Abstract
Immunotherapy, particularly immune checkpoint inhibitors (ICIs), has revolutionized cancer treatment by harnessing the host immune system to target malignancies. Melanoma, head and neck squamous cell carcinoma (HNSCC), and triple-negative breast cancer (TNBC) were among the first solid tumors to gain regulatory approval for ICIs due to their immunogenicity and unmet clinical needs. Melanoma exemplifies the success of ICI therapy, with durable responses driven by its high mutation burden and neoantigen landscape, yet both primary and acquired resistance remain major challenges. In contrast, HNSCC demonstrates clinically meaningful but modest responses in the context of a highly immunosuppressive tumor microenvironment, while TNBC derives limited benefit from ICI, often requiring combination strategies to achieve efficacy. Resistance to ICIs arises from complex tumor-intrinsic, microenvironmental, and systemic mechanisms that collectively undermine effective anti-tumor immunity. This review highlights both shared and cancer-specific mechanisms of ICI resistance across melanoma, TNBC and HNSCC. We also discuss emerging strategies, including combination therapies, neoantigen-based vaccines, adoptive T cell therapies, and precision oncology approaches, to overcome resistance and improve clinical outcomes. Together, these insights provide a framework for optimizing immunotherapy and advance durable benefit in these challenging malignancies.
Keywords
Immune checkpoint inhibitors, Immunotherapy resistance, Melanoma, Head and neck squamous cell carcinoma, Triple-negative breast cancer, Tumor microenvironment, Tumor mutation burden, Neoantigens, Oncogenic pathways, Antigen presentation
Introduction
Immunotherapy has fundamentally transformed modern oncology, offering durable clinical responses and opening new therapeutic avenues for a wide range of malignancies. By harnessing the host immune system to target and eliminate cancer cells, therapies such as immune checkpoint inhibitors (ICIs) have achieved breakthroughs in cancers previously considered treatment-refractory [1,2]. The approval of ipilimumab, a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody, for advanced cutaneous melanoma in 2011 marked the dawn of a new era in cancer treatment [3]. Subsequent regulatory approvals of programmed death-1 (PD-1) and its ligand (PD-L1) inhibitors have rapidly expanded the impact of ICIs to multiple solid tumors, including head and neck squamous cell carcinoma (HNSCC) and triple-negative breast cancer (TNBC) [2,4–6]. These cancers remain at the forefront of clinical and translational immuno-oncology.
Melanoma, HNSCC, and TNBC exemplify both the successes and limitations of current immunotherapeutic strategies. In melanoma, historically dismal outcomes with chemotherapy or interleukin-2 (IL-2) therapy have been replaced by unprecedented long-term survival in a subset of patients, driven by its high tumor mutational burden, abundant neoantigen repertoire, and a tumor microenvironment conducive to T-cell infiltration [7–9]. Despite these advances, most patients ultimately develop primary or acquired resistance (relapse after initial benefit) [10–12]. Similarly, ICIs have reshaped the therapeutic landscape in HNSCC, a cancer often associated with oncogenic viral infection, tobacco and alcohol exposure, and often marked by a profoundly immunosuppressive tumor microenvironment (TME) [13,14]. Although PD-1 blockade has provided meaningful improvements in recurrent or metastatic disease, only a subset of patients experiences durable benefit, reflecting resistance mechanisms driven by tumor heterogeneity, immune exclusion, and adaptive immunosuppression [14]. TNBC, the most aggressive breast cancer subtype characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), has likewise benefited from ICI-based combinations. The addition of pembrolizumab to chemotherapy has improved outcomes in both early-stage and metastatic settings [15–17]. Nevertheless, durable responses remain uncommon, underscoring the need to better understand tumor-immune escape and to identify strategies that extend therapeutic benefit.
Resistance across these cancers, whether primary (non-response) or acquired (relapses following initial response), emerges from a multifaceted interplay of tumor-intrinsic factors (e.g., antigen-presentation loss, signaling pathway alterations) and microenvironmental barriers (e.g. immunosuppressive cells, stromal remodeling) and systemic host-related constraints (e.g. metabolism, microbiome) that collectively blunt effective antitumor immunity [18–20].
This review examines melanoma, HNSCC, and TNBC as model immunogenic epithelial cancers responsive to ICI. We highlight both convergent and cancer-specific resistance mechanisms, explore their clinical implications, and discuss emerging therapeutic strategies—including rational ICI combinations, neoantigen-targeted vaccines, adoptive T-cell therapies, and precision-based patient selection. By integrating current insights, we aim to provide a framework for overcoming resistance and optimizing immunotherapy outcomes for these difficult-to-treat malignancies.
Current Clinical Landscape of Immunotherapy and ICI Resistance
Over the past decade, immune checkpoint blockade has become a standard of care across multiple malignancies. ICIs function by removing the inhibitory signals on T-cell activity, most prominently through pathways involving CTLA-4 (CD152) [21], PD-1 (CD279)/PD-L1 (CD274) [22]. While the number of newly identified immune checkpoint molecules is rapidly expanding, the clinically approved portfolio of ICIs remains limited, reflecting the complex biology of checkpoint regulation [23] and the highly variable efficacy of these agents across tumor types [24]. This variability is strongly influenced by tumor-intrinsic biology and the characteristics of the tumor microenvironment (TME) (Table 1).
Melanoma
Among solid tumors, cutaneous melanoma shows the highest responsiveness to ICIs, in contrast to acral and uveal melanomas, which are generally refractory to these therapies [25–28] (Table 1). Prior to ICIs, metastatic disease was associated with a median survival of less than one year, with minimal benefit from chemotherapy or high-dose interleukin-2 [29]. The introduction of anti-CTLA-4 inhibitor (ipilimumab) and subsequent PD-1 inhibitors (nivolumab, pembrolizumab) transformed outcomes for advanced disease [25] (Table 2). Landmark clinical trials, including MDX010-20 [30], KEYNOTE-006 [31], and CheckMate-067 [32], not only demonstrated objective response rates (ORRs) of ~40–45% but also achieved unprecedented long-term survival, with > 40% overall survival (OS) at 6.5 years in some cohorts [33]. Pembrolizumab has shown particularly strong activity in desmoplastic melanoma, with ORRs approaching 89%, high rates of pathological complete response (pCR), and extended disease-free survival [34]. Combination regimens (ipilimumab plus nivolumab) have further improved progression-free survival (PFS) compared with monotherapy. For patients with BRAF-mutant melanoma, the Phase III DREAMseq trial established the sequencing of targeted therapy (dabrafenib/trametinib) after combined ICI (nivolumab/ipilimumab) as the preferred treatment strategy (Table 2), demonstrating a 30% improvement in OS and a threefold improvement in PFS at 5 years compared with either therapy alone [35].
Despite these advances, resistance to ICI remains a significant clinical challenge, presenting either as primary non-response or acquired relapse. Approximately 55% of melanoma patients have primary resistance to PD-1 inhibitors, 40% to CTLA-4+PD-1 combination therapy, and 25% of initial PD-1 responders acquire resistance within two years [36]. Mechanistic drivers of resistance include loss of antigen-presentation [37], defects in interferon-γ (IFN-γ) signaling [38,39], activation of the WNT/β-catenin pathway [40–42], upregulation of compensatory inhibitory checkpoints [43], and recruitment of immunosuppressive myeloid cells [44] (Table 1). Many of these mechanisms are now being targeted with rational ICI-based combinations. For example, melanoma’s high mutational and neoantigen load made it the first tumor type to be evaluated in clinical trials of personalized neoantigen mRNA vaccine. The Phase IIb KEYNOTE-942 trial (mRNA-4157/V940, Merck and Moderna) [45] demonstrated that adding a personalized vaccine to pembrolizumab significantly improved recurrence-free survival (79% vs. 62%) and distant metastasis-free survival (92% vs. 77%) at 18 months compared with pembrolizumab alone (Table 1) [45]. The recent approval of anti-lymphocyte activation gene-3 (LAG-3) therapy (relatlimab plus nivolumab) further illustrates how rational ICI combinations are expanding the potential for durable response [46].
HNSCC
HNSCC is biologically heterogeneous, influenced by risk factors such as tobacco, alcohol, and human papillomavirus (HPV) infection, which significantly shape the tumor immune landscape [94]. Historically, platinum-based chemotherapy has served as the backbone of first-line therapy for recurrent or metastatic HNSCC. HPV-positive (HPV+) tumors are generally more inflamed and responsive to ICIs, whereas HPV-negative (HPV-) tumors often exhibit immune exclusion and profound immunosuppression [95].
The clinical efficacy of PD-1 blockade in platinum-refractory HNSCC was established through the CheckMate-141 (nivolumab) and KEYNOTE-040 (pembrolizumab) trials, confirming ICIs as a standard of care in this setting [68] (Table 2). The role of PD-1 inhibitors was later expanded to the first-line therapy in KEYNOTE-048, where pembrolizumab improved outcomes both as monotherapy for PD-L1-positive tumors and in combination with platinum/5-FU for all patients [66]. Despite durable benefits in a subset of patients, objective response rates remain modest (~15–20%) [96–98] (Table 1). KEYNOTE-012 [99] and KEYNOTE-055 [100] confirmed comparable efficacy for PD-1 blockade between HPV+ and HPV- populations in recurrent/metastatic HNSCC. Similarly, CheckMate-141 demonstrated improved response rates (13.3% vs. 5.8%) and OS in 361 platinum-refractory HNSCC patients treated with nivolumab, with no significant difference between HPV+ and HPV- status [101] (Table 2). In contrast, a PD-L1 inhibitor, durvalumab, has not demonstrated benefit in this setting. Phase III trials of durvalumab alone or in combination with tremelimumab (a CTLA-4 inhibitor) failed to improve OS compared with chemotherapy, limiting its clinical role in recurrent/metastatic HNSCC [102,103].
