 fatty acids [8]. Certain prebiotics and probiotics also aid in fortifying mucus [6]. Akkermansia muciniphilia has emerged as a promising probiotic, given its affinity for mucus and effects in stimulating mucus secretion and enhancing barrier function [19].
Role of Mucus in Gastrointestinal Diseases
Given the important role intestinal mucus plays in maintaining the gut epithelial barrier, alterations to mucus are implicated in numerous disease states. Inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn disease (CD) feature abnormalities of the epithelial barrier, including intestinal mucus specifically. Autoantibodies directed against colonic mucins, particularly MUC1 (a transmembrane mucin normally expressed on the apical borders of secretory epithelial cells), and other mucin core proteins have been demonstrated in a subset of patients with UC, particularly in 40% of those with chronic continuous UC and 19% of those with relapsing-remitting UC [20]. These antibodies contribute to mucosal injury via antibody-dependent cell-mediated cytotoxicity, depleting mucus and inducing chronic epithelial cell damage, increased intestinal permeability, and chronic inflammation [21]. Indeed, depletion of surface mucus and of goblet cells in the epithelial lining are characteristic features of colonic mucosal biopsies in UC. Moreover, visible biofilms have also been detected in 34% of patients with UC (compared to 6% of controls). This was associated with accumulation of toxic bile acids as well as dysbiosis, with enrichment of the pathobiont Ruminococcus gnavus, a known mucolytic within the biofilm of patients with UC [22,23]. Ultra-processed foods and, particularly, emulsifiers have been implicated in the pathogenesis of IBD [24].
Similar to UC, CD also features endoscopically-visible biofilms, observed in 22% of cases [22]. Pathogenic biofilms are also a known important feature of CD, enriched with organisms such as adherent invasive Escherichia coli (AIEC) [25], and fungi such as Candida species [26]. The dysbiosis and pathogenic biofilms in CD degrade mucus and damage goblet cells, contributing to barrier dysfunction, increased intestinal permeability, and chronic inflammation [27]. Even when CD is in remission, there are still demonstrable abnormalities in microbial composition and mucin production [28].
Irritable bowel syndrome (IBS), which is a disorder of gut-brain interaction, also features abnormalities relating to mucus. Patients often note the passage of mucus in the stool. Furthermore, similar to UC and CD, IBS has endoscopically-visible biofilms in 57% of patients [22]. Glycocalyx is also thinner in diarrhea-predominant IBS, and depletion of the jejunal barrier has been shown to contribute to the sensitization of enteric nerves, an important etiological factor for abdominal pain [29]. Additionally, bile acid malabsorption (BAM), which occurs in about 35.3% of patients with IBS-D [30], is also associated with alterations in mucus handling; for example, both chenodeoxycholic and deoxycholic acid are surfactants with detergent properties [31] which can contribute to mucin depletion and mucosal inflammation [6].
Abnormalities in intestinal mucus have also been implicated in celiac disease, [32] colorectal cancer, [33] small intestinal bacterial overgrowth [34], and enteric infections [35].
Role of Intestinal Mucus and Barrier Function in Neurological Diseases
The intestinal microenvironment is involved in the “gut component” of several axes involving other organs (gut-liver, gut-pancreas, gut-brain axes) that influence several organ systems via nutrition, barrier function, and microbial metabolites. Moreover, the gastrointestinal tract harbors extensive neuronal networks in the enteric nervous system. It is, therefore, not surprising that intestinal barrier function has been implicated in neurologic health and disease, and such interactions are observed in several inflammatory gastrointestinal disorders (e.g., IBD and celiac disease) as well as being associated with considerably increased risk of neurological diseases such as multiple sclerosis, Parkinson disease, Alzheimer disease, and others [36-40].
Many of the risk factors implicated for the most common neurological or neurodegenerative diseases match those documented for gastrointestinal disorders. Factors documented in neurodegenerative diseases as directly damaging the gut epithelial barrier include the Western diet [41] and exposure to environmental toxins [42]. While the effects of barrier dysfunction have been reviewed extensively elsewhere [43,44], less focus has been given to the contributions specifically from alterations in mucus. In the following section, we summarize the findings of abnormalities in intestinal permeability and intestinal mucus which have been identified in common neurologic conditions.
Multiple sclerosis
Multiple sclerosis (MS) is characterized by immune-mediated destruction of central nervous system components, particularly myelin. MS is associated with dysbiosis and increased intestinal permeability [45–50]. In fact, barrier dysfunction may precede the onset of symptoms [51] and even correlates with both disease activity and MS progression, suggesting that barrier dysfunction may directly drive neuroinflammation [48,52].
