Abstract
This study highlights a unique case of amiodarone toxicity, presenting with atypical symptoms of bilateral concentric visual field constriction and ambulation instability, without optic neuropathy or optic nerve edema. Despite no decline in visual acuity, the patient experienced significant visual field loss, along with gait instability, both of which resolved upon discontinuing amiodarone. This case underscores the importance of thorough baseline medication reconciliation and heightened awareness of unusual amiodarone-induced side effects impacting both visual fields and balance.
Keywords
Amiodarone, Visual field constriction, Whorl keratopathy, Amiodarone side effect, Amiodarone toxicity, Balance problems, Medication reconciliation, Atypical amiodarone toxicity
Introduction
Amiodarone, a commonly used antiarrhythmic drug, is commonly associated with broad spectrum toxicity affecting multiple systems. Classically, pulmonary, cardiac, and thyroid dysfunction are well described adverse effects of amiodarone and can occur from 15 and up to 52% within the first year [1,2]. Ocular toxicity frequently reported, including whorl keratopathy (aka corneal verticillate) as the most common, and less common include maculopathy, chalazion and dry eyes [1,2]. A more severe adverse effect is amiodarone induced optic neuropathy, where vision loss is marked by sudden painless, loss of vision and optic disc edema, affecting visual fields in annual incidence estimates ranging from 0.36% to 2.0% based on case reports [3-6]. Discontinuation of the medication may partially restore vision, though some deficits are permanent [3,6]. Although uncommon, a few cases have been reported in literature describing visual field constriction without optic neuropathy or optic disc edema [7]. This case exemplifies an atypical manifestation of a commonly described condition due to amiodarone toxicity. The patient initially presented with "clumsiness" eventually linked to visual field constriction and whorl keratopathy, leading to a diagnosis of amiodarone toxicity, with complete recovery observed following drug cessation.
Case
A 71-year-old retired veteran presented with a 5-month history of "clumsiness," describing frequent tripping during ambulation. Two months earlier, he had successful cataract surgery with no complications. His medical history included paroxysmal atrial fibrillation, treated with rivaroxaban and amiodarone following pacemaker implantation in 2018. Under regular care at the Veteran Administration and a community cardiology clinic, he was adherent with his medications. Initially assessed by ENT for possible vestibular issues, his vestibular function was found to be normal, leading to a referral to neuro-ophthalmology after his ophthalmologist couldn't identify the cause.
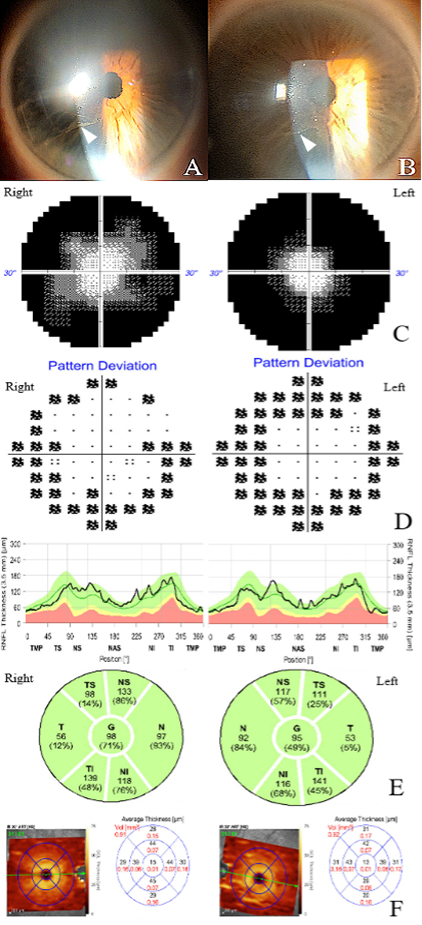
In the neuro-ophthalmology clinic, the patient had 20/20 visual acuity in both eyes, normal color vision, intact pupillary responses without a relative afferent pupillary defect, and bilateral constricted visual fields on confrontation testing. Slit-lamp examination revealed corneal verticillate in the inferior corneal regions of both eyes as seen in (Figures 1A and 1B) (Showing images of both corneas as described with verticillate indicated by arrows). The dilated fundus exam showed flat, normal optic nerves with no edema or cupping. Humphrey visual field testing confirmed significant concentric constriction bilaterally on both 30-2 and 10-2 tests as shown in (Figures 1C and 1D). Optical coherence tomography (OCT) indicated normal retinal nerve fiber and ganglion cell layers (Figure 1E and 1F – showing retinal ganglion and retinal nerve fiber layer segmentation). A prior brain MRI was unremarkable, showing no enhancements or masses.