Resistance in HNSCC is multifactorial (Table 1), including loss of MHC I expression, defective IFN-γ signaling, T-cell exclusion, and expansion of regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs) within the TME [96,104,105]. Like melanoma and TNBC, many HNSCC tumors upregulate PD-L1 in response to IFN-γ [106], and alterations in the phosphoinositide-3-kinase (PI3K) - phosphatase and tensin homolog (PTEN) pathway contribute further to tumor evasion due to the developed dysfunction of immune cells [24,107]. HNSCC exhibits unique features as HPV+ tumors evade immunity via viral oncoproteins E6/E7 [107], displaying high T-cell infiltration but increased Tregs and CTLA-4 expression [22,23]. Tobacco-associated tumors have reduced immune infiltration despite high mutational burden, consistent with poorer outcomes [109,110]. Additionally, HNSCC displays distinctive natural killer (NK)-cell biology, characterized by abundant CD56dim NK cells [13,111]. The NK activity is suppressed through killer cell immunoglobulin-like receptor (KIR) signaling and can be influenced by HPV status [13] (Table 1). Emerging treatment strategies for HNSCC include dual checkpoint blockade (PD-1 plus CTLA-4 or LAG-3) and combinations with radiation, vaccines, or targeted therapies aimed at reprogramming the immune TME [112,113].
TNBC
TNBC, defined by the absence of ER, PR, and HER2 expression, is associated with poor prognosis and limited targeted treatment options. Early-phase studies demonstrated modest efficacy of PD-1 or PD-L1 inhibitors as monotherapy (~5–20% ORR) in TNBC patients [82,114] (Table 2). The Phase I JAVELIN trial of avelumab (targeting anti-PD-L1) reported ORRs of 44.4% in PD-L1-high versus 2.6% in PD-L1-low TNBC patients [84]. Single-agent efficacy remains limited, with progression driven by intrinsic resistance mechanisms such as immune exclusion and adaptive resistance pathways [84]. TNBC is typically characterized by low TMB, limited tumor-infiltrating lymphocytes (TILs), and strong stromal barriers, making it less responsive to ICIs than melanoma [115] (Table 1). Approximately 20–30% of TNBC tumors express PD-L1, often correlating with higher TIL infiltration and higher histological grade [116], providing an additional target for ICI.
Combination strategies have proven more effective. Meta-analyses show that ICIs combined with anthracyclines and taxanes significantly increase pCR (64.8%) in early TNBC while reducing toxicity compared to platinum chemotherapy [117]. Combining anthracycline and taxane chemotherapy with durvalumab as adjuvant therapy can improve the prognosis of early TNBC [118]. The IMpassion130 trial demonstrated that atezolizumab plus nab-paclitaxel improved PFS and OS in PD-L1-positive metastatic TNBC, although IMpassion131 failed to replicate these benefits (Table 2) [117]. KEYNOTE-35 trial found that pembrolizumab plus chemotherapy extended OS in PD-L1–high (CPS≥10) metastatic TNBC patients (median OS of 23.0 vs. 16.1 months) [77]. In early disease, KEYNOTE-522 showed that pembrolizumab plus neoadjuvant chemotherapy improved pCR (64.8% vs. 51.2%) and event-free survival [119].
Despite these advances, TNBC remains largely ICI-refractory [120] (Table 1). Shared resistance mechanisms include immune exclusion, defective antigen presentation, and suppressive myeloid infiltration, but TNBC exhibits certain distinct features: low TMB, absence of pre-existing tumor-specific immunity, stromal-mediated T-cell exclusion, and macrophage-driven suppression. High genomic instability is another hallmark of TNBC [20,121,122]. While challenging, it also presents opportunities for targeted interventions, such as poly (ADP-ribose) polymerase (PARP) and Protein Kinase B (AKT) inhibitors, to enhance response rates [123]. TNBC tumors often display immune-excluded phenotypes, characterized by a lack of immune cell infiltration into the tumor parenchyma due to a dense stromal matrix and TGF-beta-induced fibrosis acting as physical barriers [121,122].
|
|
Melanoma |
HNSCC |
TNBC |
|
Common resistance mechanisms |
|
||
|
Unique cancer features |
↑ Immune infiltration |
|
↑ immune “cold” TME |
|
Response to ICI monotherapy
|
~40–45% ORR |
~15–20% ORR |
~5-20% ORR |
|
Trial Number |
Treatment |
Subject |
Reference |
|
Melanoma |
|||
|
NCT03396952 |
Pembrolizumab + Ipilimumab + High-dose Aspirin |
Advanced Metastatic Melanoma |
[47] |
|
NCT01844505 |
Nivolumab or Nivolumab + Ipilimumab vs. Ipilimumab Alone |
Advanced Melanoma |
[48] |
|
NCT03470922 |
Relatlimab + Nivolumab vs. Nivolumab Alone |
Advanced Melanoma |
[49] |
|
NCT04949113 |
Neoadjuvant Ipilimumab + Nivolumab vs. Standard Adjuvant Nivolumab |
Stage III Melanoma |
[50] |
|
NCT04274816 |
Intradermal Tremelimumab (low dose) |
Early-stage Melanoma (Stage I–II) |
[51] |
|
NCT01866319 (KEYNOTE-006) |
Pembrolizumab vs. Ipilimumab |
Metastatic Melanoma |
[31] |
|
NCT00323206 |
Intratumoral IL-12 plasmid + electroporation |
Metastatic Melanoma (Phase I dose escalation) |
[52] |
|
NCT02275416 |
UV1 peptide vaccine + Ipilimumab |
Unresectable Metastatic Melanoma |
[53] |
|
NCT02752074 (ECHO-301 / KEYNOTE-252) |
Epacadostat + Pembrolizumab vs. Pembrolizumab alone |
Unresectable/Metastatic Melanoma |
[54] |
|
NCT02475213 |
Enoblituzumab + Pembrolizumab |
Advanced Solid Tumors (including Melanoma) |
[55] |
|
NCT03693612 (INDUCE-2) |
Feladilimab + Tremelimumab |
Advanced Solid Tumors (including Melanoma) |
[56] |
|
NCT03776136 (LEAP-004) |
Lenvatinib + Pembrolizumab |
Unresectable Stage III/IV Melanoma with progression on prior PD-1/PD-L1 therapy |
[57] |
|
NCT00179608 |
Lenalidomide + Dacarbazine |
Chemo-naïve Metastatic Melanoma patients |
[58] |
|
NCT00864253 |
Nab-paclitaxel vs. Dacarbazine |
Metastatic Melanoma |
[59] |
|
NCT03178851 |
Cobimetinib + Atezolizumab |
BRAF V600 WT Advanced Melanoma, post–PD-1 therapy |
[60] |
|
NCT00086489 |
Tremelimumab |
Advanced Melanoma |
[61] |
|
NCT01656642 |
Atezolizumab + Vemurafenib ± Cobimetinib |
Metastatic Melanoma (BRAF V600–mutant) |
[62] |
|
NCT00616564 |
ch14.18 + R24 antibodies combined with IL-2 |
Metastatic Melanoma (23 patients) and Sarcoma (4 patients) |
[63] |
|
NCT00631072 |
Autologous iNKT cell infusion |
Stage IIIB–IV Melanoma |
[64] |
|
NCT04551352 |
TYRP1-TCB (RO7293583) — bispecific antibody targeting TYRP1 + CD3 |
Metastatic Melanoma (cutaneous, uveal, mucosal; TYRP1-positive) |
[65] |
|
Head and Neck Cancers |
|||
|
KEYNOTE-048 |
Pembrolizumab mono; Pembro + chemo + 5-FU Cetuximab + chemo + 5-FU |
Recurrent/Metastatic HNSCC |
[66]
|
|
NCT02741570 |
Nivolumab + ipilimumab vs. EXTREME regimen |
Recurrent/Metastatic HNSCC |
[67] |
|
NCT02252042 |
Pembrolizumab vs. methotrexate, docetaxel or cetuximab |
Recurrent/Metastatic HNSCC |
[68] |
|
NCT03342911
|
Nivolumab + carboplatin + paclitaxel |
Stage III-IV HNSCC |
[69] |
|
NCT04282109
|
Nivolumab + paclitaxel |
Recurrent/Metastatic HNSCC |
[70] |
|
NCT02179918 |
PF-05082566 + pembrolizumab (anti-PD-1) |
Advanced Solid Tumors |
[71] |
|
NCT02110082 |
Urelumab (4-1BB agonist) and cetuximab |
Advanced/Metastatic HNSCC |
[72] |
|
Triple Negative Breast Cancer |
|||
|
NCT02622074 |
Pembrolizumab + Chemotherapy as Neoadjuvant |
Early-Stage TNBC |
[73,74] |
|
NCT04613674 |
Camrelizumab + Chemotherapy vs. placebo + chemotherapy |
Early or Locally Advanced TNBC |
[75] |
|
NCT03289819 |
Neoadjuvant Pembrolizumab/Nab-Paclitaxel Followed by Pembrolizumab/Epirubicin/Cyclophosphamide |
Early-Stage TNBC |
[76]
|
|
NCT02819518 |
Pembrolizumab combinations vs. Placebo + Chemotherapy |
Previously untreated locally recurrent Metastatic TNBC |
[77,78]
|
|
NCT03487666 |
Nivolumab and Capecitabine combined vs. alone |
TNBC |
[79] |
|
NCT03125902 |
Atezolizumab + Paclitaxel vs. Atezolizumab Placebo + Paclitaxel |
Previously Untreated Inoperable Locally Advanced or Metastatic TNBC |
[80] |
|
NCT02413320 |
Carboplatin + Paclitaxel then Doxorubicin + Cyclophosphamide vs. Carboplatin + Docetaxel |
Stage I-III TNBC |
[81] |
|
NCT02447003 |
Pembrolizumab |
Metastatic TNBC |
[82] |
|
NCT01375842 |
Atezolizumab |
Metastatic TNBC |
[83] |
|
NCT01772004 |
Avelumab |
Metastatic TNBC |
[84] |
|
NCT02657889 |
Pembrolizumab + Niraparib |
Advanced/Metastatic TNBC |
[85] |
|
NCT03330405 |
ICIs + Avelumab + Talazoparib |
Advanced TNBC |
[86] |
|
NCT02555657 |
Pembrolizumab vs. TPCe |
Metastatic TNBC |
[87] |
|
NCT02734004 |
Olaparib + Durvalumab |
Metastatic TNBC |
[88] |
|
NCT01042379 |
Paclitaxel with or without Pembrolizumab + adjuvant chemotherapy |
Early-stage TNBC |
[89] |
|
NCT01633970 |
Nab-paclitaxel + Atezolizumab |
Metastatic TNBC |
[90] |
|
NCT04129996 |
Angiogenesis inhibitor + Camrelizumab + |
Advanced immunomodulatory TNBC patients |
[91] |
|
NCT02425891 |
Nabpaclitaxel + |
Metastatic TNBC |
[92] |
|
NCT02299999 |
Durvalumab vs. Chemotherapy |
Metastatic TNBC |
[93] |
|
Trials are categorized by cancer type, treatment regimen, patient population, and corresponding reference. Included studies highlight both monotherapy and combination strategies that have shaped current clinical practice and informed emerging approaches to overcome resistance. |
|||
Mechanisms of Resistance to ICI Therapy
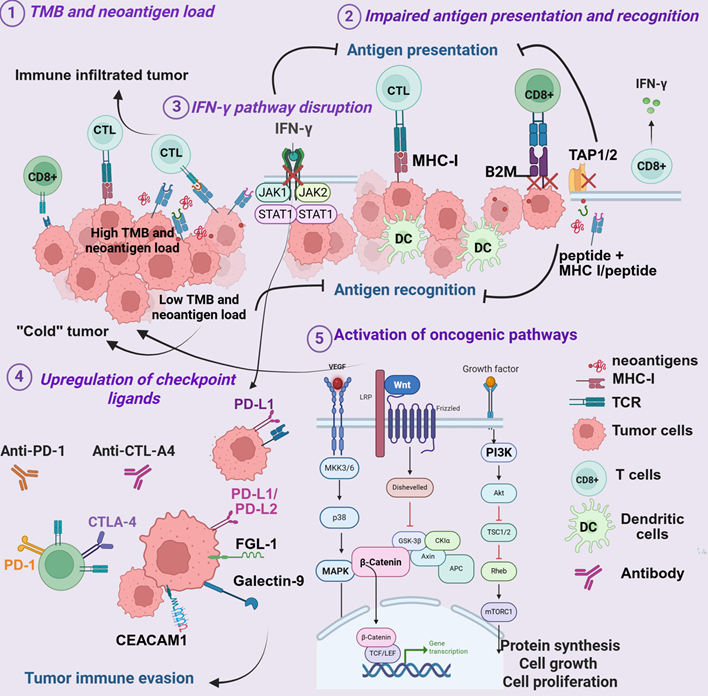
Resistance to ICIs can be broadly divided into tumor-intrinsic and -extrinsic mechanisms. Intrinsic mechanisms arise from genetic and signaling alterations within tumor cells that impair immune recognition or effector function. Extrinsic mechanisms occur in the TME, where cellular and soluble factors create an immunosuppressive milieu. In practice, these mechanisms are highly interconnected and often overlap, collectively shaping the degree and durability of response [124,125] (Figures 1 and 2).