Table 1 shows a summary of studies of intestinal permeability in diverse neurological diseases [45,54–70]. The published literature provides some evidence of altered barrier function in MS, but Table 1 also shows that the observations are controversial and any conclusions are speculative, particularly since measurements of serum zonulin (a protein critical for regulation of intestinal permeability via regulation of epithelial cell tight junctions) using the commercial ELISA are of questionable significance since it has been reported that the assay does not detect a precursor of haptoglobin2 (which is a function of zonulin), but it recognizes another protein, possibly properdin [53].
|
Population |
Barrier dysfunction |
Epithelial damage |
Comment |
Ref. |
|
Multiple sclerosis |
||||
|
20 MS and 8 with MS and IBD |
25% of MS patients with high LMR |
|
|
[54] |
|
22 RRMS and 18 HC |
Increased LMR but reduced mannitol concentration in MS vs. HC |
- |
Unclear why reduced mannitol absorption if LMR increased |
[45] |
|
57 RRMS; 69 progressive MS |
Serum zonulin higher when a concurrent MRI confirmed blood-brain barrier (BBB) disruption and correlated with MS progression at 1y |
IBABP elevated in RRMS |
Zonulin assay may measure properdin, not zonulin |
[48] |
|
49 newly diagnosed RRMS and 58 healthy controls |
Out of panel of 30 biomarkers for inflammation and epithelial barrier function, MS had higher fecal calprotectin and soluble urokinase plasminogen activator. No difference in zonulin, soluble intercellular adhesion molecule 1, or soluble vascular adhesion molecule-1 |
- |
[55] |
|
|
72 newly diagnosed patients with RRMS or CID and 50 healthy controls |
Higher plasma occludin levels higher in MS; levels improved by 25% on therapy |
Occludin and zonulin: do not correlate with biomarkers of inflammation or predict disease activity at baseline or after 12 months |
[56] |
|
|
25 MS before and after treatment with dimethyl-fumarate |
64% with high LMR at baseline; these patients had 15x greater odds of MRI improvement on therapy. However, there was no consistent effect of therapy on permeability. |
- |
[57] |
|
|
18 RRMS and 18 controls |
Serum zonulin increased in MS compared with controls and positively correlated with disease duration |
- |
[49] |
|
|
18 RRMS and 10 Healthy controls |
Increased LMR |
Normal fecal zonulin |
[58]
|
|
|
9 MS treated with monthly FMT for up to 6 months |
LMR was elevated in 2/5 at baseline which improved following FMT |
- |
|
[59] |
|
26 RRMS, 19 secondary progressive MS in relapse, 18 with RRMS in remission, 20 healthy controls |
Serum zonulin highest in relapsed RRMS, significantly higher than all other groups |
- |
|
[60] |
|
Parkinson disease |
||||
|
15 PD and 15 HC |
LMR significantly higher in PD |
|
|
[61] |
|
9 PD with no GI symptoms and 10 HC |
Increased sucralose permeability; normal lactulose, mannitol and LMR results; reduced plasma LPS binding protein; no difference in endotoxin or sCD14 |
- |
Suggests colonic not small bowel increased permeability |
[62] |
|
34 PD and 28 controls matched for age |
Increased fecal zonulin |
Increased fecal markers of damage, α-1 AT and calprotectin |
Zonulin assay may measure properdin not zonulin |
[63] |
|
12 unselected PD |
4 abnormal LMR or sucralose permeability |
- |
Minority with increased permeability in small bowel or colon |
[64] |
|
22 sporadic PD 16 unmatched HC |
Increased serum and fecal zonulin |
Increased fecal calprotectin |
Zonulin assay may measure properdin, not zonulin |
[65] |
|
64 PD and 64 HC |
beta-defensin 2, zonulin and lactoferrin significantly higher in PD compared to healthy subjects |
β-defensin 2 and lactoferrin higher in PD vs. healthy subjects |
Zonulin assay may measure properdin, not zonulin; possible protective role of β-defensin 2 and lactoferrin |
[66] |
|
19 PD, 19 controls |
Increased plasma LBP levels |
Increased fecal TNF and IL-1β |
|
[67] |
|
Alzheimer disease |
||||
|
45 AD and 27 HC |
Higher fecal zonulin in AD |
- |
|
[68] |
|
71 mild and 66 moderate AD and 74 geriatric controls |
Higher plasma zonulin in AD, associated with poor balance. Moderate AD had higher zonulin than mild AD |
- |
|
[69] |
|
45 AD, 31 MCI, 35 HC |
AD and MCI had barrier dysfunction as evidenced by higher levels of blood diamine oxidase, D-lactic acid, and endotoxin |
- |
|
[70] |
|
AD: Alzheimer Dementia; AT: Anti-Trypsin; BBB: Blood-Brain Barrier; FMT: Fecal Microbial Transplantation; HC: Healthy Controls; IBABP: Ileal Bile Acid Binding Protein; IL: Interleukin; MCI: Mild Cognitive Impairment; LBP: Lipopolysaccharide Binding Protein; LMR: Lactulose to Mannitol Ratio; MS: Multiple Sclerosis; RRMS: Relapsing-Remitting Multiple Sclerosis; TNF: Tumor Necrosis Factor |
||||
Experimental models of autoimmune encephalomyelitis (prototypic animal model of MS) have shown that barrier dysfunction, manifested as increased intestinal permeability, overexpression of the tight junction protein, zonulin, and alterations in intestinal morphology (increased crypt depth and thickness of the submucosa and muscularis layers), precedes the development of neuroinflammation and supports disease progression [71].
On the other hand, there appears to be more convincing evidence regarding alterations in intestinal mucus in MS. Thus, among patients with relapsing and progressive MS, fecal levels of MUC2 are reduced, and this is associated with an overabundance of mucus-degrading bacteria and increased serum zonulin (measured by commercial ELISA) in progressive MS compared to relapsing-remitting MS, and with no increased fecal zonulin in either group compared to healthy controls [72].
