Abstract
Background: Sepsis remains a critical global health challenge with high mortality. This review summarizes current understanding of the intricate molecular mechanisms governing sepsis pathogenesis and highlights emerging therapeutic approaches.
Main body of the abstract: The dysregulated immune response in sepsis, involving both excessive inflammation and immunosuppression, is mediated through cytokines, pattern recognition receptors, and disturbances in immune cell function. Endothelial dysfunction, coagulation abnormalities, microbiome dysbiosis, and metabolic/mitochondrial alterations also critically contribute to sepsis progression. Preclinical models have facilitated detailed study of these pathways and identification of potential therapeutic targets, including immunomodulators, microbiome-directed therapies, endothelial modulators, anticoagulants, and metabolic/mitochondrial agents. Combination therapies targeting multiple pathogenic aspects may be necessary. However, translating preclinical findings to clinical applications remains challenging. Heterogeneity among sepsis patients is a key issue, underscoring the need for precision medicine approaches. Potential adverse effects and optimal treatment regimens must be rigorously evaluated.
Short conclusion: Further research should focus on validating preclinical discoveries, understanding sepsis subtypes, developing predictive biomarkers and innovative therapies including artificial intelligence-based tools, and bridging knowledge gaps through enhanced academia-industry collaboration. Comprehensive efforts spanning from unraveling molecular pathways to clinical translation hold promise for improving outcomes in this deadly syndrome.
Keywords
Sepsis, Molecular mechanisms, Inflammation, Immunomodulation, Endothelial dysfunction, Microbiome, Precision medicine
Background
Sepsis is a life-threatening condition that arises when the body's response to an infection becomes dysregulated, leading to widespread inflammation and organ dysfunction. It is a critical medical emergency that affects millions of people worldwide, making it a significant global health concern [1]. The epidemiology of sepsis reveals its alarming prevalence and impact on healthcare systems. Each year, millions of individuals are diagnosed with sepsis, and it is estimated to be responsible for over 11 million deaths globally. Sepsis can affect individuals of all ages, from neonates to the elderly, and can arise from various types of infections, including bacterial, viral, fungal, or parasitic. H. pylori infection has been associated with extra-digestive disorders including cardiovascular, hematological, metabolic, autoimmune, and other diseases. Recent evidence suggests H. pylori may also contribute to sepsis pathogenesis through direct and indirect effects on the host immune response [2]. It can occur in both community and healthcare settings, with hospital-acquired sepsis posing a particular challenge [3]. Understanding the molecular pathways underlying sepsis is of paramount importance in effectively managing and treating this complex condition. Extensive research has shed light on the intricate mechanisms involved in the pathogenesis of sepsis. The dysregulated immune response, specifically the overactivation of pro-inflammatory pathways and inadequate control of anti-inflammatory processes, plays a central role in the development and progression of sepsis [4]. The consequences of sepsis extend beyond the initial infection, affecting multiple organ systems and leading to severe complications. Sepsis is associated with decreased multiple leukocyte functions (MLF) including impaired phagocytosis, cytokine production, and antigen presentation by immune cells, which can worsen outcomes in stroke patients [5]. Vascular complications arise due to endothelial dysfunction, microvascular thrombosis, and impaired blood flow regulation, which can result in tissue hypoperfusion and organ damage. The immunological consequences involve immune system dysregulation, leading to both hyperinflammation and immunosuppression, rendering the body susceptible to secondary infections. Hematological abnormalities, such as coagulopathy and disseminated intravascular coagulation (DIC), can further complicate the clinical picture [6]. Sepsis also poses a significant burden on renal function, leading to acute kidney injury (AKI) due to altered blood flow, inflammation, and direct tubular injury. Neurological complications, ranging from delirium to encephalopathy and cognitive impairments, frequently manifest in septic patients. Respiratory dysfunction, including acute respiratory distress syndrome (ARDS), is another common consequence of sepsis, often necessitating mechanical ventilation and intensive care [7].
Despite advances, sepsis remains a critical challenge with persistently high mortality. Elucidating intricate molecular mechanisms and translating findings to improve patient outcomes is imperative. This review comprehensively summarizes current understanding of sepsis pathogenesis, from inflammatory cascades to metabolic derangements, while underscoring knowledge gaps and emerging therapeutic avenues. It provides an expansive overview of this multifaceted syndrome, emphasizing recent developments and future directions needed to combat sepsis morbidity and mortality. This analysis intends to inform clinicians and researchers on progress and persisting barriers in addressing this complex condition.