1.Tumor-intrinsic resistance mechanisms
Tumor mutation burden and neoantigen load: One of the strongest correlates of ICI efficacy is TMB and the associated generation of non-synonymous mutations that produce immunogenic neoantigens [126]. High TMB, particularly observed in mismatch repair-deficient tumors, is associated with improved ICI responsiveness [127–129] (Figure 1.1). Melanoma exemplifies this phenomenon: ultraviolet-induced mutagenesis produces one of the highest TMBs among solid tumors, generating a rich neoantigen landscape that drives robust immune recognition [130,131]. Consistent with this concept, recent clinical analyses have shown that patients receiving biomarker-guided dual-matched therapies (combining targeted agents with ICIs) can experience durable clinical benefit, including long-term survival exceeding 1.5 years in some cases [132]. While the overall TMB in HNSCC is intermediate compared to melanoma, a significant subset of HNSCC patients have elevated TMB, and this is predictive of better responses to ICIs [133,134].
HPV status significantly shapes the neoantigen repertoire with HPV+ tumors, in addition to tumor-derived neoantigens, presenting viral antigens that enhance immune responses, whereas HPV- tumors often show neoantigen loss [135]. By contrast, TNBC exhibits lower TMB, limiting neoantigen-driven immunity and contributing to modest ICI response rates [136]. Highly immunogenic tumors such as melanoma, HNSCC, and non-small cell lung carcinoma, with enriched TMB and neoantigen landscapes, are more responsive to ICIs than tumors with low TMB, such as TNBC, prostate, and pancreatic cancers. Consequently, low-TMB tumors often exhibit poor T cell infiltration and a "cold" tumor immune TME [131,137,138] (Figure 1.1). However, TMB is not universally predictive of ICI response [139]. Tumors with high neoantigen load may still develop resistance if these neoantigens are weakly immunogenic [140,141] or actively suppress immune responses (inhibitory neoantigens) [141–145]. Current strategies focus on radiotherapy, chemotherapy, and vaccines in combination with immunotherapy to increase neoantigen availability and enhance immunogenicity [146–148].
Recent evidence indicates that neoantigens can also arise from post-translational modifications, including glycosylation (O-linked β-N-acetylglucosamine), phosphorylation (phospho-neoantigens), and alternative RNA splicing [149–151]. These modifications expand antigenic diversity, create unique epitopes, and provide an immunological signature of the "transformed self" recognized by T cells [152,153]. Some phospho-neoantigens are shared across multiple tumor types and patients, offering the potential for immunotherapeutic targeting beyond personalized approaches [154].
Impaired antigen processing and presentation: Defects in antigen presentation are a well-documented mechanism of ICI resistance [155] (Figure 1.2). Effective CD8+ T-cell recognition requires intact MHC-I-mediated antigen processing and presentation of tumor antigens [156]. Loss of MHC-I surface expression or structural disruption allows tumors to evade T-cell surveillance [157]. Mutations in β2-microglobulin (B2M) destabilize MHC-I complexes [158], while deficiencies in transporters associated with antigen processing (TAP1/2) or endoplasmic reticulum (ER) aminopeptidases (ERAP1/2) impair peptide translocation and loading [159,160].
These alterations occur across melanoma, HNSCC, and TNBC, with context-dependent contributions [161,162]. In melanoma, B2M mutations and MHC-I downregulation are strongly linked to acquired resistance after initial PD-1 blockade, and IFN-γ pathway defects further reduce antigen presentation [163,164] (Figure 1.2). In HNSCC, antigen presentation status is influenced by HPV status, with HPV+ tumors generally retaining intact MHC-I expression and an inflamed TME, whereas HPV- tumors more often lack MHC-I, correlating with poor ICI response [124]. Beyond MHC-I, impaired MHC-II presentation by tumor or myeloid cells attenuates CD4+ T-cell-mediated immunity, adding another layer of immune evasion [165]. In TNBC, B2M mutations are less common, with resistance often driven by transcriptional or epigenetic repression of antigen presentation machinery [161,166].
Disruption of IFN-γ signaling: The IFN-γ pathway is central to tumor immune recognition (Figure 1.3), driving expression of MHC-I and immunoregulatory molecules such as PD-L1 via Janus kinases 1 and 2 (JAK1 and JAK2) and the signal transducer and activator of transcription 1 (STAT1) activation [167]. Disruption of this pathway by tumor-intrinsic alterations is a well-established mechanism of primary resistance to ICIs. Loss-of-function mutations or epigenetic silencing of JAK1/JAK2, and downstream transcriptional regulators impair the IFN-γ–mediated induction of the antigen presentation machinery and checkpoint ligands, enabling tumor immune evasion by rendering tumors “invisible” to cytotoxic T cells, even in the context of high TMB [164,168]. Consequently, in melanoma, HNSCC, and TNBC, JAK/STAT pathway inactivation prevents IFN-γ-induced upregulation of MHC-I and PD-L1, contributing to primary resistance despite an increased neoantigen burden [163,169] (Figure 1.3).
In HNSCC (particularly HPV+) and TNBC, preserved IFN-γ signaling drives strong PD-L1 induction (Figure. 1.3), limiting T-cell activity and promoting adaptive resistance [170]. HPV- and tobacco-associated tumors often harbor JAK/STAT defects, reducing IFN-γ signaling, diminishing antigen presentation, and promoting tumor immune evasion [171]. Amplification of negative regulators, such as suppressor of cytokine signaling 1 (SOCS1) and protein inhibitor of activated STAT4 (PIAS4), further suppresses IFN-γ signaling [169], thereby facilitating immune evasion.
Paradoxically, intact IFN-γ signaling can also drive adaptive resistance, as chronic exposure induces chronic PD-L1 expression, dampening T-cell activity and fostering immune evasion [170]. Thus, IFN-γ signaling exerts a dual influence: loss abrogates immune recognition and drives primary resistance, while persistent activation promotes adaptive resistance through PD-L1-mediated suppression.
Upregulation of immune checkpoint ligands by tumor cells: Tumor cells evade immune pressure by upregulating several inhibitory checkpoint ligands on their surface, effectively suppressing activation of the T-cells expressing cognate receptors (Figure 1.4). The expression of such ligands within the TME can quench immune effector functions, promote regulatory or suppressive subsets of cells, and allow the tumor to evade immune attack [172]. This mechanism is central to the processes of immune escape and contributes to both primary and acquired resistance to ICI [173]. PD-L1 expression has been extensively studied as a predictive biomarker for response to PD-1/PD-L1 blockade across multiple cancer types, including melanoma, HNSCC, NSCLC, and TNBC [97,135,162,170,174]. Higher PD-L1 expression is generally associated with increased response rates to checkpoint inhibitors; however, substantial clinical benefit is also observed in PD-L1-negative tumors. This indicates that PD-L1 status is neither a sufficient nor necessary condition for therapeutic response [124,175]. The limitations of PD-L1 as a biomarker reflect tumor heterogeneity, emphasizing the need for additional predictive indicators beyond PD-L1 alone [174].