Other studies have similarly shown dysbiosis, particularly featuring a shift toward mucus-degrading bacteria [43,73]. Akkermansia muciniphila has consistently emerged as overrepresented in most of these studies, and has even been shown to induce inflammation [73]. However, in other studies, A. muciniphilia abundance has been regarded as protective [74]. This phenomenon underscores the central tenet in microbiome science where microbes are not universally “good” or “bad”, but instead exert context-specific biological effects, dependent on numerous host factors such as genetics, diet, environment, epithelial barrier status, etc [75]. In summary, in the context to MS, A. muciniphila can either be protective or cause excess mucus degradation and barrier dysfunction, depending on broader host factors.
Parkinson disease
Parkinson disease (PD) is a neurodegenerative disease characterized by progressive degeneration of dopaminergic neurons in the substantia nigra, ultimately depleting dopamine in the striatum, and manifesting primarily as progressive motor dysfunction and cognitive impairment. Gastrointestinal dysfunction in PD is common and often precedes the development of the characteristic motor symptoms of the disease [76,77]. Intestinal barrier dysfunction is well documented in PD, and a prevailing hypothesis for the pathogenesis of PD (i.e., the Braak hypothesis) is that an initial insult within the gastrointestinal tract (such as an enteric infection, environmental toxin, or dietary components) increases intestinal permeability, allowing harmful agents to access the enteric nervous system, trigger α-synuclein misfolding, and subsequently propagate to the brainstem via the vagus nerve, thereby initiating chronic neurodegeneration which ultimately manifests as PD [78,79]. Numerous studies document dysbiosis, intestinal inflammation, and increased intestinal permeability in PD; moreover, these abnormalities may precede the onset of PD symptoms [62,63,65,66].
In addition to the dysfunctional intestinal epithelial barrier in PD, specific abnormalities in intestinal mucus have also been reported. Histological assessment of colonic epithelium in PD has demonstrated a decrease in epithelial neutral mucins and an increase in acidic mucins, reflecting altered architecture of the mucus layers [67]. Interestingly, it has been demonstrated that α-synuclein translocates across mucus barriers, and it is conceivable that this might be a critical step in the infection of the gastrointestinal epithelium and the eventual development of Parkinson disease [80].
As in MS, patients with PD also exhibit higher abundance of A. muciniphilia [43], and this is thought to degrade the mucus layer and promote increased intestinal permeability, especially in the absence of dietary fiber, which is common in PD, as reflected by studies consistently finding an absence of short-chain fatty acids and short-chain fatty acid-producing organisms such as Faecalibaceterium and Roseburia [81].
Alzheimer disease
Alzheimer disease (AD) is a progressive neurodegenerative disease which causes cognitive impairment. There is strong evidence from both experimental and human studies that intestinal barrier dysfunction is important in the pathophysiology of AD. Patients with AD have consistently been found to have dysbiosis, intestinal inflammation, and increased intestinal permeability [68–70,82–84]. The barrier dysfunction (based on increased plasma endotoxin), epithelial damage (based on serum diamine oxidase), and oxidative stress (revealed from increased serum D-lactic acid) have been shown to be significantly correlated with cognitive impairment in a cross-sectional study of 45 patients with AD, 38 patients with mild cognitive impairment, and 35 normal controls [70]. These data suggest that barrier dysfunction may also precede the development of cognitive impairment [70]. In AD, there is an overabundance of A. muciniphilia along with other mucus-degrading bacteria [43,85,86]. While these are the only studies relating to intestinal mucus in humans, several animal models of AD have demonstrated altered mucus secretion, reduced mucin glycoprotein content, and impaired barrier-forming capacity [87,88].
Psychiatric disorders
Several psychiatric disorders have also been reported to feature dysbiosis, intestinal barrier dysfunction, and increased intestinal permeability. The disorders include anxiety, depression, post-traumatic stress disorder, and schizophrenia, and barrier dysfunction may precede development of these disorders [89]. However, little attention has been paid to mucus specifically [90,91]. There is some evidence implicating abnormalities in gut mucus in view of the finding that schizophrenia is associated with fungal dysbiosis and reduced blood MUC2 expression [92]. The mucus layer has also been implicated in depression, with a proposal of direct causative links between stress, mucus depletion, dysbiosis, and depression [93].
Several other neurological disorders have been documented to feature dysbiosis, abnormal intestinal barrier function, and increased permeability. These disorders include amyotrophic lateral sclerosis [94], epilepsy [95], and autism spectrum disorder [96], but there has not been specific focus on the contribution of the integrity of the mucus layer. Nevertheless, there is some indirect evidence implicating the barrier and mucus, such as observations that fecal A. muciniphilia is overabundant in epilepsy and increases the risk of epilepsy [97], or the converse in autism, where there is a depletion of A. muciniphilia [98].
Conclusions
Intestinal epithelial barrier function is crucial for neurologic homeostasis. Damage to the barrier, leading to increased intestinal permeability, results in neuronal exposure to microbes, inflammatory mediators, and toxins. These pathophysiological mechanisms create an environment of chronic neuroinflammation, a key mechanism underlying many common neuropsychiatric disorders. Intestinal mucus is an important component of the intestinal barrier, and there is evidence of abnormalities in mucus and mucus-degrading bacteria in several neuropsychiatric disorders (Figure 1). The role of A. muciniphilia appears to be context-dependent, likely affected by host diet, among other factors. Future studies should seek to evaluate interventions targeted at intestinal mucus (such as diet, prebiotics, and probiotics) in the prevention and treatment of neuropsychiatric diseases.