Molecular Pathways Underlying Sepsis
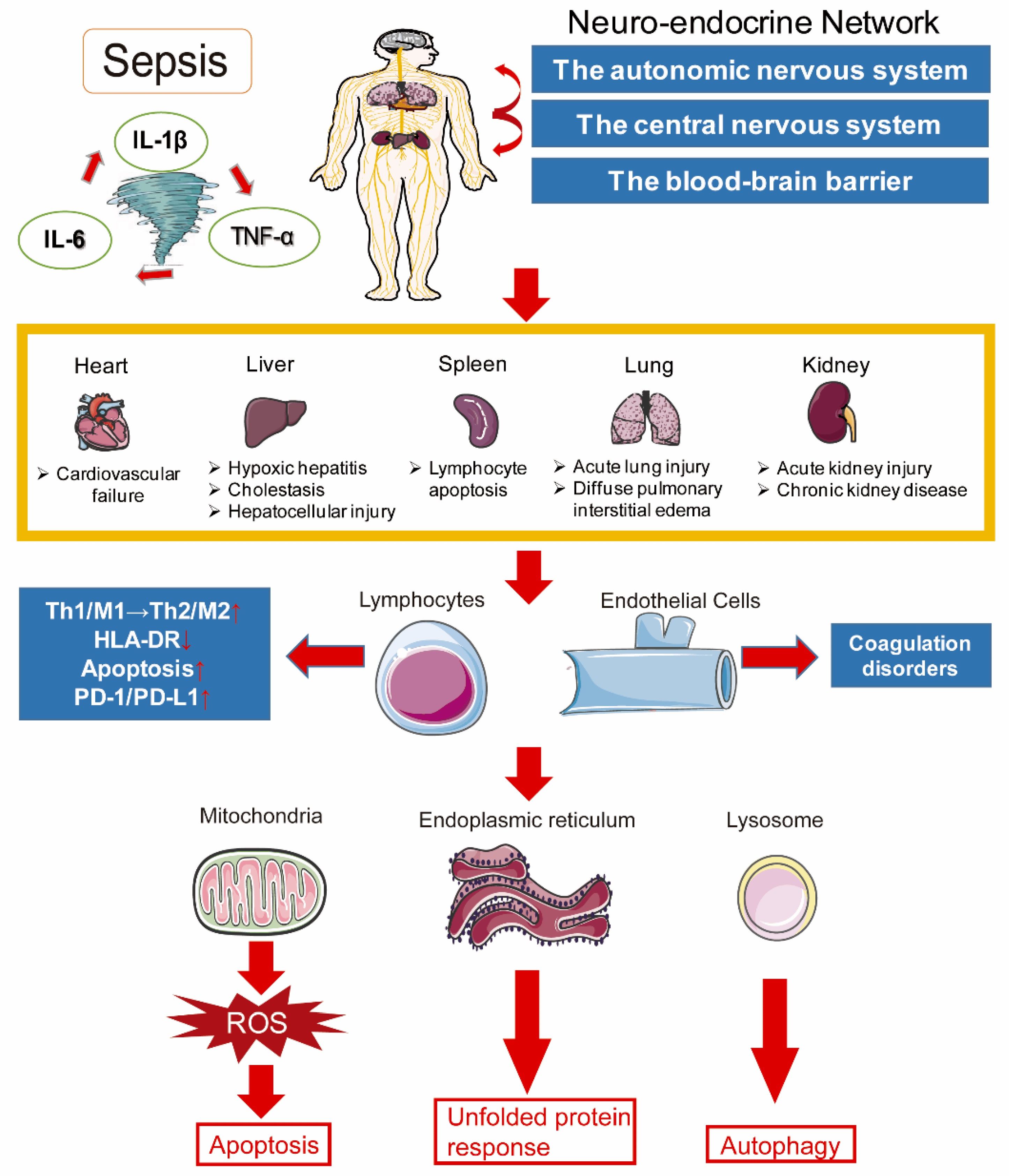
Sepsis, a complex and life-threatening condition, involves dysregulated immune responses and molecular pathways that contribute to its pathophysiology. Understanding these underlying mechanisms is crucial for developing effective treatments and improving patient outcomes. The immune system plays a central role in sepsis, orchestrating the body's response to infection. In sepsis, an initially appropriate immune response becomes dysregulated, resulting in a cascade of pro-inflammatory and anti-inflammatory processes [8]. As shown in Table 1, the release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), triggers an excessive immune response, leading to tissue damage and organ dysfunction. Simultaneously, anti-inflammatory mediators, such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β), attempt to counterbalance the inflammation but can contribute to immunosuppression in sepsis [9]. The microbiome, consisting of the trillions of microorganisms residing in the human body, also plays a significant role in sepsis. Disturbances in the composition and diversity of the microbiome, known as dysbiosis, can contribute to the development and progression of sepsis. Dysbiosis can disrupt the delicate balance between commensal and pathogenic microorganisms, impairing immune function, and promoting the translocation of harmful bacteria into the bloodstream [10]. Endothelial dysfunction is a hallmark of sepsis and contributes to its pathogenesis. Endothelial cells line the blood vessels and regulate vascular tone, permeability, and coagulation. In sepsis, the endothelium becomes activated, leading to increased vascular permeability, impaired regulation of blood flow, and microvascular thrombosis. These alterations result in tissue hypoperfusion, organ damage, and the development of septic shock [11]. Coagulation abnormalities are another critical aspect of sepsis. The dysregulated immune response and endothelial dysfunction lead to a procoagulant state, characterized by excessive clotting and consumption of clotting factors. Simultaneously, an impaired anticoagulant system and fibrinolysis further contribute to coagulation abnormalities. This intricate balance between procoagulant and anticoagulant factors can result in disseminated intravascular coagulation (DIC), a life-threatening condition associated with organ dysfunction and increased mortality in septic patients [12]. Metabolic and mitochondrial dysfunction have emerged as significant contributors to sepsis pathology. In sepsis, there is a shift in cellular metabolism, with a transition from oxidative phosphorylation to glycolysis, known as the Warburg effect. This metabolic reprogramming aims to meet the increased energy demands of the immune response but can lead to mitochondrial dysfunction, reactive oxygen species (ROS) production, and cellular damage. Impaired mitochondrial function further exacerbates organ dysfunction in sepsis [13]. Understanding the molecular pathways underlying sepsis, including the dysregulated immune response, microbiome dysbiosis, endothelial dysfunction, coagulation abnormalities, and metabolic and mitochondrial dysfunction, is crucial for developing targeted therapies and improving patient outcomes. Further research in these areas holds promise for identifying novel therapeutic targets and interventions to mitigate the devastating consequences of sepsis [14]. At the molecular level, sepsis involves a complex interplay of inflammatory imbalance, immune abnormalities, mitochondrial dysfunction, coagulation disorders, neuroendocrine-immune disturbances, endoplasmic reticulum stress, autophagy, and other processes that ultimately culminate in multi-organ dysfunction. The intricate pathogenesis spans cellular to organismal levels, highlighting the need for a nuanced, systems-based perspective when investigating and treating sepsis as depicted in Figure 1 [15].