Beyond PD-L1, many tumors express ligands for other checkpoint receptors (Figure 1.4), for instance: LAG-3, T cell immunoglobulin and mucin-domain-containing-3 (TIM-3), T cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif domain (TIGIT) and V-domain Ig suppressor of T cell activation (VISTA) [176,177]. TIM3 interacts with several ligands, including galectin-9, phosphatidylserine, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), and high mobility group protein B1 (HMGB1), as well as HLA-B-associated transcript 3 (BAT3). LAG-3 binds fibrinogen-like protein 1 (FGL1), lectins galectin-3 (Gal-3), and lymph node sinusoidal endothelial cell C-type lectin (LSECtin). TIGIT interacts with CD155 (PVR) and CD112 (PVRL2) [178,179], expressed on tumor cells and competing with ligand-expressing APCs [180,181]. In melanoma [179] and HNSCC [182], the CD155/TIGIT axis is prominent, contributing to ICI resistance despite highly immune TME. TNBC cells express CD155 and CD112 as well, promoting TIGIT-mediated immunosuppression, which is linked to poor prognosis with anti-PD-1 therapy [183].
Tumor-intrinsic mechanisms can include PD-L1-enriched exosomes, which extend immunosuppression systemically by inhibiting T-cell activation, promoting apoptosis, and enhancing Treg function [184]. A reduction in exosomal PD-L1 during treatment correlates with improved responses, suggesting its potential as a liquid biopsy biomarker [184].
Oncogenic pathway activation: Oncogenic signaling pathways play a central role in immune evasion and ICI resistance across melanoma, HNSCC, and TNBC. Activation of PI3K/AKT/mammalian target of rapamycin (mTOR), WNT/β-catenin, and mitogen-activated protein kinase (MAPK) pathways reshapes the TME and suppresses immune infiltration (Figure. 1.5) [185–188]. However, these pathways are often characterized by complex feedback loops and compensatory mechanisms that sustain tumor growth and contribute to immune escape [189–191]. Current efforts focus on optimizing drug combinations, dosing schedules, and patient selection to maximize therapeutic benefit. Rationally combining these pathway inhibitors with ICIs represents a promising approach to overcome immune exclusion and treatment resistance [192,193].
Aberrant PI3K/AKT/mTOR activation is common in TNBC and HNSCC, often resulting from PTEN loss or PI3K mutations [194,195]. This pathway supports tumor proliferation and immune escape by reducing CTL infiltration and enriching immunosuppressive myeloid populations [196]. Some PI3K/AKT/mTOR inhibitors have demonstrated promising preclinical activity in breast cancer [197,198], but their therapeutic efficacy has been partially limited by acquired resistance, as well as by substantial adverse effects [199]. Preclinical and clinical studies show that combining PI3K inhibitors with ICIs improves antitumor responses in melanoma and TNBC [200,201], with ongoing trials investigating similar strategies in HNSCC [24]. While pharmacological AKT inhibition showed no impressive effects, genetic silencing of all AKT paralogs triggered mTOR-dependent melanoma cell death, rescuable by kinase-active AKT1 [191]. A novel dual PI3K/mTOR inhibitor suppressed both proliferation and growth of MAPK inhibitor-resistant melanoma in vitro and in vivo, showing promise as a well-tolerated therapy for frontline and resistant disease [202,203]. Key strategies also include leveraging pan-PI3K inhibitors for broader pathway targeting in HNSCC, as well as incorporating epigenetic modifiers such as histone deacetylase inhibitors (HDACi) or DNA methyltransferase inhibitors to disrupt alternative signaling routes and overcome compensatory resistance mechanisms [195,196].
Aberrant WNT/β-catenin signaling is not only a key mechanism of tumorigenesis but a significant modulator of TME, contributing to immune exclusion and resistance to ICIs across several cancers [190,204]. This phenomenon has been extensively demonstrated in melanoma and is gaining recognition in HNSCC and TNBC [42,205]. In preclinical models and clinical settings, the WNT/β-catenin pathway prevents dendritic cell and T cell infiltration, generating a "cold" immune TME and driving resistance to PD-1/CTLA-4 blockade [41] (Figure 1.5). Mechanistically, β-catenin activation suppresses CCL4, impairing the recruitment of CD103+ dendritic cells essential for CD8+ T-cell priming [188]. The WNT/β-catenin pathway contributes to the preservation or expansion of Tregs via IL-10 release, thereby reinforcing an immunosuppressive TME [206]. In TNBC, characterized by a generally "cold" immune landscape, the WNT/β-catenin pathway's influence on immune exclusion is significant, making it an essential target for therapeutic intervention. Furthermore, the interplay between the WNT/β-catenin pathway and other metabolic pathways, such as those involving IDO and adenosine, can further solidify an immunosuppressive microenvironment, presenting additional challenges for immune cell function in the face of ICI treatment [207].
Mutations in the MAPK pathway (e.g., BRAF-V600E in melanoma, diverse mutations in HNSCC) contribute to ICI resistance via cytokine induction, diminished antigen presentation, and expansion of regulatory cells [192,208]. In melanoma, acquired resistance to MAPK-targeted therapy is associated with decreased MHC-I expression, reduced T-cell infiltration, and diminished immunotherapy efficacy through IL-6/IL-10-mediated suppression and Treg expansion [209,210]. In contrast, HNSCC displays a more nuanced signaling context, where some MAPK-mutant tumors exhibit better CD8+ T-cell infiltration and improved ICI outcomes. In TNBC, MAPK dysregulation similarly contributes to immune escape, limiting responses to combination therapy [211].
Figure 1. Tumor-intrinsic mechanisms of resistance to ICIs. (1) Variations in tumor mutational burden (TMB) and neoantigen load limit tumor recognition and reduce immune cell infiltration; (2) loss of antigen processing and presentation machinery, including MHC I, β2-microglobulin (B2M), and transporter-associated antigen processing (TAP1/2), impairs T-cell–mediated cytotoxicity; (3) defects in IFN-γ signaling, such as JAK1/2 mutations or STAT1 inactivation, blunt immune activation and antigen presentation; (4) overexpression of inhibitory checkpoint ligands (PD-L1, galectin-9, CEACAM1, FGL1, CD155, CD112, among others) dampens T-cell function; and (5) activation of oncogenic pathways (PI3K/AKT/mTOR, WNT/β-catenin, MAPK) promotes immune exclusion and supports the survival of immunosuppressive cells.
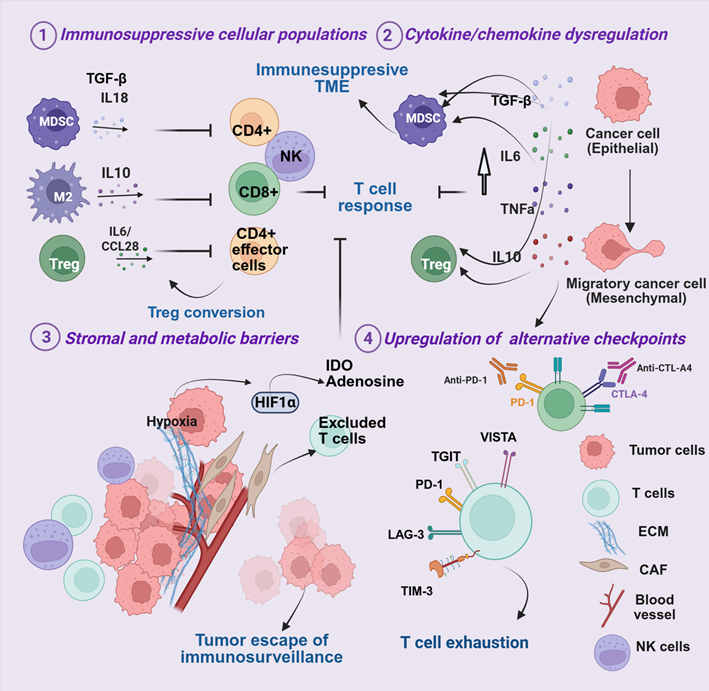
2. Tumor-extrinsic resistance mechanisms
The TME is highly heterogeneous, consisting of malignant cells, immune populations, stromal elements, vasculature, and extracellular matrix. Emerging evidence highlights the complex crosstalk among these components critically shaping immunosuppression, remodeling anti-tumor immune responses, and dictating therapeutic sensitivity [25,212]. Resistance mechanisms within the TME arise from both cellular and non-cellular factors that suppress local immunity, including expansion of inhibitory immune populations, physical exclusion of effector T cells, and metabolic constraints that induce T-cell exhaustion [213] (Figure 2).
Immunosuppressive cell populations: Resistance across solid tumors is reinforced by immunosuppressive subsets such as Tregs, MDSCs, and M2-polarized tumor-associated macrophages (TAMs), which are key mediators in melanoma, HNSCC, and TNBC (Figure 2.1). These cells inhibit cytotoxic T-cell activity by secreting IL-10, transforming growth factor-beta (TGF-β), and other suppressive molecules [214,215]. In melanoma, tumors arise within a “hot” immune milieu enriched in CD8+ T cells [216] (Figure 2.1). However, abundant Tregs blunt T cell cytotoxic activity, enforce tolerance, and promote therapy resistance. In HNSCC, the immune landscape is shaped by etiological diversity. HPV+ tumors display dense T-cell infiltration with frequent Tregs and stromal activation [217], whereas tobacco-associated HNSCC exhibits immune desertification and poor ICI responses [109,171]. Across both HPV+ and HPV- tumors, cytotoxic CD56dim NK cells, though abundant, are suppressed by KIR signaling [111]. HPV+ tumors also exploit E6/E7 oncoproteins to impair antigen presentation, dampen NK activity, and reprogram cytokine signaling [13,218]. In contrast, TNBC is often immunologically "cold" and characterized by scarce CTLs and an enrichment with immunosuppressive MDSCs and M2-polarized macrophages, which may limit ICI responsiveness [219]. Within these tumor contexts, several immunosuppressive cell populations are central drivers of immunotherapy resistance [214,215].
Cytokine/chemokine dysregulation: Cytokine dysregulation in the TME is typically initiated by oncogenic signaling, hypoxia-induced stress responses, and innate immune activation, which together establish self-sustaining cytokine loops that shape an immunosuppressive microenvironment (Figure 2.2). IL-6 drives MDSC expansion via STAT3 and IDO signaling [220], skewing T cell differentiation toward Th17 phenotypes [221]. Tumor necrosis factor-alpha (TNF-α) signaling, despite activation of CTL, enhances MDSC-mediated immunosuppression by promoting the survival and suppressive function of these cells [222]. TGF-β reprograms immune and stromal metabolism, promoting epithelial-to-mesenchymal transition (EMT) [223]. Together, IL-6, TNF-α, and TGF-β drive T-cell exhaustion, enhance the expression of PD-1 and CTLA-4, expand Tregs, and impair NK activity [221,223,224]. These cells subsequently release inhibitory mediators that block effector T-cell infiltration into the tumor, establishing a suppressive niche and fostering immune exclusion [225,226].