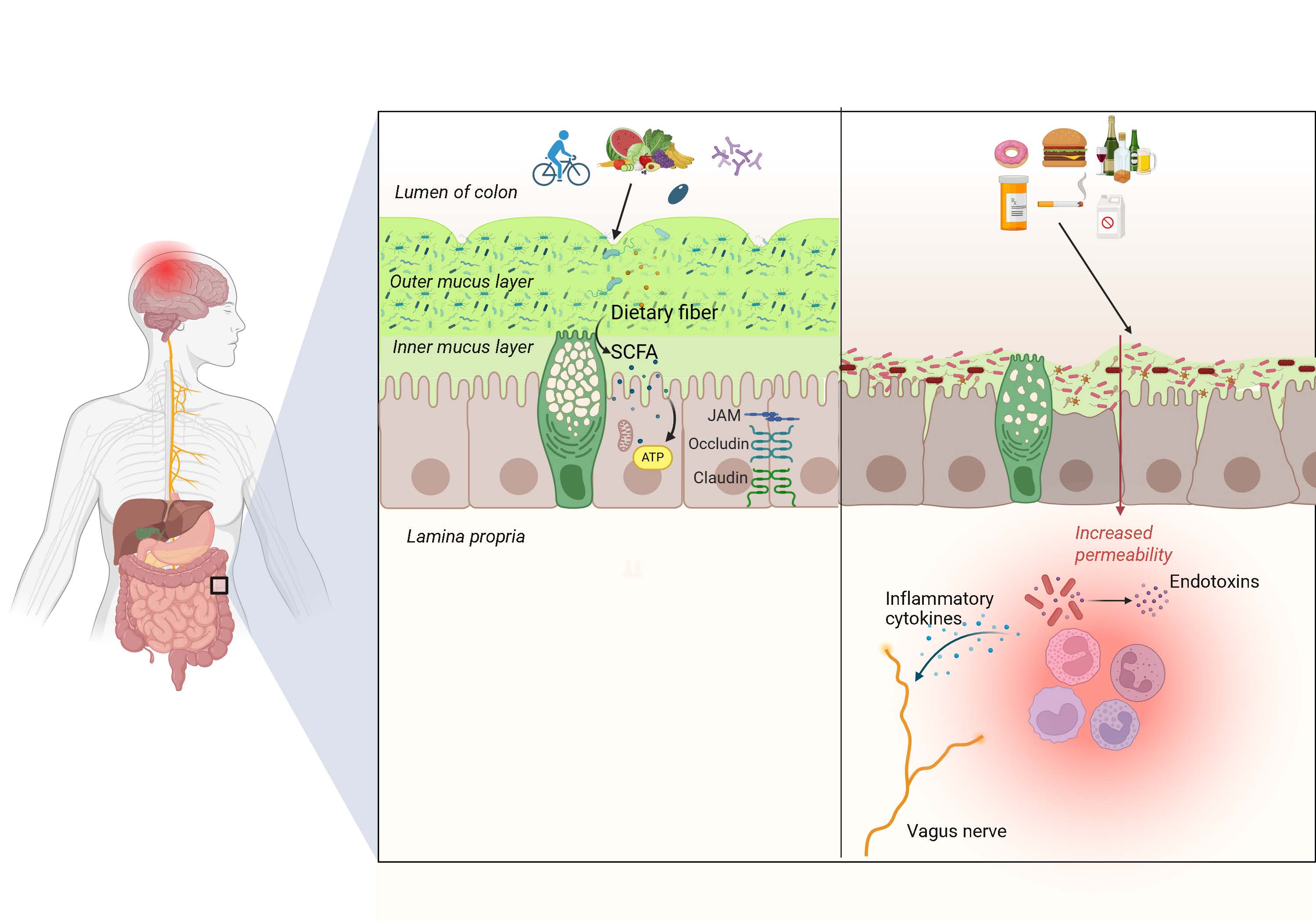
Figure 1. Role of intestinal mucus in neurologic health. In the colon, a healthy bilayer of mucus, fortified by microbial-derived short-chain fatty acids (SCFA) in response to dietary fiber, reinforces the gut epithelial barrier. Conversely, when mucus is degraded, such as in fiber deficient or high emulsifier diet states, microbes infiltrate the inner layer and deplete mucus, and the barrier is compromised, allowing microbes and toxins to access the subepithelium, inciting an inflammatory cascade that causes neuroinflammation, a central mechanism in neurological diseases
Funding
Michael Camilleri is funded by a National Institute of Health grant R01-DK135440 for research on gastrointestinal pathobiology in Parkinson disease.
Disclosures
The authors have no conflicts of interest.
Authors’ Contributions
John Damianos: Writing and revising the manuscript; literature review.
Michael Camilleri: Development of study concept; writing and revising the manuscript; literature review.
References
2. Martel J, Chang SH, Ko YF, Hwang TL, Young JD, Ojcius DM. Gut barrier disruption and chronic disease. Trends Endocrinol Metab. 2022 Apr;33(4):247–65.
3. Minaga K, Watanabe T, Hara A, Yoshikawa T, Kamata K, Kudo M. Gut Microbiota Involved in the Immunopathogenesis of Autoimmune Pancreatitis. Gut Liver. 2025 Mar 15;19(2):171–76.
4. Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010 Feb;105(2):323–27.
5. Tilg H, Adolph TE, Trauner M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022 Nov 1;34(11):1700–18.
6. Damianos J, Abdelnaem N, Camilleri M. Gut Goo: Physiology, Diet, and Therapy of Intestinal Mucus and Biofilms in Gastrointestinal Health and Disease. Clinical Gastroenterology and Hepatology 2025;23:205–15.
7. Jandl B, Dighe S, Baumgartner M, Makristathis A, Gasche C, Muttenthaler M. Gastrointestinal Biofilms: Endoscopic Detection, Disease Relevance, and Therapeutic Strategies. Gastroenterology. 2024 Nov;167(6):1098–112.e5.
8. Matar A, Damianos JA, Jencks KJ, Camilleri M. Intestinal Barrier Impairment, Preservation, and Repair: An Update. Nutrients. 2024 Oct 15;16(20):3494.
9. Suriano F, Nyström EEL, Sergi D, Gustafsson JK. Diet, microbiota, and the mucus layer: The guardians of our health. Front Immunol. 2022 Sep 13;13:953196.
10. Schroeder BO, Birchenough GMH, Ståhlman M, Arike L, Johansson MEV, Hansson GC, et al. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe. 2018 Jan 10;23(1):27–40.e7.
11. Liu Q, Niu X, Li Y, Zhang JR, Zhu SJ, Yang QY, et al. Role of the mucin-like glycoprotein FCGBP in mucosal immunity and cancer. Front Immunol. 2022 Jul 22;13:863317.
12. Jawhara M, Sørensen SB, Heitmann BL, Halldórsson ÞI, Pedersen AK, Andersen V. The Relation between Red Meat and Whole-Grain Intake and the Colonic Mucosal Barrier: A Cross-Sectional Study. Nutrients. 2020 Jun 12;12(6):1765.
13. Lock JY, Carlson TL, Wang CM, Chen A, Carrier RL. Acute Exposure to Commonly Ingested Emulsifiers Alters Intestinal Mucus Structure and Transport Properties. Sci Rep. 2018 Jul 3;8(1):10008.
14. Chassaing B, Compher C, Bonhomme B, Liu Q, Tian Y, Walters W, et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology. 2022 Mar;162(3):743–56.
15. Qin X, Deitch EA. Dissolution of lipids from mucus: a possible mechanism for prompt disruption of gut barrier function by alcohol. Toxicol Lett. 2015 Jan 22;232(2):356–62.
16. Melis M, Tang XH, Mai K, Gudas LJ, Trasino SE. Fenretinide Reduces Intestinal Mucin-2-Positive Goblet Cells in Chronic Alcohol Abuse. Pharmacology. 2022;107(7-8):406–16.
17. Dobani S, Kirsty Pourshahidi L, Ternan NG, McDougall GJ, Pereira-Caro G, Bresciani L, et al. A review on the effects of flavan-3-ols, their metabolites, and their dietary sources on gut barrier integrity. Food Funct. 2025 Feb 3;16(3):815–30.
18. Roussel C, Anunciação Braga Guebara S, Plante PL, et al. Short-term supplementation with ω-3 polyunsaturated fatty acids modulates primarily mucolytic species from the gut luminal mucin niche in a human fermentation system. Gut Microbes 2022;14:2120344.
19. Roshanravan N, Bastani S, Tutunchi H, Kafil B, Nikpayam O, Mesri Alamdari N, et al. A comprehensive systematic review of the effectiveness of Akkermansia muciniphila, a member of the gut microbiome, for the management of obesity and associated metabolic disorders. Arch Physiol Biochem. 2023 Jun;129(3):741–51.
20. Takaishi H, Ohara S, Hotta K, Yajima T, Kanai T, Inoue N, et al. Circulating autoantibodies against purified colonic mucin in ulcerative colitis. J Gastroenterol. 2000;35(1):20–7.
21. Hayashi T, Ishida T, Motoya S, Itoh F, Takahashi T, Hinoda Y, Imai K. Mucins and immune reactions to mucins in ulcerative colitis. Digestion. 2001;63 Suppl 1:28–31.
22. Baumgartner M, Lang M, Holley H, Crepaz D, Hausmann B, Pjevac P, et al. Mucosal Biofilms Are an Endoscopic Feature of Irritable Bowel Syndrome and Ulcerative Colitis. Gastroenterology. 2021 Oct;161(4):1245–56.e20.
23. Crost EH, Tailford LE, Monestier M, Swarbreck D, Henrissat B, Crossman LC, et al. The mucin-degradation strategy of Ruminococcus gnavus: The importance of intramolecular trans-sialidases. Gut Microbes. 2016 Jul 3;7(4):302–12.
24. Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. 2022 Dec;7(12):1128–40.
25. Martinez-Medina M, Aldeguer X, Lopez-Siles M, González-Huix F, López-Oliu C, Dahbi G, et al. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm Bowel Dis. 2009 Jun;15(6):872–82.
26. Gerard R, Sendid B, Colombel JF, Poulain D, Jouault T. An immunological link between Candida albicans colonization and Crohn's disease. Crit Rev Microbiol. 2015 Jun;41(2):135–9.