Given the presence of corneal verticillate and constricted visual field loss, an extensive review of his medical history and medications revealed that he did not have a family history of Fabry’s disease and that the patient received care from multiple care facilities outside of each other’s network and did not communicate with each other. Upon receiving records from his multiple physician teams, it was discovered that the patient was initiated on amiodarone by cardiology clinic within the last 6 months. Due to lapses in communication between his multiple care teams, including a pacemaker placement and prescriptions from different facilities, this link had gone unnoticed. After communication with the cardiologist, amiodarone was stopped immediately.
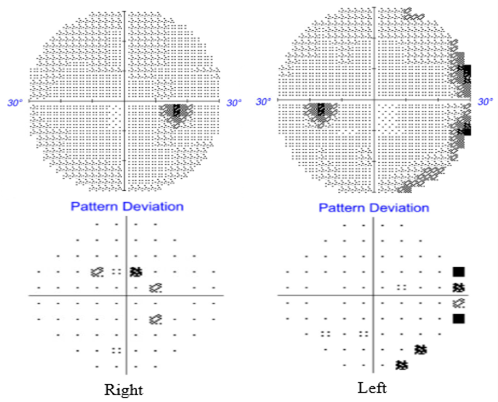
Eleven days after stopping amiodarone, the patient returned for follow-up, reporting significant improvement in ambulation and repeat automated Humphrey 30-2 visual field testing showed with complete resolution of the previously observed peripheral constriction as seen in the full visual field in (Figure 2) showing a complete improvement of his constricted visual field compared to the previous testing in Figure 1C (automated visual field with constriction in both eyes).
Figure 1. Initial examination and testing. Whorl keratopathy (corneal verticillate, white arrowheads) seen in corneas of the right eye (A) and left eye (B). Humphrey visual field testing (30-2, greyscale plot shown) showed severe concentric constriction in both eyes (C) in the initial visit. Humphrey visual field 10-2 testing (pattern deviation plot shown) showed similar peripheral constriction in each eye (D). Normal optical coherence tomography of the retinal nerve fiber layer was seen in each eye (E). Normal ganglion cell layer thickness was seen on optical coherence tomography as well in each eye (F).
Figure 2. Follow up testing. Humphrey visual field 30-2 showed essentially normal visual fields in each eye following cessation of amiodarone. Showing a significant, almost complete resolution of the visual field constriction after amiodarone cessation.
Discussion
This case presents some unique challenges and teaching points:
First, this case emphasizes the need to consider non-classical findings in conditions traditionally characterized in literature. Our patient presents with concentric visual field constriction without optic nerve edema or pallor highlights the variability in presentation that can delay diagnosis. The most common ocular finding for amiodarone side effect are corneal epithelial opacities caused by intracytoplasmic deposits called corneal verticillate [8]. These resemble “cat’s whiskers” and are reported in 50-60% of patients [8]. Although helpful to clue in on the diagnosis, whorl keratopathy (aka corneal verticillate), does not cause decreased vision and thus did not explain for his visual field loss, adding to the case that this is a non-classical presentation of a previously described condition [1,8].
Second, the case emphasizes the critical role of thorough medication reconciliation and medical record review in preventing diagnostic delays and medical errors. A comprehensive review of a patient’s medical history and medication list helps identify key diagnostic information, reduce errors, and resolve discrepancies between healthcare providers [9]. Medication omissions, particularly with cardiovascular drugs like amiodarone, are common errors in hospitalized patients [9]. It is important to recognize that complete medication reconciliation involves two key steps: reviewing accessible records within the system and obtaining external records, which can be time-consuming and contribute to delays in care. In this case, incomplete knowledge of the patient's medication regimen across multiple healthcare systems led to misinterpretation of symptoms which may contribute to medical errors and a delay in diagnosis as was the case in this patient [10].