Stromal and metabolic barriers: Spatial heterogeneity across melanoma, HNSCC, and TNBC creates barriers to immune infiltration and ICI efficacy (Figure 2.3). Beyond purely “hot” or “cold” classifications, many solid tumors exhibit immune-excluded phenotypes, characterized by immune cells—especially CD8+ T cells—localized to the tumor periphery or stroma but unable to penetrate the tumor parenchyma [227,228]. This spatial immune segregation, frequently driven by dense extracellular matrix (ECM) deposition, cancer-associated fibroblast (CAF) activation, and TGF-β–mediated signaling, creates physical and biochemical barriers that prevent effective cytotoxic engagement [226].
Such immune-excluded environments are especially prominent in TNBC, where stromal TGF-β signaling and myofibroblast expansion contribute to peripheral T-cell trapping and therapeutic resistance [229]. Similarly, subsets of HNSCC show collagen crosslinking and stromal niche formation driven by CAFs, contributing to immune exclusion [230]. Together, these features underscore that stromal remodeling, not only cellular immunosuppression, represents a complementary axis of immune evasion across solid tumors.
Dense desmoplastic stroma, enriched in CAFs, collagen, and hyaluronan, further restricts T-cell infiltration in HNSCC and TNBC [231,232]. CAF-derived IL-6 and JAK2/STAT3 activation promote fibroblast proliferation, Th17 polarization, and immunosuppressive cytokine release [218,233]. In melanoma, resistance is compounded by metabolic rewiring, including activation of indoleamine 2,3-dioxygenase (IDO) and adenosine accumulation, which suppresses T- and NK-cell function and blunts PD-1 blockade efficacy [231,233,234].
Epithelial–mesenchymal transition (EMT) remodeling further strengthens stromal barriers (Figure 2.3), particularly in HNSCC and TNBC, where TGF-β, IL-6, Wnt, Notch, and hypoxia pathways collectively drive immune exclusion and therapeutic resistance [235,236]. Hypoxia, a common feature across all three cancers, stabilizes hypoxia-inducible factor-1α (HIF-1α), thereby promoting angiogenesis, PD-L1 expression, recruitment of suppressive cells, and activation of metabolic checkpoints such as IDO and adenosine [237–239] (Figure 2.3).
HNSCC and TNBC are particularly hypoxic due to dense stroma and high metabolic demand, whereas melanoma harbors localized hypoxic niches driving immune escape [240–242]. In HNSCC, hypoxia is particularly pronounced due to the high metabolic demand of rapidly proliferating tumor cells and extensive stromal fibrosis. Hypoxia in HNSCC promotes angiogenesis through vascular endothelial growth factor (VEGF), induces PD-L1 upregulation, and facilitates recruitment of MDSCs and TAMs, thereby driving tumor immune evasion [233]. In TNBC, elevated HIF-1α enhances VEGF secretion and stromal fibrosis, fostering metastasis and resistance [243]. Accumulation of lactate and adenosine under hypoxic stress also suppresses T and NK cell function, limiting ICI efficacy [244]. In melanoma, although global hypoxia is less pronounced, localized niches activate CCL28 and CXCL12, attracting Tregs and MDSCs and reinforcing immune suppression [245]. Collectively, stromal remodeling, metabolic reprogramming, and hypoxia form an interlinked network that shapes the TME, restricts immune infiltration, and drives resistance to ICI therapies across melanoma, HNSCC, and TNBC.
Alternative immune checkpoints: Beyond PD-1 and CTLA-4, several alternative inhibitory pathways—including TIM-3, LAG-3, TIGIT, and VISTA—contribute to sustained immune exhaustion and tumor immune evasion [172,246]. These pathways signal through unique mechanisms to suppress T cell proliferation and cytokine production, fostering a state of chronic exhaustion and reduced cytotoxic activity. The engagement of these alternative checkpoints as compensatory mechanisms in response to ICI therapy underscores the development of secondary acquired resistance.
TIM-3, often co-expressed with PD-1 on T cells, NK cells, and Tregs, suppresses effector functions and is associated with poor survival [247,248]. Dual blockade of PD-1 and TIM-3 has shown potential to restore T-cell activity [249].
LAG-3 is widely expressed on activated and exhausted T cells, NK cells, B cells, and plasmacytoid dendritic cells. It synergizes with PD-1—particularly in melanoma and HNSCC—leading to profound T-cell exhaustion and resistance to anti-PD-1/PD-L1 therapy. In TNBC, LAG-3 expression demonstrates a context-dependent role, but combined targeting of PD-1 and LAG-3 offers promise for overcoming immunosuppression [181,250].
TIGIT interacts with CD155 or CD112 to suppress CTL and NK cell activity, enhancing IL-10 secretion and Treg expansion [251]. High TIGIT levels predict resistance in melanoma and TNBC, and TIGIT inhibition can enhance responses to PD-1 blockade [180].
VISTA, expressed on myeloid cells and T cells, dampens T-cell activation and promotes immunotherapy resistance, particularly in inflamed tumors such as HNSCC and TNBC [252,253]. Collectively, these alternative checkpoints interact with cytokine networks and stromal barriers, establishing a multifaceted immunosuppressive tumor microenvironment. Their cooperative roles support clinical investigation of dual or triple checkpoint blockade strategies to overcome resistance [177,249].
Figure 2. Tumor-extrinsic mechanisms of resistance to ICIs. (1) Recruitment of immunosuppressive cell populations (Tregs, MDSCs, and M2 macrophages) suppresses T-cell cytotoxicity and limits effective anti-tumor responses; (2) dysregulated cytokine and chemokine signaling (e.g., TGF-β, TNF-α, IL-6, IL-10) enhances immune suppression and reinforces T-cell dysfunction; (3) stromal and metabolic alterations—including hypoxia, abnormal vasculature, and extracellular matrix (ECM) remodeling—create physical barriers to immune cell infiltration and promote tumor immune escape; and (4) upregulation of alternative immune checkpoints (LAG-3, TIM-3, TIGIT, VISTA) drives T-cell exhaustion and limits response to PD-1/PD-L1 blockade.
3. Systemic mechanisms
Resistance to ICIs is shaped by systemic host determinants [19,20,254]. Host-related factors, including chronic inflammation, nutritional and metabolic status, and the microbiome, modulate both intrinsic (mutational landscape, cytokine signaling, and metabolism) and tumor extrinsic mechanisms (immune cell trafficking and effector function within the TME) [233,255]. These systemic influences differ in relative importance across melanoma, HNSCC, and TNBC, yet collectively define immune competence, treatment tolerance, and the durability of anti-tumor responses.
Chronic inflammation and comorbidities: Systemic inflammation, driven by smoking, alcohol use, obesity, chronic infections, and aging, impairs antigen presentation, blunts T-cell priming, accelerates immune senescence, and thereby reduces ICI efficacy [256]. In melanoma, chronic ultraviolet-driven inflammation and age-related immune-senescence reduce naive T-cell pools and cytokine fitness, limiting response durability in older or frail patients. Body-composition metrics in melanoma patients further forecast ICI outcomes: low skeletal muscle index, high subcutaneous adipose tissue density, and sarcopenia correlate with inferior progression-free and overall survival on ICI therapy. Conversely, better pre-diagnosis diet quality (e.g., higher Healthy Eating Index) has been linked to thinner primary tumors at presentation, underscoring the role of modifiable host determinants in shaping immunity.
In HNSCC, tobacco and alcohol exposure drive inflammation, myeloid skewing, and frailty, all of which correlate with inferior ICI outcomes [257]. HPV+ HNSCC is generally more immunogenic, yet systemic comorbidities and malnutrition remain detrimental. For instance, cachexia and sarcopenia impose substantial metabolic stress that impairs T-cell priming and effector cytokine production [258,259]. Dysphagia, mucositis, and treatment-related catabolism frequently result in weight loss and immune dysfunction. Validated tools such as patient-generated subjective global assessment (PG-SGA) link poor nutritional status to advanced stage and worse survival; structured interventions (dietary counseling, prophylactic feeding tubes) mitigate severe toxicities and preserve immune competence during chemoradiation [260].
In TNBC, systemic inflammation is often linked to obesity, insulin resistance, and adipokine dysregulation (elevated leptin, reduced adiponectin), which increase circulating IL-6 and TNF-α, promote myelopoiesis, and skew toward suppressive myeloid phenotypes [261,262]. Systemic metabolism strongly influences TNBC aggressiveness. Preclinical studies show that Western-style diets accelerate tumor growth and blunt chemotherapy, whereas fasting-mimicking or ketogenic diets enhance immune fitness and prolong survival in murine TNBC [263]. Clinically, lower circulating glucose has been associated with improved outcomes in some TNBC cohorts [264], consistent with tumor–immune metabolic competition. Nutritional and metabolic interventions are now being evaluated as adjunctive strategies to potentiate ICI efficacy [260].
Microbiome dysbiosis: Melanoma and HNSCC are uniquely shaped by their interaction with microbiota, given their interface with heavily colonized barrier surfaces—the skin and oral cavity, respectively—which profoundly influence tumor-immune dynamics. The distinct microbial ecology at these sites plays a pivotal role in local immunity [265]. Microbial metabolites, particularly short-chain fatty acids, modulate chemokine production (CCL5, CXCL10), enhance T-cell metabolism, and promote intra-tumoral trafficking [266]. In contrast, dysbiosis impairs antigen presentation, reduces CD8+ T cell activation, and promotes expansion of Tregs, creating an environment conducive to immune evasion [267].