27. Wang Z, Shen J. The role of goblet cells in Crohn' s disease. Cell Biosci. 2024 Apr 1;14(1):43.
28. Magro DO, Santos A, Guadagnini D, de Godoy FM, Silva SHM, Lemos WJF, et al. Remission in Crohn's disease is accompanied by alterations in the gut microbiota and mucins production. Sci Rep. 2019 Sep 13;9(1):13263.
29. Pardo-Camacho C, Ganda Mall JP, Martínez C, Pigrau M, Expósito E, Albert-Bayo M, et al. Mucosal Plasma Cell Activation and Proximity to Nerve Fibres Are Associated with Glycocalyx Reduction in Diarrhoea-Predominant Irritable Bowel Syndrome: Jejunal Barrier Alterations Underlying Clinical Manifestations. Cells. 2022 Jun 28;11(13):2046.
30. Slattery SA, Niaz O, Aziz Q, Ford AC, Farmer AD. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther. 2015 Jul;42(1):3–11.
31. Camilleri M. Bile acid detergency: permeability, inflammation, and effects of sulfation. Am J Physiol Gastrointest Liver Physiol. 2022 May 1;322(5):G480–8.
32. Bertolazzi S, Lanzarotto F, Zanini B, Ricci C, Villanacci V, Lanzini A. Bio-physical characteristics of gastrointestinal mucosa of celiac patients: comparison with control subjects and effect of gluten free diet-. BMC Gastroenterol. 2011 Nov 7;11:119.
33. Loktionov A. Colon mucus in colorectal neoplasia and beyond. World J Gastroenterol. 2022 Aug 28;28(32):4475–92.
34. Quigley EM, Abu-Shanab A. Small intestinal bacterial overgrowth. Infect Dis Clin North Am. 2010 Dec;24(4):943–59, viii-ix.
35. Melhem H, Regan-Komito D, Niess JH. Mucins Dynamics in Physiological and Pathological Conditions. Int J Mol Sci. 2021 Dec 20;22(24):13642.
36. El-Hakim Y, Bake S, Mani KK, Sohrabji F. Impact of intestinal disorders on central and peripheral nervous system diseases. Neurobiol Dis. 2022 Apr;165:105627.
37. Zong J, Yang Y, Wang H, Zhang H, Yang X, Yang X. The two-directional prospective association between inflammatory bowel disease and neurodegenerative disorders: a systematic review and meta-analysis based on longitudinal studies. Front Immunol. 2024 Apr 24;15:1325908.
38. Szandruk-Bender M, Wiatrak B, Szeląg A. The Risk of Developing Alzheimer's Disease and Parkinson's Disease in Patients with Inflammatory Bowel Disease: A Meta-Analysis. J Clin Med. 2022 Jun 27;11(13):3704.
39. Giuffrè M, Gazzin S, Zoratti C, Llido JP, Lanza G, Tiribelli C, et al. Celiac Disease and Neurological Manifestations: From Gluten to Neuroinflammation. Int J Mol Sci. 2022 Dec 8;23(24):15564.
40. Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol. 2018 Sep;136(3):345–61.
41. Barbaresko J, Lellmann AW, Schmidt A, Lehmann A, Amini AM, Egert S, Schlesinger S, Nöthlings U. Dietary Factors and Neurodegenerative Disorders: An Umbrella Review of Meta-Analyses of Prospective Studies. Adv Nutr. 2020 Sep 1;11(5):1161–73.
42. Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015 Apr 10;9:124.
43. Herath M, Hosie S, Bornstein JC, Franks AE, Hill-Yardin EL. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front Cell Infect Microbiol. 2020 May 28;10:248.
44. Pellegrini C, Fornai M, D'Antongiovanni V, Antonioli L, Bernardini N, Derkinderen P. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol Hepatol. 2023 Jan;8(1):66–80.
45. Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler. 2017 Mar;23(3):442–46.
46. Buscarinu MC, Romano S, Mechelli R, Pizzolato Umeton R, Ferraldeschi M, Fornasiero A, et al. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics. 2018 Jan;15(1):68–74.
47. Camara-Lemarroy CR, Metz L, Meddings JB, Sharkey KA, Wee Yong V. The intestinal barrier in multiple sclerosis: implications for pathophysiology and therapeutics. Brain. 2018 Jul 1;141(7):1900–16.
48. Camara-Lemarroy CR, Silva C, Greenfield J, Liu WQ, Metz LM, Yong VW. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult Scler. 2020 Oct;26(11):1340–50.
49. Pellizoni FP, Leite AZ, Rodrigues NC, Ubaiz MJ, Gonzaga MI, Takaoka NNC, Mariano VS, Omori WP, Pinheiro DG, Matheucci Junior E, Gomes E, de Oliveira GLV. Detection of Dysbiosis and Increased Intestinal Permeability in Brazilian Patients with Relapsing-Remitting Multiple Sclerosis. Int J Environ Res Public Health. 2021 Apr 27;18(9):4621.
50. Yadav SK, Ito K, Dhib-Jalbut S. Interaction of the Gut Microbiome and Immunity in Multiple Sclerosis: Impact of Diet and Immune Therapy. Int J Mol Sci. 2023 Sep 29;24(19):14756.