|
Cytokine |
Type |
Major Sources |
Actions in Sepsis |
|
TNF-α |
Pro-inflammatory |
Macrophages, monocytes, NK cells, T cells |
Induces systemic inflammation, fever, apoptosis, cellular activation, endothelial dysfunction |
|
IL-1β |
Pro-inflammatory |
Macrophages, monocytes, dendritic cells |
Stimulates release of secondary inflammatory mediators, fever, leukocyte recruitment |
|
IL-6 |
Pro-inflammatory |
Macrophages, T cells, endothelium |
Stimulates acute phase response, fever, leukocytosis, endothelial activation |
|
IL-10 |
Anti-inflammatory |
Monocytes, macrophages, T cells, B cells |
Inhibits antigen presentation, pro-inflammatory cytokine production, counter-regulates inflammation |
|
TGF-β |
Anti-inflammatory |
Macrophages, T cells, platelets |
Limits T cell proliferation, suppresses macrophage activation, promotes tissue repair |
Figure 1. Unraveling the Intricate Pathogenesis of Sepsis [13].
Preclinical Research into Sepsis
Preclinical research plays a pivotal role in advancing our understanding of sepsis, providing valuable insights into its pathogenesis and potential therapeutic interventions as shown in Table 2. Animal models have been instrumental in studying sepsis; however, they come with inherent limitations and challenges [16]. Animal models of sepsis have significantly contributed to our understanding of the disease. These models typically involve inducing infection or bacterial toxins in animals, allowing researchers to study the complex immune responses and molecular pathways involved. Rodent models, such as mice and rats, remain the most commonly used models due to their genetic similarity to humans and the availability of genetic manipulation tools. Large animal models, such as pigs and non-human primates, offer closer physiological resemblance to humans and allow for more clinically relevant observations [17]. However, it is important to acknowledge the limitations of animal models in sepsis research. The complexity of sepsis and its heterogeneity in human patients cannot be fully replicated in animal models. The translational gap between preclinical findings and clinical outcomes remains a challenge. Furthermore, ethical considerations and the high cost associated with large animal models limit their widespread use [18]. Despite these challenges, preclinical research has made significant strides in unraveling the molecular pathways underlying sepsis. Advances in technologies such as genomics, transcriptomics, proteomics, and metabolomics have enabled the comprehensive analysis of the molecular changes occurring during sepsis. These omics technologies have identified potential biomarkers and therapeutic targets, providing a more nuanced understanding of sepsis pathophysiology [19]. Omics approaches have revealed dysregulated gene expression, altered protein profiles, and metabolic disturbances in septic patients and animal models. These findings have shed light on critical pathways involved in immune dysregulation, endothelial dysfunction, coagulation abnormalities, and metabolic alterations. By identifying key molecules and pathways, researchers can develop targeted interventions to modulate these processes and potentially improve patient outcomes [20]. In addition to traditional infection models, there has been a growing interest in alternative approaches to modeling sepsis. These include models that incorporate microbial infections and models that focus on tissue injury. Microbial infection models aim to replicate the complex interactions between the infecting pathogen and the host immune system, providing insights into the microbiome-host interactions and their impact on sepsis development. Tissue injury models simulate the damage caused by trauma, surgery, or ischemia-reperfusion, which can predispose individuals to sepsis. These models help elucidate the interplay between tissue injury, immune responses, and subsequent infections [21]. Preclinical research continues to be a vital component in the quest to unravel the complexities of sepsis. Despite the challenges and limitations of animal models, they remain essential tools for studying the disease. Advances in omics technologies and the exploration of innovative modeling approaches provide valuable opportunities to deepen our understanding of sepsis pathophysiology, identify biomarkers, and develop novel therapeutic strategies. By bridging the gap between preclinical research and clinical practice, we can strive towards effective interventions that improve outcomes for septic patients.