Third, is to highlight the association between vision loss and balance. Many studies have demonstrated statistical correlation that vision loss increases the odds of balance problems [11]. The ability to maintain balance is an integration of several systems that include vision, vestibular, and somatosensory system [12]. Dysfunction in any of these components can destabilize balance, with vision playing a key role in postural stability. The patient's initial symptoms of "clumsiness" and difficulty ambulating were mistakenly not attributed to vision loss, despite a history of cataract surgery. Understanding that balance issues may stem from visual dysfunction allows for accurate diagnosis and prevents unnecessary interventions [13-15].
Conclusion
This case underscores the complexities of diagnosing amiodarone-induced toxicity, particularly when it presents with atypical features, such as concentric visual field constriction without optic disc edema or pallor. It highlights the importance of maintaining a high level of suspicion for non-classical presentations of well-known conditions and emphasize the critical role of thorough medication reconciliation and comprehensive medical records review, especially when patients receive care from multiple providers across different health systems. Additionally, the case illustrates the strong connection between vision and balance, reminding physicians that visual dysfunction can manifest as balance problems, which may be misinterpreted as vestibular or cerebellar disorders. A timely and accurate diagnosis, based on a careful evaluation of both symptoms and medical history, can prevent unnecessary interventions and lead to better patient outcomes.
Author Contributions
Conceptualization, P.R.C. and M.T.; methodology, M.T., P.R.C.; formal analysis, B.Z. and P.R.C.; investigation, B.Z., P.R.C., A.H., M.T.; resources, P.R.C.; writing—original draft preparation, P.R.C.; writing—review and editing, B.Z., A.H., and M.T.; visualization, B.Z. and P.R.C.; supervision, A.H. and M.T.; project administration, A.H. and M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Texas Southwestern Medical Center.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
2. Florek JB, Lucas A, Girzadas D. Amiodarone. [Updated 2023 Nov 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan.
3. Passman RS, Bennett CL, Purpura JM, Kapur R, Johnson LN, et al. Amiodarone-associated optic neuropathy: a critical review. Am J Med. 2012 May;125(5):447-53.
4. Wang AG, Cheng HC. Amiodarone-Associated Optic Neuropathy: Clinical Review. Neuroophthalmology. 2016;41(2):55-8.
5. Mindel JS. Amiodarone and optic neuropathy. Am Heart J. 2008;156(3):411-3.
6. Johnson LN, Krohel GB, Thomas ER. The clinical spectrum of amiodarone-associated optic neuropathy. J Natl Med Assoc. 2004 Nov;96(11):1477-91.
7. Speicher MA, Goldman MH, Chrousos GA. Amiodarone optic neuropathy without disc edema. J Neuroophthalmol. 2000 Sep;20(3):171-2.
8. Mäntyjärvi M, Tuppurainen K, Ikäheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998 Jan-Feb;42(4):360-6.
9. Porcelli PJ, Waitman LR, Brown SH. A review of medication reconciliation issues and experiences with clinical staff and information systems. Appl Clin Inform. 2010 Dec 1;1(4):442-61.
10. Sameera V, Bindra A, Rath GP. Human errors and their prevention in healthcare. J Anaesthesiol Clin Pharmacol. 2021 Jul-Sep;37(3):328-35.
11. Kahiel Z, Grant A, Aubin MJ, Buhrmann R, Kergoat MJ, Freeman EE. Vision, Eye Disease, and the Onset of Balance Problems: The Canadian Longitudinal Study on Aging. Am J Ophthalmol. 2021 Nov;231:170-8.
12. Collings R, Paton J, Glasser S, Marsden J. The effect of vision impairment on dynamic balance. Journal of Foot and Ankle Research. 2015 Dec;8(Suppl 1):A6.
13. Aartolahti E, Häkkinen A, Lönnroos E, Kautiainen H, Sulkava R, Hartikainen S. Relationship between functional vision and balance and mobility performance in community-dwelling older adults. Aging Clin Exp Res. 2013 Oct;25(5):545-52.
14. Redfern MS, Yardley L, Bronstein AM. Visual influences on balance. J Anxiety Disord. 2001 Jan-Apr;15(1-2):81-94.
15. Freeman EE, Muñoz B, Rubin G, West SK. Visual field loss increases the risk of falls in older adults: the Salisbury eye evaluation. Invest Ophthalmol Vis Sci. 2007 Oct;48(10):4445-50.