In melanoma, multiple studies demonstrate that fecal microbiota transplantation (FMT) from ICI responders into non-responders restores intra-tumoral immune infiltration and improves clinical outcomes, with OSR approaching 65%, including 20% complete responses [268–270]. In HNSCC, the oral microbiome exerts both cancer- and therapy-relevant effects [271,272]. In resected HNSCC patients, a shift toward health-associated taxa (e.g., Streptococcus, Rothia) and away from Capnocytophaga, Prevotella, and Leptotrichia correlated with improved three-year disease-specific survival [272,273]. However, direct evidence linking oral or gut microbiome modulation to ICI efficacy in HNSCC remains limited and warrants prospective studies [274]. In TNBC, baseline gut microbial diversity correlates with longer PFS in patients treated with atezolizumab plus chemotherapy [275]. Preclinical TNBC models further suggest that restoring beneficial microbial metabolites, such as branched-chain amino acids, enhances PD-1-mediated immunity [276]. Collectively, the microbiome functions both as a biomarker of ICI benefit and as a modifiable co-therapeutic target across melanoma, HNSCC, and advanced TNBC.
Conclusions and Lessons from a Decade of Forefront Therapy
A decade of clinical experience with ICI has fundamentally shifted the therapeutic paradigm in cancer, particularly for melanoma, HNSCC, and TNBC. These advances have redefined survival outcomes and established immunotherapy as a core pillar of modern oncology. However, the management of immune-related adverse events (irAEs) remains a critical challenge [277]. irAEs can affect virtually any organ system—most commonly the skin, gastrointestinal tract, liver, and endocrine glands—and range from mild to life-threatening [278]. Severe, multi-organ toxicities limit the broader application of ICIs, particularly in frail or comorbid patients. Several prospective clinical strategies are under active investigation—notably IL-6/IL-6R blockade (e.g., tocilizumab) and TNF-α or gut-selective agents (infliximab, vedolizumab) for severe or steroid-refractory irAEs, as well as targeted approaches such as abatacept, JAK inhibitors, IL-1 blockade, and microbiome modulation—which aim to reduce toxicity severity and dependence on high-dose corticosteroids while maintaining efficacy [221–226]. Early clinical signals are encouraging, but larger randomized studies with survival and quality-of-life endpoints are required to establish standard mitigation strategies [279].
Recent clinical and translational research increasingly emphasizes combination strategies that concurrently target multiple resistance mechanisms (Table 2,3). Key developments include dual checkpoint blockade, targeting PD-1 together with LAG-3 [280], TIGIT [281], or other inhibitory receptors [282], which has demonstrated efficacy in treating refractory tumors. Neoadjuvant immunotherapy, involving administration of ICIs before surgical intervention, has shown improved pathological responses and survival outcomes in melanoma and TNBC, offering insight into early tumor–immune dynamics [50,283].
|
Target/Agent |
Mechanism of Action |
Clinical Trial Phase Status |
Tumor Type(s) |
Key Outcome |
References |
|
EGFR inhibitors |
Inhibition of EGFR promotes antigen presentation and enhances immune response to tumor cells |
Phase II/III |
HPV-related cancers |
Improved ORR and overall survival in combination with PD-1 inhibitors |
[68,291] |
|
STAT3 inhibitors |
Blockade of STAT3 suppresses an immunosuppressive transcription factor |
Phase I |
Advanced Solid Tumors |
Tolerable safety profile; preliminary anti-tumor activity |
[292] |
|
CXCR2 inhibitors |
Blockade of the chemokine receptor CXCR2 (primarily binds IL-8) reduces neutrophil recruitment |
Phase I/II |
Solid tumors |
Combination with durvalumab did not improve ORR and had high adverse event rates. |
[293] |
|
IDO1 inhibitors |
Blockade reduces immunosuppressive tryptophan metabolism and restores T and NK cell proliferation. |
Phase I–III |
Melanoma, Solid Tumors |
Epacadostat + pembrolizumab: safe but failed phase III melanoma trial. Navoximod + atezolizumab: phase I ongoing |
[207] |
|
NKG2A inhibitors |
Blockade restores CD8+ T cell and NK cell function. |
Phase II |
HNSCC |
Combination with durvalumab + SOC did not improve PFS; results are pending. |
[294] |
|
B7-H3 inhibitors |
Blockade enhances CD8+ T cell–mediated anti-tumor activity. |
Phase II/III |
Solid Tumors |
Combination with retifanlimab improved ORR; acceptable safety profile. |
[55] |
|
VEGF inhibitors |
Blockade of VEGF signaling reduces tumor angiogenesis and alleviates hypoxia-driven immunosuppression. |
Phase III |
Endometrial, Renal, Solid Tumors |
Lenvatinib + pembrolizumab did not improve survival outcomes [126,127]. |
[295,296] |
|
OX40 agonists |
Costimulatory receptor activation enhances T cell proliferation and survival. |
Phase I/II |
Solid Tumors |
Well tolerated; modest activity as monotherapy; combination trials with PD-1 ongoing. |
[297] |
|
4-1BB (CD137) agonists |
Costimulatory receptor activation enhances T and NK cell activation. |
Phase I/II |
Solid Tumors |
Some clinical activity; hepatotoxicity limited development; newer agents in trials with PD-1 inhibitors. |
[298] |
|
TLR agonists |
Toll-like receptor activation stimulates innate immunity and dendritic cell function. |
Phase I/II |
Melanoma, HNSCC |
Early signals of efficacy in combination with PD-1 inhibitors. |
[299] |
|
STING agonists |
Activates STING pathway, inducing type I interferons and innate immune activation. |
Phase I |
Solid Tumors, Lymphoma |
Safe but limited responses as monotherapy; PD-1 combinations under investigation. |
[300] |
|
CSF1R inhibitors |
Reprograms tumor-associated macrophages from immunosuppressive to pro-inflammatory phenotype. |
Phase I/II |
Pancreatic and Solid Tumors |
Combination with nivolumab showed limited clinical activity. |
[301] |
|
This table summarizes investigational approaches combining PD-1 inhibitors with other immune-modulating therapies. Each entry highlights the molecular target, its immunological mechanism of action, and the clinical outcome reported to date, ranging from early-phase safety and efficacy signals to negative or inconclusive phase II results. |
|||||
Novel immunomodulatory agents such as vidutolimod (TLR9 agonist) [284] and WTX-124 (tumor-activated IL-2 prodrug) [285] are designed to enhance both innate and adaptive immunity while minimizing systemic toxicity. Adoptive T-cell therapies, particularly TIL products such as lifileucel (Amtagvi™), along with personalized cancer vaccines, offer promising avenues for patients with ICI-refractory disease [286–288]. Similarly, antibody–drug conjugates (ADCs) such as sacituzumab govitecan, a Trop-2-directed ADC, have shown substantial benefit in heavily pretreated TNBC [289]. These targeted therapies address tumor-specific vulnerabilities and contribute to a multi-modal framework for overcoming tumor heterogeneity and resistance.
In addition to these approaches, next-generation immunotherapies are being developed to further enhance clinical outcomes [290]. Oncolytic viruses represent a promising avenue for overcoming resistance to ICIs. The FDA-approved talimogene laherparepvec (T-VEC), a genetically modified herpes simplex virus, has demonstrated durable responses and improved overall survival in advanced melanoma by promoting tumor lysis and systemic immune activation [302]. Ongoing trials are evaluating T-VEC in combination with ICIs and radiotherapy to further augment antitumor immunity [303]. In HNSCC, clinical studies are investigating intratumoral viral delivery and combination regimens, particularly in HPV-positive or immunologically “cold” tumors where viral priming may enhance immune infiltration [304]. For TNBC, oncolytic HSV and reoviruses are under clinical evaluation for their ability to induce immunogenic cell death and reshape the TME [305].
Epigenetic reprogramming provides an additional avenue to overcome immune resistance. In melanoma, inhibitors of DNA methyltransferases (DNMTs) and HDACs can restore antigen presentation, reactivate silenced immune genes, and enhance checkpoint blockade efficacy [306,307]. In HNSCC and TNBC, epigenetic therapies are under investigation to reverse tumor-induced immunosuppression and restore effective immune signaling, potentially sensitizing tumors to chemotherapy and ICIs [308,309]. Moreover, targeting DNA damage response pathways has emerged as a complementary approach. PARP inhibitors, such as Olaparib, have been approved for BRCA-mutated TNBC [310], with ongoing trials exploring combinations with ICIs, AKT inhibitors, and ADC [311–313].
The integration of these next-generation therapies with current ICIs underscores a dynamic shift towards personalized cancer treatments. Strategies such as optimizing nutrition, preserving metabolic and immune fitness, and modulating the microbiome through dietary fiber, pre/probiotics, or FMT are being investigated as adjunctive therapies to enhance immune competence, reduce toxicities, and extend the durability of checkpoint blockade [266,314]. Recent progress in single-cell transcriptomics, spatial multi-omics, and AI-assisted response prediction is also refining patient selection and guiding personalized combinations [315–318].
Collectively, these developments highlight a translational shift toward precision immunotherapy—integrating genomic, metabolic, and immunologic profiling to tailor treatments while mitigating toxicity. Lessons from the past decade underscore that durable benefit from ICIs requires not only overcoming resistance but also mastering the balance between immune activation and immune tolerance, ensuring that the next generation of immunotherapies achieves maximal efficacy with minimal harm.
Conflicts of Interest
M.K. serves on the scientific advisory boards of Genentech and Merck and Co. and received research support from Merck Sharp & Dohme Corp., (a subsidiary of Merck and Co., Inc.), Genentech, Biogen, Novartis and the Mark Foundation. The other authors declare no competing interests.
Author Contributions Statement
Conceptualization: S.I., N.W., T.A., M.H., A.M., F.T., F.K., D.F., and C.W. conducted the literature search and collected all necessary information for this manuscript. I.V., A.T., S.I., N.W., T.A., and M.H. wrote the manuscript and created figures and tables. Y.P. and M.K. edited and supervised the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the grants R01CA243486, R01CA284604, and P50CA225450 (to M.K.), Merck Oncology Translational Program Concept grant 60974 (to M.K.), as well as the Perlmutter Cancer Center Support Grant (CCSG), NCI P30 P30CA016087 (to M.K. and Y.P.).
Acknowledgments
Figures in this manuscript were created with BioRender.com.
References
2. Shiravand Y, Khodadadi F, Kashani SM, Hosseini-Fard SR, Hosseini S, Sadeghirad H, et al. Immune checkpoint inhibitors in cancer therapy. Curr Oncol. 2022 Apr 24;29(5):3044–60.