|
Model |
Examples |
Key Features |
Advantages |
Limitations |
|
Rodent models |
Mice, rats |
Genetic similarity to humans, available transgenic strains, low cost |
Allow detailed study of molecular pathways, easy genetic manipulation |
Do not fully replicate human sepsis complexity and heterogeneity |
|
Large animal models |
Pigs, sheep, dogs, primates |
Closer physiological resemblance to humans |
Allow more clinically relevant study of therapies and monitoring |
High cost, limited availability, ethical constraints |
|
Bacterial infusion |
LPS, viable bacteria |
Mimics hyperinflammatory response |
Simple, reproducible |
May not reflect full complexity of human sepsis |
|
Microbial infection |
CLP, pneumonia, peritonitis |
Incorporates live pathogen-host interactions |
Mimics important microbiome-immune interactions |
Variable infection course, technically challenging |
|
Tissue injury |
Burn, trauma, ischemia-reperfusion |
Models’ common sepsis predisposing factors |
Relevant for studying sterile inflammation in sepsis |
Difficult to standardize injury, indirect model of infection |
Potential Therapeutic Avenues for Sepsis
Sepsis remains a critical medical challenge, and the development of effective therapeutic strategies is of utmost importance as presented in Table 3. Over the years, extensive research has identified several potential avenues for therapeutic intervention in sepsis. These approaches target various aspects of the disease, including microbial infection, immune dysregulation, endothelial dysfunction, microbiome modulation, coagulation abnormalities, and metabolic and mitochondrial dysfunction [22]. Antibiotics and antimicrobial peptides play a crucial role in the management of sepsis by directly targeting the underlying infectious pathogens. Prompt administration of appropriate antibiotics is vital in controlling the initial infection and preventing its progression to severe sepsis or septic shock [23]. Antimicrobial peptides, naturally occurring molecules with antimicrobial properties, have shown promise as potential therapeutics due to their broad-spectrum activity against a range of pathogens [24]. Immunomodulatory agents have emerged as a potential avenue for sepsis treatment. Cytokine inhibitors, such as interleukin-1 receptor antagonists and anti-TNF-α antibodies, aim to attenuate the excessive pro-inflammatory response observed in sepsis [25]. Toll-like receptor (TLR) agonists or antagonists, which regulate the immune response to microbial components, are being explored as potential immunomodulatory agents to restore immune balance in sepsis [26]. Targeting endothelial dysfunction is another promising therapeutic approach in sepsis. Nitric oxide (NO) and endothelial nitric oxide synthase (eNOS) play crucial roles in regulating vascular tone and endothelial function [27]. Therapies aimed at increasing NO bioavailability or modulating eNOS activity hold potential for improving microcirculatory function and mitigating organ damage in sepsis [28]. Vaccination plays a critical role in sepsis prevention by protecting against infections that can trigger sepsis, especially in high-risk populations like the elderly and immunocompromised [29]. Modulating the microbiome has gained increasing attention as a potential therapeutic avenue in sepsis. Fecal microbiota transplantation (FMT), a procedure involving the transfer of fecal material from a healthy donor to a recipient, aims to restore a balanced and diverse microbial community. Probiotics, live microorganisms with potential health benefits, have also shown promise in modulating the gut microbiota and improving outcomes in septic patients [30]. Coagulation-targeted therapies have been explored to address the dysregulated coagulation observed in sepsis. Antithrombin, a natural anticoagulant, and tissue factor pathway inhibitors aim to counteract the procoagulant state and prevent excessive clot formation. These therapies hold potential for improving microcirculatory function and reducing the risk of organ dysfunction [31]. Metabolic and mitochondrial-targeted therapies focus on restoring cellular energy metabolism and mitigating mitochondrial dysfunction in sepsis [32]. Strategies such as glucose control, aiming to maintain optimal blood glucose levels, and antioxidants, which counteract oxidative stress, have shown promise in preclinical and clinical studies [33]. To effectively combat sepsis, a multifaceted approach that combines these therapeutic avenues may be necessary. The integration of personalized medicine, considering individual patient characteristics and biomarkers, holds promise for tailoring therapies to specific sepsis subtypes or patient populations.
|
Pathogenesis Mechanism |
Potential Therapeutic Targets |
Example Interventions |
|
Immune dysregulation |
Pro- and anti-inflammatory cytokines, cytokine receptors, TLRs, immune cell receptors |
Cytokine inhibitors (anti-TNF, IL-1Ra), TLR agonists/antagonists, immune cell modulators |
|
Endothelial dysfunction |
NO, NOS enzymes, VEGF, Ang-Tie system, adhesion molecules |
NO donors, eNOS modulators, VEGF mimetics, Ang-Tie inhibitors, anti-adhesion molecules |
|
Coagulation abnormalities |
Tissue factor, thrombin, platelets, natural anticoagulants |
Anti-thrombin, activated protein C, anticoagulants, platelet inhibitors |
|
Microbiome dysbiosis |
Gut microbiota, pathogens, pathogen virulence factors |
Probiotics, prebiotics, FMT, antimicrobial peptides, anti-virulence drugs |
|
Mitochondrial dysfunction |
Oxidative stress, metabolism, mtDNA |
Antioxidants, metabolic modulators, mtDNA-targeted therapies |
Consequences and Post-Sepsis Syndrome
Sepsis, a complex and life-threatening condition, not only poses immediate risks to patients but can also have long-lasting consequences that persist even after the initial infection has been treated. Understanding these consequences and the development of post-sepsis syndrome is crucial for comprehensive patient care and rehabilitation. Vascular consequences are prominent in sepsis and can lead to severe complications. Septic shock, a state of profound circulatory dysfunction, can result in organ failure and even death. It is characterized by persistent hypotension, inadequate tissue perfusion, and multiorgan dysfunction. Acute respiratory distress syndrome (ARDS) is another vascular consequence frequently associated with sepsis. It involves severe lung inflammation and impaired oxygenation, leading to respiratory failure. ARDS significantly contributes to the morbidity and mortality of septic patients [34]. Hematological consequences play a significant role in sepsis pathology. Thrombocytopenia, a decrease in platelet count, is commonly observed in septic patients and can contribute to bleeding complications [35]. Disseminated intravascular coagulation (DIC) is a critical hematological consequence characterized by widespread clotting and subsequent consumption of clotting factors, leading to both bleeding and thrombotic events. DIC is associated with poor outcomes and increased mortality in sepsis [36]. Renal consequences are frequently observed in septic patients, with acute kidney injury (AKI) being a common complication. Sepsis-induced AKI involves a complex interplay of inflammatory and hemodynamic factors, leading to impaired kidney function. AKI significantly impacts patient prognosis and is associated with increased morbidity and mortality [37]. Neurological consequences of sepsis can manifest as delirium and cognitive impairment. Delirium, a state of acute mental confusion and altered consciousness, is prevalent in septic patients, particularly in the intensive care unit (ICU) setting [38]. Cognitive impairment, including memory deficits and executive dysfunction, can persist even after recovery from sepsis [39]. These neurological consequences can have long-term impacts on patients' quality of life [40]. Respiratory consequences are also frequently encountered in sepsis. In addition to ARDS, septic patients are at an increased risk of developing pneumonia, a severe lung infection that can further exacerbate respiratory dysfunction. Pneumonia can prolong hospital stays, increase the need for mechanical ventilation, and contribute to overall morbidity and mortality in septic patients [41].