51. Parodi B, Kerlero de Rosbo N. The Gut-Brain Axis in Multiple Sclerosis. Is Its Dysfunction a Pathological Trigger or a Consequence of the Disease? Front Immunol. 2021 Sep 21;12:718220.
52. Buscarinu MC, Fornasiero A, Romano S, Ferraldeschi M, Mechelli R, Reniè R, et al. The Contribution of Gut Barrier Changes to Multiple Sclerosis Pathophysiology. Front Immunol. 2019 Aug 28;10:1916.
53. Scheffler L, Crane A, Heyne H, Tönjes A, Schleinitz D, Ihling CH, et al. Widely Used Commercial ELISA Does Not Detect Precursor of Haptoglobin2, but Recognizes Properdin as a Potential Second Member of the Zonulin Family. Front Endocrinol (Lausanne). 2018 Feb 5;9:22.
54. Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci. 1996 Dec;41(12):2493–8.
55. Olsson A, Gustavsen S, Hasselbalch IC, Langkilde AR, Sellebjerg F, Oturai AB, et al. Biomarkers of inflammation and epithelial barrier function in multiple sclerosis. Mult Scler Relat Disord. 2020 Nov;46:102520.
56. Olsson A, Gustavsen S, Langkilde AR, Hansen TH, Sellebjerg F, Bach Søndergaard H, et al. Circulating levels of tight junction proteins in multiple sclerosis: Association with inflammation and disease activity before and after disease modifying therapy. Mult Scler Relat Disord. 2021 Sep;54:103136
57. Buscarinu MC, Gargano F, Lionetto L, Capi M, Morena E, Fornasiero A, et al. Intestinal Permeability and Circulating CD161+CCR6+CD8+T Cells in Patients With Relapsing-Remitting Multiple Sclerosis Treated With Dimethylfumarate. Front Neurol. 2021 Aug 26;12:683398.
58. Sjöström B, Bredberg A, Mandl T, Alonso-Magdalena L, Ohlsson B, Lavasani S, et al. Increased intestinal permeability in primary Sjögren's syndrome and multiple sclerosis. J Transl Autoimmun. 2021 Jan 6;4:100082.
59. Al KF, Craven LJ, Gibbons S, Parvathy SN, Wing AC, Graf C, et al. Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial. Mult Scler J Exp Transl Clin. 2022 May 11;8(2):20552173221086662.
60. Nassef PD, ElNabil LM, Fouad MM, Moussa AA. Assessment of serum zonulin as a marker of altered intestinal permeability in patients with multiple sclerosis. Multiple Sclerosis and Related Disorders 2024 Dec;92:106001.
61. Davies KN, King D, Billington D, Barrett JA. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson's disease. Postgrad Med J. 1996 Mar;72(845):164-7.
62. Forsyth CB, Shannon KM, Kordower JH, Voigt RM, Shaikh M, Jaglin JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. 2011;6(12):e28032.
63. Schwiertz A, Spiegel J, Dillmann U, Grundmann D, Bürmann J, Faßbender K, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson's disease. Parkinsonism Relat Disord. 2018 May;50:104–7.
64. Salat-Foix D, Tran K, Ranawaya R, Meddings J, Suchowersky O. Increased intestinal permeability and Parkinson disease patients: chicken or egg? Can J Neurol Sci. 2012 Mar;39(2):185–8.
65. Dumitrescu L, Marta D, Dănău A, Lefter A, Tulbă D, Cozma L, et al. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson's Disease. Front Neurosci. 2021 Jun 18;15:689723.
66. Rajkovaca Latic I, Popovic Z, Mijatovic K, Sahinovic I, Pekic V, Vucic D, et al. Association of intestinal inflammation and permeability markers with clinical manifestations of Parkinson's disease. Parkinsonism Relat Disord. 2024 Jun;123:106948.
67. Bellini G, Benvenuti L, Ippolito C, Frosini D, Segnani C, Rettura F, et al. Intestinal histomorphological and molecular alterations in patients with Parkinson's disease. Eur J Neurol. 2023 Nov;30(11):3440–3450.
68. Kowalski K, Mulak A. Small intestinal bacterial overgrowth in Alzheimer's disease. J Neural Transm (Vienna). 2022 Jan;129(1):75–83.
69. Qaisar R, Karim A, Iqbal MS, Ahmad F, Shaikh A, Kamli H, et al. A leaky gut contributes to postural dysfunction in patients with Alzheimer's disease. Heliyon. 2023 Aug 25;9(9):e19485.
70. Pei Y, Lu Y, Li H, Jiang C, Wang L. Gut microbiota and intestinal barrier function in subjects with cognitive impairments: a cross-sectional study. Front Aging Neurosci. 2023 Jun 7;15:1174599.
71. Nouri M, Bredberg A, Weström B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One. 2014 Sep 3;9(9):e106335.
72. Schwerdtfeger LA, Montini F, Chitnis T, Cox LM, Weiner HL. Faecal mucoprotein MUC2 is decreased in multiple sclerosis and is associated with mucin degrading bacteria. EBioMedicine. 2025 Jun;116:105721.
73. Correale J, Hohlfeld R, Baranzini SE. The role of the gut microbiota in multiple sclerosis. Nat Rev Neurol. 2022 Sep;18(9):544–58.
74. Zancan V, Nasello M, Bigi R, Reniè R, Buscarinu MC, Mechelli R, et al. Gut Microbiota Composition Is Causally Linked to Multiple Sclerosis: A Mendelian Randomization Analysis. Microorganisms. 2024 Jul 19;12(7):1476.