3. Mansh M. Ipilimumab and cancer immunotherapy: a new hope for advanced stage melanoma. Yale J Biol Med. 2011 Dec;84(4):381.
4. Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, et al. High response rate to PD-1 blockade in desmoplastic melanomas. Nature. 2018 Jan 18;553(7688):347–50.
5. Botticelli A, Cirillo A, Strigari L, Valentini F, Cerbelli B, Scagnoli S, et al. Anti–PD-1 and anti–PD-L1 in head and neck cancer: a network meta-analysis. Front Immunol. 2021 Aug 9;12:705096.
6. Wang Z, You P, Yang Z, Xiao H, Tang X, Pan Y, et al. PD-1/PD-L1 immune checkpoint inhibitors in the treatment of unresectable locally advanced or metastatic triple negative breast cancer: a meta-analysis on their efficacy and safety. BMC cancer. 2024 Oct 31;24(1):1339.
7. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014 Sep 20;384(9948):1109–17.
8. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015 Jan 22;372(4):320–30.
9. Ning B, Liu Y, Wang M, Li Y, Xu T, Wei Y. The predictive value of tumor mutation burden on clinical efficacy of immune checkpoint inhibitors in melanoma: a systematic review and meta-analysis. Front Pharmacol. 2022 Mar 9;13:748674.
10. Lauss M, Phung B, Borch TH, Harbst K, Kaminska K, Ebbesson A, et al. Molecular patterns of resistance to immune checkpoint blockade in melanoma. Nat Commun. 2024 Apr 9;15(1):3075.
11. Liu D, Jenkins RW, Sullivan RJ. Mechanisms of resistance to immune checkpoint blockade. Am J Clin Dermatol. 2019 Feb 13;20(1):41–54.
12. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017 Dec 21;377(25):2500–1.
13. Mandal R, Şenbabaoğlu Y, Desrichard A, Havel JJ, Dalin MG, Riaz N, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI insight. 2016 Oct 20;1(17):e89829.
14. Meci A, Goyal N, Slonimsky G. Mechanisms of resistance and therapeutic perspectives in immunotherapy for advanced head and neck cancers. Cancers. 2024 Feb 7;16(4):703.
15. Yi H, Li Y, Tan Y, Fu S, Tang F, Deng X. Immune checkpoint inhibition for triple-negative breast cancer: current landscape and future perspectives. Front Oncol. 2021 May 19;11:648139.
16. Debien V, De Caluwé A, Wang X, Piccart-Gebhart M, Tuohy VK, Romano E, et al. Immunotherapy in breast cancer: an overview of current strategies and perspectives. NPJ breast cancer. 2023 Feb 13;9(1):7.
17. Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020 Jun 9;22(1):61.
18. Mantovani A, Marchesi F, Jaillon S, Garlanda C, Allavena P. Tumor-associated myeloid cells: diversity and therapeutic targeting. Cell Mol Immunol. 2021 Mar;18(3):566–78.
19. Spada S, Mukherjee S. Progression of the Immune Escape Mechanism in Tumors. Biology. 2024 Nov 4;13(11):898.
20. Ikeda H, Kawase K, Nishi T, Watanabe T, Takenaga K, Inozume T, et al. Immune evasion through mitochondrial transfer in the tumour microenvironment. Nature. 2025 Feb 6;638(8049):225–36.
21. Woods DM, Laino AS, Winters A, Alexandre J, Freeman D, Rao V, et al. Nivolumab and ipilimumab are associated with distinct immune landscape changes and response-associated immunophenotypes. JCI insight. 2020 Jun 4;5(11):e137066.
22. Gaikwad S, Agrawal MY, Kaushik I, Ramachandran S, Srivastava SK. Immune checkpoint proteins: Signaling mechanisms and molecular interactions in cancer immunotherapy. Semin Cancer Biol. 2022 Nov;86(Pt 3):137-150.
23. Zamani MR, Šácha P. Immune checkpoint inhibitors in cancer therapy: what lies beyond monoclonal antibodies?. Med Oncol. 2025 Jun 19;42(7):273.
24. Saladi SV, Park JC, Basak N, Useche M, Park JS, Ganesh M, et al. Impact of Pi3k Pathway Alterations on Response to Immune Checkpoint Inhibitors in Hpv-Negative Head and Neck Squamous Cell Carcinoma. Available at SSRN 5237193.
25. Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022 May;23(5):660–70.
26. Thornton J, Chhabra G, Singh CK, Guzmán-Pérez G, Shirley CA, Ahmad N. Mechanisms of immunotherapy resistance in cutaneous melanoma: recognizing a shapeshifter. Front Oncol. 2022 Apr 19;12:880876.
27. Yamada K, Takeuchi M, Fukumoto T, Suzuki M, Kato A, Mizuki Y, et al. Immune checkpoint inhibitors for metastatic uveal melanoma: A meta-analysis. Sci Rep. 2024 Apr 3;14(1):7887.
28. Vos JL, Traets JJ, Qiao X, Seignette IM, Peters D, Wouters MW, et al. Diversity of the immune microenvironment and response to checkpoint inhibitor immunotherapy in mucosal melanoma. JCI insight. 2024 Nov 8;9(21):e179982.
29. Dillman RO, DePriest C, McClure SE. High-dose IL2 in metastatic melanoma: better survival in patients immunized with antigens from autologous tumor cell lines. Cancer Biother Radiopharm. 2014 Mar 1;29(2):53–7.
30. McDermott D, Haanen J, Chen TT, Lorigan P, O'day S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol. 2013 Oct 1;24(10):2694–8.
31. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015 Jun 25;372(26):2521–32.
32. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018 Nov 1;19(11):1480–92.
33. Noringriis IM, Donia M, Madsen K, Schmidt H, Haslund CA, Bastholt L, et al. Long-term clinical outcome of patients with metastatic melanoma and initial stable disease during anti-PD-1 checkpoint inhibitor immunotherapy with pembrolizumab: Epidemiology. Br J Cancer. 2025 May 26:1–9.
34. Kendra KL, Bellasea SL, Eroglu Z, Hu-Lieskovan S, Campbell KM, Carson III WE, et al. Anti-PD-1 therapy in unresectable desmoplastic melanoma: the phase 2 SWOG S1512 trial. Nat Med. 2025 Aug 14:1–7.
35. Atkins MB, Lee SJ, Chmielowski B, Tarhini AA, Cohen GI, Truong TG, et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: the DREAMseq trial—ECOG-ACRIN EA6134. J Clin Oncol. 2023 Jan 10;41(2):186–97.
36. Lim SY, Shklovskaya E, Lee JH, Pedersen B, Stewart A, Ming Z, et al. The molecular and functional landscape of resistance to immune checkpoint blockade in melanoma. Nat Commun. 2023 Mar 18;14(1):1516.
37. Pereira C, Gimenez-Xavier P, Pros E, Pajares MJ, Moro M, Gomez A, et al. Genomic profiling of patient-derived xenografts for lung cancer identifies B2M inactivation impairing immunorecognition. Clin Cancer Res. 2017 Jun 14;23(12):3203–13.
38. Jacquelot N, Yamazaki T, Roberti MP, Duong CP, Andrews MC, Verlingue L, et al. Sustained Type I interferon signaling as a mechanism of resistance to PD-1 blockade. Cell Res. 2019 Oct;29(10):846–61.
39. Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov. 2018 Sep 1;8(9):1156–75.
40. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017 Mar;36(11):1461–73.
41. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti–tumour immunity. Nature. 2015 Jul 9;523(7559):231–5.
42. Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. 2019 May 15;25(10):3074–83.
43. Wang B, Han Y, Zhang Y, Zhao Q, Wang H, Wei J, et al. Overcoming acquired resistance to cancer immune checkpoint therapy: potential strategies based on molecular mechanisms. Cell Biosci. 2023 Jun 30;13(1):120.
44. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020 Aug 17;30(16):R921–5.
45. Weber JS, Carlino MS, Khattak A, Meniawy T, Ansstas G, Taylor MH, et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet. 2024 Feb 17;403(10427):632–44.
46. Albrecht LJ, Livingstone E, Zimmer L, Schadendorf D. The latest option: nivolumab and relatlimab in advanced melanoma. Curr Oncol Rep. 2023 Jun;25(6):647–57.
47. Quandt Z, Jacob S, Fadlullah MZ, Wu C, Wu C, Huppert L, et al. Phase II trial of pembrolizumab, ipilimumab, and aspirin in melanoma: clinical outcomes and translational predictors of response. BJC reports. 2024 Jun 24;2(1):46.
48. Wolchok JD, Chiarion-Sileni V, Rutkowski P, Cowey CL, Schadendorf D, Wagstaff J, et al. Final, 10-year outcomes with nivolumab plus ipilimumab in advanced melanoma. The N Engl J Med. 2024 Sep 15;392(1):11.
49. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022 Jan 6;386(1):24–34.
50. Blank CU, Lucas MW, Scolyer RA, van de Wiel BA, Menzies AM, Lopez-Yurda M, et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N Engl J Med. 2024 Nov 7;391(18):1696–708.
51. van Pul KM, Notohardjo JCL, Fransen MF, Koster BD, Stam AGM, Chondronasiou D, et al. Local delivery of low-dose anti-CTLA-4 to the melanoma lymphatic basin leads to systemic Treg reduction and effector T cell activation. Sci Immunol. 2022 Jul 15;7(73):eabn8097.
52. Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008 Dec 20;26(36):5896–903.
53. Ellingsen EB, Bounova G, Kerzeli I, Anzar I, Simnica D, Aamdal E, et al. Characterization of the T cell receptor repertoire and melanoma tumor microenvironment upon combined treatment with ipilimumab and hTERT vaccination. J Transl Med. 2022 Sep 11;20(1):419
54. Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019 Aug;20(8):1083–97.
55. Aggarwal C, Prawira A, Antonia S, Rahma O, Tolcher A, Cohen RB, et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: interim results from a multicenter phase I/II trial. J Immunother Cancer. 2022 Apr;10(4):e004424.
56. Hilton JF, Ott PA, Hansen AR, Li Z, Mathew M, Messina CH, et al. INDUCE-2: A Phase I/II, open-label, two-part study of feladilimab in combination with tremelimumab in patients with advanced solid tumors. Cancer Immunol Immunother. 2024 Feb 13;73(3):44.