Post-sepsis syndrome encompasses the long-term consequences that sepsis survivors may experience. This syndrome can include physical, psychological, and cognitive impairments that persist beyond the acute phase of the illness. Fatigue, muscle weakness, chronic pain, anxiety, depression, and post-traumatic stress disorder (PTSD) are among the common manifestations of post-sepsis syndrome. These long-term consequences can significantly impact patients' functional status, quality of life, and ability to return to their pre-sepsis level of functioning [42]. Recognizing and addressing the consequences of sepsis, including vascular complications, hematological abnormalities, renal dysfunction, neurological impairments, respiratory complications, and post-sepsis syndrome, is crucial for comprehensive patient care. Rehabilitation and support services play a vital role in aiding sepsis survivors in their recovery and improving long-term outcomes. Continued research efforts are needed to better understand the mechanisms underlying these consequences and to develop targeted interventions to mitigate their impact on septic patients.
Challenges and Future Directions in Sepsis Research
Despite significant advances in sepsis research, several challenges and opportunities lie ahead as we strive to improve outcomes for septic patients. Addressing these challenges and exploring new directions will be critical in shaping the future of sepsis management and treatment. Translating preclinical research findings into clinical practice remains a significant challenge. While preclinical studies have provided valuable insights into sepsis pathophysiology and potential therapeutic targets, the translational gap between bench and bedside persists. Bridging this gap requires robust clinical trials that validate the efficacy and safety of interventions identified in preclinical models. Collaboration between researchers, clinicians, and regulatory bodies is essential to facilitate the translation of promising preclinical findings into effective clinical therapies [43]. Heterogeneity among sepsis patients presents another challenge. Sepsis is a complex syndrome with diverse underlying causes, clinical presentations, and treatment responses. Developing personalized therapies that consider patient characteristics, such as genetic factors, comorbidities, and biomarker profiles, is crucial [44]. Precision medicine approaches, incorporating molecular profiling and advanced diagnostics, hold promise for identifying patient subgroups and tailoring interventions to individual needs. By understanding and addressing the heterogeneity of sepsis, we can optimize treatment outcomes and improve patient care [45]. Identifying and addressing potential adverse effects of therapies is of paramount importance. Many sepsis treatments, such as immunomodulatory agents and coagulation-targeted therapies, can have unintended consequences [46]. Balancing the potential benefits with the risk of adverse effects is crucial in optimizing patient outcomes. Comprehensive monitoring, rigorous safety assessments, and long-term follow-up are necessary to identify and mitigate any potential harm associated with sepsis therapies [47]. Developing combination therapies and optimizing treatment regimens is an area of active investigation. Given the complexity of sepsis pathophysiology, a single therapeutic approach may not be sufficient. Combination therapies that target multiple aspects of the disease, such as simultaneously modulating the immune response and addressing endothelial dysfunction, hold promise in improving treatment efficacy [48]. Additionally, optimizing treatment regimens, including timing, dosing, and duration of therapies, is crucial for maximizing their effectiveness and minimizing the development of resistance [49]. Incorporating new technologies and approaches, such as machine learning and artificial intelligence (AI), can revolutionize sepsis research and clinical practice [50]. These advancements offer opportunities for data integration, predictive modeling, and clinical decision support. Machine learning algorithms can analyze large datasets, identify patterns, and aid in early sepsis detection, risk stratification, and treatment optimization [51]. AI-powered tools can enhance clinical decision-making, facilitate real-time monitoring, and improve patient outcomes. Embracing these technologies and integrating them into sepsis research and healthcare systems can accelerate progress in the field [52].
The Importance of Continued Preclinical Research into Sepsis
Preclinical research plays a crucial role in advancing our understanding of sepsis and developing novel therapies to improve patient outcomes and quality of life. While clinical studies are essential for validating the safety and efficacy of interventions, preclinical research provides a foundation for these clinical investigations by unraveling the underlying mechanisms of sepsis and identifying potential therapeutic targets. Continued preclinical research into sepsis is vital for several reasons. First, it allows for a detailed exploration of the complex pathophysiology of sepsis. By using animal models or in vitro systems, researchers can study the intricate interactions between pathogens, the immune system, endothelial cells, and other relevant factors involved in sepsis development and progression. These studies provide valuable insights into the molecular and cellular processes underlying sepsis, guiding the development of targeted therapies [53]. Second, preclinical research enables the identification and validation of potential therapeutic targets. By investigating the key players in sepsis, such as cytokines, immune receptors, signaling pathways, and coagulation factors, researchers can identify molecules or biological processes that can be modulated to mitigate sepsis-induced organ damage and dysfunction. Preclinical studies also allow for the screening and testing of drug candidates, including small molecules, antibodies, and gene therapies, to assess their efficacy and safety before advancing to human trials. Furthermore, preclinical models provide a platform to investigate the effects of potential interventions on various aspects of sepsis, such as immune responses, endothelial function, coagulation abnormalities, and organ dysfunction. Researchers can assess the impact of different therapeutic strategies, optimize dosing regimens, and evaluate potential adverse effects. These studies help prioritize the most promising interventions for further clinical evaluation and provide valuable insights into the potential benefits and limitations of different treatment approaches [54]. Moreover, preclinical research allows for the exploration of innovative treatment modalities and technologies. Researchers can investigate the use of nanomedicine, gene editing techniques, cell-based therapies, and other cutting-edge approaches for sepsis management. These advancements hold the potential to revolutionize sepsis treatment by providing more targeted, efficient, and personalized therapies [55]. Lastly, preclinical research contributes to the development of predictive models and biomarkers for early sepsis detection, risk stratification, and monitoring treatment response. By identifying specific biomarkers or patterns of gene expression, researchers can develop diagnostic tools that enable early intervention and personalized treatment strategies. These biomarkers can also serve as indicators of treatment efficacy or disease progression, aiding in clinical decision-making and patient management.