75. Panzetta ME, Valdivia RH. Akkermansia in the gastrointestinal tract as a modifier of human health. Gut Microbes. 2024 Jan-Dec;16(1):2406379.
76. Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson's disease. Lancet Neurol. 2015 Jun;14(6):625–39.
77. Travagli RA, Browning KN, Camilleri M. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2020 Nov;17(11):673–85.
78. Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003 May;110(5):517–36
79. Houser MC, Tansey MG. The gut-brain axis: is intestinal inflammation a silent driver of Parkinson's disease pathogenesis? NPJ Parkinsons Dis. 2017 Jan 11;3:3.
80. Marczynski M, Rickert CA, Semerdzhiev SA, van Dijk WR, Segers-Nolten IMJ, Claessens MMAE, et al. α-Synuclein Penetrates Mucin Hydrogels Despite Its Mucoadhesive Properties. Biomacromolecules. 2019 Dec 9;20(12):4332–44.
81. Hirayama M, Nishiwaki H, Hamaguchi T, Ohno K. Gastrointestinal disorders in Parkinson's disease and other Lewy body diseases. NPJ Parkinsons Dis. 2023 May 5;9(1):71.
82. Kowalski K, Mulak A. Brain-Gut-Microbiota Axis in Alzheimer's Disease. J Neurogastroenterol Motil. 2019 Jan 31;25(1):48–60.
83. Megur A, Baltriukienė D, Bukelskienė V, Burokas A. The Microbiota-Gut-Brain Axis and Alzheimer's Disease: Neuroinflammation Is to Blame? Nutrients. 2020 Dec 24;13(1):37.
84. Ahmad Fadzuli NI, Lim SM, Neoh CF, Majeed ABA, Tan MP, Khor HM, et al. Faecal intestinal permeability and intestinal inflammatory markers in older adults with age-related disorders: A systematic review and meta-analysis. Ageing Res Rev. 2024 Nov;101:102506.
85. Jemimah S, Chabib CMM, Hadjileontiadis L, AlShehhi A. Gut microbiome dysbiosis in Alzheimer's disease and mild cognitive impairment: A systematic review and meta-analysis. PLoS One. 2023 May 24;18(5):e0285346.
86. Li H, Cui X, Lin Y, Huang F, Tian A, Zhang R. Gut microbiota changes in patients with Alzheimer's disease spectrum based on 16S rRNA sequencing: a systematic review and meta-analysis. Front Aging Neurosci. 2024 Aug 8;16:1422350.
87. He J, Liu Y, Li J, Zhao Y, Jiang H, Luo S, et al. Intestinal changes in permeability, tight junction and mucin synthesis in a mouse model of Alzheimer's disease. Int J Mol Med. 2023 Dec;52(6):113.
88. Homolak J, De Busscher J, Zambrano-Lucio M, Joja M, Viragos D, Babic Perhoc A, et al. Altered Secretion, Constitution, and Functional Properties of the Gastrointestinal Mucus in a Rat Model of Sporadic Alzheimer's Disease. ACS Chem Neurosci. 2023 Aug 2;14(15):2667-82.
89. Paton SEJ, Solano JL, Coulombe-Rozon F, Lebel M, Menard C. Barrier-environment interactions along the gut-brain axis and their influence on cognition and behaviour throughout the lifespan. J Psychiatry Neurosci. 2023 May 30;48(3):E190–E208.
90. Wasiak J, Gawlik-Kotelnicka O. Intestinal permeability and its significance in psychiatric disorders - A narrative review and future perspectives. Behav Brain Res. 2023 Jun 25;448:114459.
91. Ioannou M, Borkent J, Severance EG, Yolken RH, Fasano A, Sommer IEC, et al. Biomarkers of intestinal permeability in major psychiatric disorders: Distinct biological roles call for a more nuanced application. Prog Neuropsychopharmacol Biol Psychiatry. 2025 Jun 20;139:111405.
92. Yuan X, Li X, Hei G, Zhang X, Song X. Intestinal mycobiota dysbiosis associated inflammation activation in chronic schizophrenia. Behav Brain Res. 2024 Aug 24;472:115149.
93. Rivet-Noor C, Gaultier A. The Role of Gut Mucins in the Etiology of Depression. Front Behav Neurosci. 2020 Nov 5;14:592388.
94. Martin S, Battistini C, Sun J. A Gut Feeling in Amyotrophic Lateral Sclerosis: Microbiome of Mice and Men. Front Cell Infect Microbiol. 2022 Mar 11;12:839526.
95. De Caro C, Iannone LF, Citraro R, Striano P, De Sarro G, Constanti A, et al. Can we 'seize' the gut microbiota to treat epilepsy? Neurosci Biobehav Rev. 2019 Dec; 107:750-64.
96. Karagözlü S, Dalgıç B, İşeri E. The Relationship of Severity of Autism with Gastrointestinal Symptoms and Serum Zonulin Levels in Autistic Children. J Autism Dev Disord. 2022 Feb;52(2):623–29.
97. He X, Zhang Y. Changes in gut flora in patients with epilepsy: a systematic review and meta-analysis. Front Microbiol. 2024 Nov 14;15:1480022.
98. Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011 Sep;77(18):6718-21.