Conclusion
Sepsis remains a critical challenge in healthcare, necessitating continued efforts to unravel the intricate molecular pathways governing its pathogenesis and translate findings into improved patient outcomes. Significant progress has been made in elucidating the dysregulated inflammatory cascades, endothelial dysfunction, coagulation abnormalities, microbiome alterations and metabolic derangements underlying sepsis progression. Preclinical research has been instrumental in modeling sepsis pathophysiology, identifying biomarkers and therapeutic targets, and assessing innovative treatments from antimicrobials to stem cell therapies. However, barriers persist in translating preclinical discoveries to clinical applications. Heterogeneity among sepsis patients and subtypes warrants a precision medicine approach. Potential adverse effects of novel therapies require rigorous evaluation. Multidisciplinary collaboration and embracing new technologies like machine learning will be key to advancing sepsis research and management.
Overall, combating sepsis demands a multifaceted strategy spanning from in-depth investigation of molecular mechanisms to pragmatic clinical translation efforts. Continued preclinical research provides a critical foundation for clinical trials of emerging therapies. By addressing current challenges, investing in research, and fostering a culture of scientific inquiry and collaboration, significant headway can be made against this deadly syndrome. Sepsis remains a puzzling and problematic condition, but persistent efforts to unravel its intricacies and translate findings to patient-centric solutions provide hope for improving outcomes in this complex disease.
Recommendations
Sepsis remains a critical health challenge worldwide, necessitating robust efforts across the research-to-practice spectrum to improve patient outcomes. Key recommendations include establishing a collaborative framework between academia, industry, policy makers, and clinicians to accelerate research translation and consensus-building around best practices. Supporting preclinical sepsis research is vital to advance understanding of molecular mechanisms, identify therapeutic targets, and assess innovative treatments from a rigorous evidence-based foundation. Implementation of precision diagnostics, combinatorial therapies, and artificial intelligence to address the complexity and heterogeneity of sepsis should be prioritized. Increased awareness, early recognition programs, and optimized sepsis management protocols are needed to enable timely intervention. Post-sepsis rehabilitation and support services require greater attention.
In summary, a multifaceted strategy is essential to reduce the burden of sepsis globally. This encompasses sustaining investments in preclinical research, clinical validation of emerging therapies, access to advanced diagnostics, education for improved recognition, combination treatment regimens, enhanced recovery/rehabilitation programs, and an integrated framework of stakeholders dedicated to patient-centric solutions. Sepsis inflicts an immense toll worldwide, but a concerted effort across the spectrum from pathophysiology to policy offers hope for transforming outcomes. Continued progress requires perseverance, ingenuity, resources, and a collaborative spirit. By boldly tackling the remaining challenges, we can envision a future where sepsis-related morbidity and mortality are significantly reduced.
Abbreviations List
SIRS: Systemic Inflammatory Response Syndrome; PRRs: Pattern Recognition Receptors; TLRs: Toll-Like Receptors; TNF-α: Tumor Necrosis Factor alpha; IL-1β: Interleukin 1 beta; IL-10: Interleukin 10; TGF-β: Transforming Growth Factor Beta; DIC: Disseminated Intravascular Coagulation; AKI: Acute Kidney Injury; ARDS: Acute Respiratory Distress Syndrome; ICU: Intensive Care Unit; PTSD: Post-Traumatic Stress Disorder; NO: Nitric Oxide; Endothelial eNOS: Nitric Oxide Synthase; FMT: Fecal Microbiota Transplantation; AI: Artificial Intelligence
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data are available, and sharing is available as well as publication.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript. Lastly, the authors (TA, IE, MA, YW, AE, AK, and NE) reviewed and confirmed the final version of the manuscript.
Acknowledgements
The authors thank all the researchers who have made great efforts in their studies. The authors would also like to thank the Deanships of all the participating Universities for supporting this work. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Addissouky TA, Ali MM, El Sayed IE, Wang Y. Recent Advances in Diagnosing and Treating Helicobacter pylori through Botanical Extracts and Advanced Technologies. Archives of Pharmacology and Therapeutics. 2023;5(1):53-66.
3. Tirupakuzhi Vijayaraghavan BK, Adhikari NK. Sepsis Epidemiology and Outcomes in Asia: Advancing the Needle. American Journal of Respiratory and Critical Care Medicine. 2022 Nov 1;206(9):1059-60.
4. Elbakkoush AA, Khaleel A, Mohamed AN, Alathamneh A. Pathway analysis of sepsis-induced changes gene expression. Egyptian Journal of Medical Human Genetics. 2022 Dec;23(1):1-8.
5. Liu Y, Deng S, Song Z, Zhang Q, Guo Y, Yu Y, et al. MLIF modulates microglia polarization in ischemic stroke by targeting eEF1A1. Frontiers in Pharmacology. 2021 Sep 7;12:725268.
6. Wiersinga WJ, van der Poll T. Immunopathophysiology of human sepsis. EBioMedicine. 2022 Dec 1;86:104363.
7. Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nature Reviews Nephrology. 2023 Feb 23:401-7.
8. Wang A, Zhang S, Peng G, Tang Y, Yang Y. ICU and sepsis: role of myeloid and lymphocyte immune cells. Journal of Oncology. 2022 Sep 25;2022:1-7.
9. Tang H, Qin S, Li Z, Gao W, Tang M, Dong X. Early immune system alterations in patients with septic shock. Frontiers in Immunology. 2023 Feb 9;14:1126874.
10. Aggarwal N, Kitano S, Puah GR, Kittelmann S, Hwang IY, Chang MW. Microbiome and human health: Current understanding, engineering, and enabling technologies. Chemical Reviews. 2022 Nov 1;123(1):31-72.
11. Maneta E, Aivalioti E, Tual-Chalot S, Emini Veseli B, Gatsiou A, Stamatelopoulos K, et al. Endothelial dysfunction and immunothrombosis in sepsis. Frontiers in Immunology. 2023 Apr 4;14:1144229.
12. Tsantes AG, Parastatidou S, Tsantes EA, Bonova E, Tsante KA, Mantzios PG, et al. Sepsis-Induced Coagulopathy: An Update on Pathophysiology, Biomarkers, and Current Guidelines. Life. 2023 Jan 28;13(2):350.
13. Nedel W, Deutschendorf C, Portela LV. Sepsis-induced mitochondrial dysfunction: A narrative review. World Journal of Critical Care Medicine. 2023 Jun 9;12(3):139-52.
14. Mohanty T, Karlsson CA, Chao Y, Malmström E, Bratanis E, Grentzmann A, et al. A pharmacoproteomic landscape of organotypic intervention responses in Gram-negative sepsis. Nature Communications. 2023 Jun 17;14(1):3603.
15. Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. International Journal of Molecular Sciences. 2019 Oct 29;20(21):5376.
16. Addissouky TA, Ali MM, El Sayed IE, Wang Y, El Baz A, Elarabany N, et al. Preclinical Promise and Clinical Challenges for Innovative Therapies Targeting Liver Fibrogenesis. Archives of Gastroenterology Research. 2023 Nov 14;4(1):14-23.
17. Zurek-Leffers FM, Lehmann F, Brabenec L, Kintrup S, Hellenthal KE, Mersjann K, et al. A model of porcine polymicrobial septic shock. Intensive Care Medicine Experimental. 2023 Dec;11(1):1-8.
18. Zhang M, Fergusson DA, Sharma R, Khoo C, Mendelson AA, McDonald B, et al. Sex-based analysis of treatment responses in animal models of sepsis: a preclinical systematic review protocol. Systematic Reviews. 2023 Mar 21;12(1):50.
19. Cano-Gamez E, Burnham KL, Goh C, Allcock A, Malick ZH, Overend L, et al. An immune dysfunction score for stratification of patients with acute infection based on whole-blood gene expression. Science translational medicine. 2022 Nov 2;14(669):eabq4433.
20. Mu A, Klare WP, Baines SL, Ignatius Pang CN, Guérillot R, Harbison-Price N, et al. Integrative omics identifies conserved and pathogen-specific responses of sepsis-causing bacteria. Nature Communications. 2023 Mar 18;14(1):1530.
21. Reddy P. Clinical approach to nosocomial bacterial sepsis. Cureus. 2022 Aug 30;14(8):e28601.
22. Marques A, Torre C, Pinto R, Sepodes B, Rocha J. Treatment Advances in Sepsis and Septic Shock: Modulating Pro-and Anti-Inflammatory Mechanisms. Journal of Clinical Medicine. 2023 Apr 15;12(8):2892.
23. Lyu Z, Yang P, Lei J, Zhao J. Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics (Basel). 2023 Jun 10;12(6):1037.
24. Addissouky TA, Wang Y, El Sayed IE, Baz AE, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef University Journal of Basic and Applied Sciences. 2023 Sep 2;12(1):80.
25. Marshall JC, Leligdowicz A. Gaps and opportunities in sepsis translational research. EBioMedicine. 2022 Dec 1;86.
26. Walsh TJ, Bright RA, Ahuja A, McCarthy MW, Marfuggi RA, Simpson SQ. Meeting the Challenges of Sepsis in Severe Coronavirus Disease 2019: A Call to Arms. Open Forum Infectious Diseases. 2023 Jan 1;10(1):ofac645.
27. Singh J, Lee Y, Kellum JA. A new perspective on NO pathway in sepsis and ADMA lowering as a potential therapeutic approach. Critical Care. 2022 Dec;26(1):1-8.
28. Garduno A, Cusack R, Leone M, Einav S, Martin-Loeches I. Multi-Omics Endotypes in ICU Sepsis-Induced Immunosuppression. Microorganisms. 2023 Apr 25;11(5):1119.
29. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Can Vaccines Stop Cancer Before It Starts? Assessing the Promise of Prophylactic Immunization Against High-Risk Preneoplastic Lesions. Journal of Cellular Immunology. 2023 Nov 29;5(4):127-40.
30. Lou X, Xue J, Shao R, Yang Y, Ning D, Mo C, et al. Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Frontiers in Immunology. 2023 Jan 11;13:1063543.
31. Iba T, Helms J, Connors JM, Levy JH. The pathophysiology, diagnosis, and management of sepsis-associated disseminated intravascular coagulation. Journal of Intensive Care. 2023 Dec;11(1):1-10.
32. Bachman LO, Zwezdaryk KJ. Targeting the Host Mitochondria as a Novel Human Cytomegalovirus Antiviral Strategy. Viruses. 2023 Apr 28;15(5):1083.
33. Chellappan DK, Paudel KR, Tan NW, Cheong KS, Khoo SS, Seow SM, et al. Targeting the mitochondria in chronic respiratory diseases. Mitochondrion. 2022 Nov 1;67:15-37.
34. Cusack R, Bos LD, Povoa P, Martin-Loeches I. Endothelial dysfunction triggers acute respiratory distress syndrome in patients with sepsis: a narrative review. Frontiers in Medicine. 2023 Jun 2;10:1203827.
35. Daniel M, Bedoui Y, Vagner D, Raffray L, Ah-Pine F, Doray B, et al. Pathophysiology of sepsis and genesis of septic shock: the critical role of mesenchymal stem cells (MSCs). International Journal of Molecular Sciences. 2022 Aug 17;23(16):9274.
36. Dixit S, Arora JK, Kumar R, Arora R. Role of Routine Blood Parameters in Predicting Mortality Among Surgical Patients With Sepsis. Cureus. 2023 Apr 10;15(4):e37413.
37. White K, Serpa-Neto A, Hurford R, Clement P, Laupland K, See E, et al. Sepsis-associated acute kidney injury in the intensive care unit: Incidence, Patient Characteristics, Timing, Trajectory, Treatment, and Associated Outcomes. A multicenter, observational study. Intensive Care Medicine. 2023 49; 1079-1089.
38. Pan S, Lv Z, Wang R, Shu H, Yuan S, Yu Y, et al. Sepsis-induced brain dysfunction: pathogenesis, diagnosis, and treatment. Oxidative Medicine and Cellular Longevity. 2022 Aug 24;2022:1-13.
39. Piva S, Bertoni M, Gitti N, Rasulo FA, Latronico N. Neurological complications of sepsis. Current Opinion in Critical Care. 2023 Apr;29(2):75-84.
40. Dumbuya JS, Li S, Liang L, Zeng Q. Paediatric sepsis-associated encephalopathy (SAE): a comprehensive review. Molecular Medicine. 2023 Dec;29(1):1-24.
41. Geyer-Roberts E, Lacatusu DA, Kester J, Foster-Moumoutjis G, Sidiqi M. Preventative management of sepsis-induced acute respiratory distress syndrome in the geriatric population. Cureus. 2023 Feb 6;15(2):e34680.
42. Póvoa P, Coelho L, Dal-Pizzol F, Ferrer R, Huttner A, Conway Morris A, et al. How to use biomarkers of infection or sepsis at the bedside: guide to clinicians. Intensive care medicine. 2023 Feb;49(2):142-53.
43. Michels KR, Sheih A, Hernandez SA, Brandes AH, Parrilla D, Irwin B, et al. Preclinical proof of concept for VivoVec, a lentiviral-based platform for in vivo CAR T-cell engineering. Journal for Immunotherapy of Cancer. 2023;11(3):e006292.
44. Wang W, Liu CF. Sepsis heterogeneity. World Journal of Pediatrics. 2023 Feb 3:1-9.
45. Gao RY, Jia HM, Han YZ, Qian BS, You P, Zhang XK, et al. Calprotectin as a diagnostic marker for sepsis: A meta-analysis. Frontiers in Cellular and Infection Microbiology. 2022 Nov 28;12:1726.
46. Fan J, Shi S, Qiu Y, Liu M, Shu Q. Analysis of signature genes and association with immune cells infiltration in pediatric septic shock. Frontiers in Immunology. 2022 Nov 10;13:1056750.
47. Czempik PF, Wiórek A. Management Strategies in Septic Coagulopathy: A Review of the Current Literature. InHealthcare 2023 Jan 12; 11:227.
48. Schrijver DP, Röring RJ, Deckers J, de Dreu A, Toner YC, Prevot G, et al. Resolving sepsis-induced immunoparalysis via trained immunity by targeting interleukin-4 to myeloid cells. Nature Biomedical Engineering. 2023 Jun 8:1-6.
49. Gallenstein N, Tichy L, Weigand MA, Schenz J. Notch Signaling in Acute Inflammation and Sepsis. International Journal of Molecular Sciences. 2023 Feb 9;24(4):3458.
50. Schinkel M, van der Poll T, Wiersinga WJ. Artificial Intelligence for Early Sepsis Detection: A Word of Caution. American Journal of Respiratory and Critical Care Medicine. 2023 Apr 1;207(7):853-4.
51. van der Vegt AH, Scott IA, Dermawan K, Schnetler RJ, Kalke VR, Lane PJ. Deployment of machine learning algorithms to predict sepsis: systematic review and application of the SALIENT clinical AI implementation framework. Journal of the American Medical Informatics Association. 2023 Jul 1;30(7):1349-61.
52. Iqbal F, Chandra P, Lewis LE, Acharya D, Purkayastha J, Shenoy PA, et al. Application of artificial intelligence to predict the sepsis in neonates admitted in neonatal intensive care unit. Journal of Neonatal Nursing. 2023 Aug 8.
53. Yang S, Zhang K, Hou J, Liu X, Xu D, Chen X, et al. Protective properties of extracellular vesicles in sepsis models: a systematic review and meta-analysis of preclinical studies. Journal of Translational Medicine. 2023 Dec;21(1):262.
54. Guan J, Liao Y, Guo Y, Yu S, Wei R, Niu M, et al. Adjunctive granisetron therapy in patients with sepsis or septic shock (GRANTISS): A single-center, single-blinded, randomized, controlled clinical trial. Frontiers in Pharmacology. 2022 Dec 13;13:1013284.
55. Fitzgerald JC, Reddy AR, Woods-Hill CZ. Let's get loud: Amplifying female voices in sepsis research. EBioMedicine. 2022 Dec 1;86:104370.