57. Arance A, De La Cruz-Merino L, Petrella TM, Jamal R, Ny L, Carneiro A, et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on a programmed cell death protein-1 or programmed death ligand 1 inhibitor given as monotherapy or in combination. J Clin Oncol. 2023 Jan 1;41(1):75–85.
58. Hwu WJ, Knight RD, Patnana M, Bassett R, Papadopoulos NE, Kim KB, et al. Phase I safety study of lenalidomide and dacarbazine in patients with metastatic melanoma previously untreated with systemic chemotherapy. Melanoma Res. 2010 Dec;20(6):501–6.
59. Hersh EM, Del Vecchio M, Brown MP, Kefford R, Loquai C, Testori A, et al. A randomized, controlled phase III trial of nab-Paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann Oncol. 2015 Nov;26(11):2267–74.
60. Sandhu S, Atkinson V, Cao MG, Medina T, Rivas AS, Menzies AM, et al. Phase 1b study of cobimetinib plus atezolizumab in patients with advanced BRAFV600 wild-type melanoma progressing on prior anti-programmed death-1 therapy. Eur J Cancer. 2023 Jan;178:180–90.
61. Comin-Anduix B, Lee Y, Jalil J, Algazi A, de la Rocha P, Camacho LH, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008 May 1;6:22
62. Sullivan RJ, Hamid O, Gonzalez R, Infante JR, Patel MR, Hodi FSet al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med. 2019 Jun;25(6):929–35.
63. Choi BS, Sondel PM, Hank JA, Schalch H, Gan J, King DM, et al. Phase I trial of combined treatment with ch14.18 and R24 monoclonal antibodies and interleukin-2 for patients with melanoma or sarcoma. Cancer Immunol Immunother. 2006 Jul;55(7):761–74.
64. Exley MA, Friedlander P, Alatrakchi N, Vriend L, Yue S, Sasada T, et al. Adoptive Transfer of Invariant NKT Cells as Immunotherapy for Advanced Melanoma: A Phase I Clinical Trial. Clin Cancer Res. 2017 Jul 15;23(14):3510–9.
65. Spreafico A, Couselo EM, Irmisch A, Bessa J, Au-Yeung G, Bechter O, et al. Phase 1, first-in-human study of TYRP1-TCB (RO7293583), a novel TYRP1-targeting CD3 T-cell engager, in metastatic melanoma: active drug monitoring to assess the impact of immune response on drug exposure. Front Oncol. 2024 Mar 21;14:1346502
66. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019 Nov 23;394(10212):1915–28.
67. Haddad RI, Harrington K, Tahara M, Ferris RL, Gillison M, Fayette J, et al. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J Clin Oncol. 2023 Apr 20;41(12):2166–80.
68. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019 Jan 12;393(10167):156–67.
69. Zinner R, Johnson JM, Tuluc M, Curry JM, Luginbuhl A, Fundakowski CC, et al. Neoadjuvant nivolumab (N) plus weekly carboplatin (C) and paclitaxel (P) in resectable locally advanced head and neck cancer. Journal of Clinical Oncology, 2020. 38:6583.
70. Iglesias L, Carral a, Bernal MO, Martinez-Trufero j, Castaño AG, Calderon VG, et al. Phase II multicenter randomized trial to assess the efficacy and safety of first line nivolumab in combination with paclitaxel in subjects with R/M HNSCC unable for cisplatin-based chemotherapy (NIVOTAX): A TTCC study. Journal of Clinical Oncology, 2021. 39: p. TPS6086.
71. Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, et al. Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2017 Sep 15;23(18):5349–57.
72. Khushalani NI, Ott PA, Ferris RL, Cascone T, Schadendorf D, Le DT, et al. Final results of urelumab, an anti-CD137 agonist monoclonal antibody, in combination with cetuximab or nivolumab in patients with advanced solid tumors. J Immunother Cancer. 2024 Mar 7;12(3):e007364
73. Schmid P, Salgado R, Park YH, Muñoz-Couselo E, Kim SB, Sohn J, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020 May;31(5):569–81.
74. Dent R, Cortés J, Park YH, Muñoz-Couselo E, Kim SB, Sohn J, et al. Molecular determinants of response to neoadjuvant pembrolizumab plus chemotherapy in patients with high-risk, early-stage, triple-negative breast cancer: exploratory analysis of the open-label, multicohort phase 1b KEYNOTE-173 study. Breast Cancer Res. 2025 Mar 11;27(1):35
75. Chen L, Li H, Zhang H, Yang H, Qian J, Li Zet al. Camrelizumab vs Placebo in Combination With Chemotherapy as Neoadjuvant Treatment in Patients With Early or Locally Advanced Triple-Negative Breast Cancer: The CamRelief Randomized Clinical Trial. JAMA. 2025 Feb 25;333(8):673–81.
76. Fasching PA, Hein A, Kolberg HC, Häberle L, Uhrig S, Rübner M, et al. Pembrolizumab in combination with nab-paclitaxel for the treatment of patients with early-stage triple-negative breast cancer - A single-arm phase II trial (NeoImmunoboost, AGO-B-041). Eur J Cancer. 2023 May;184:1–9.
77. Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2022 Jul 21;387(3):217–26.
78. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020 Dec 5;396(10265):1817–28.
79. Lynce F, Mainor C, Donahue RN, Geng X, Jones G, Schlam I, et al. Adjuvant nivolumab, capecitabine or the combination in patients with residual triple-negative breast cancer: the OXEL randomized phase II study. medRxiv [Preprint]. 2023 Dec 4:2023.12.04.23297559.
80. Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021 Aug;32(8):994–1004.
81. Sharma P, Kimler BF, O'Dea A, Nye L, Wang YY, Yoder R, et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin Cancer Res. 2021 Feb 15;27(4):975–82
82. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019 Mar 1;30(3):397–404.
83. Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019 Jan 1;5(1):74–82.
84. Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018 Feb;167(3):671–86.
85. Vinayak S, Tolaney SM, Schwartzberg L, Mita M, McCann G, Tan AR, et al. Open-label Clinical Trial of Niraparib Combined With Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol. 2019 Aug 1;5(8):1132–40.
86. Yap TA, Bardia A, Dvorkin M, Galsky MD, Beck JT, Wise DR, et al. Avelumab Plus Talazoparib in Patients With Advanced Solid Tumors: The JAVELIN PARP Medley Nonrandomized Controlled Trial. JAMA Oncol. 2023 Jan 1;9(1):40–50.
87. Winer EP, Lipatov O, Im SA, Goncalves A, Muñoz-Couselo E, Lee KS, et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):499–511.
88. Domchek SM, Postel-Vinay S, Im SA, Park YH, Delord JP, Italiano A, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol. 2020 Sep;21(9):1155–64.
89. Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020 May 1;6(5):676–84.
90. Adams S, Diamond JR, Hamilton E, Pohlmann PR, Tolaney SM, Chang CW, et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019 Mar 1;5(3):334–42
91. Wu SY, Xu Y, Chen L, Fan L, Ma XY, Zhao S, et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: concept exploration and biomarker analysis in the FUTURE-C-Plus trial. Mol Cancer. 2022 Mar 25;21(1):84.
92. Emens LA, Adams S, Barrios CH, Diéras V, Iwata H, Loi S, et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021 Aug;32(8):983–93
93. Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc Fet al. Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med. 2021 Feb;27(2):250–5.
94. Canning M, Guo G, Yu M, Myint C, Groves MW, Byrd JK, et al. Heterogeneity of the Head and Neck Squamous Cell Carcinoma Immune Landscape and Its Impact on Immunotherapy. Front Cell Dev Biol. 2019 Apr 9;7:52.
95. Borcoman E, Marret G, Le Tourneau C. Paradigm change in first-line treatment of recurrent and/or metastatic head and neck squamous cell carcinoma. Cancers. 2021 May 24;13(11):2573.
96. Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC, Shivdasani P, Frieden A, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018 Feb 22;3(4):e98811.
97. Levy DA, Patel JJ, Nguyen SA, Nicholas Jungbauer W, Neskey DM, Cohen EEW, et al. Programmed death 1 (PD-1) and ligand (PD-L1) inhibitors in head and neck squamous cell carcinoma: A meta-analysis. World J Otorhinolaryngol Head Neck Surg. 2022 Apr 18;8(3):177–86.
98. Orland MD, Ullah F, Yilmaz E, Geiger JL. Immunotherapy for Head and Neck Squamous Cell Carcinoma: Present and Future Approaches and Challenges. JCO Oncol Pract. 2024 Dec;20(12):1588–95.
99. Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018 Jul;119(2):153–9.
100. Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J Clin Oncol. 2017 May 10;35(14):1542–9.
101. Gillison ML, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. CheckMate 141: 1-Year Update and Subgroup Analysis of Nivolumab as First-Line Therapy in Patients with Recurrent/Metastatic Head and Neck Cancer. Oncologist. 2018 Sep;23(9):1079–82.
102. Wang BC, Li PC, Fan JQ, Lin GH, Liu Q. Durvalumab and tremelimumab combination therapy versus durvalumab or tremelimumab monotherapy for patients with solid tumors: A systematic review and meta-analysis. Medicine (Baltimore). 2020 Jul 10;99(28):e21273.
103. Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer. 2019 Jan;107:142–52.
104. Jhaveri N, Ben Cheikh B, Nikulina N, Ma N, Klymyshyn D, DeRosa J, et al. Mapping the spatial proteome of head and neck tumors: key immune mediators and metabolic determinants in the tumor microenvironment. Gen Biotechnology. 2023 Oct 1;2(5):418–34.
105. Wang J, Sun H, Zeng Q, Guo XJ, Wang H, Liu HH, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep. 2019 Sep 16;9(1):13404.
106. Meliante PG, Barbato C, Zoccali F, Ralli M, Greco A, de Vincentiis M, et al. Programmed Cell Death-Ligand 1 in Head and Neck Squamous Cell Carcinoma: Molecular Insights, Preclinical and Clinical Data, and Therapies. Int J Mol Sci. 2022 Dec 6;23(23):15384.
107. Shen B, Huang D, Ramsey AJ, Ig-Izevbekhai K, Zhang K, Lajud SA, et al. PD-L1 and MRN synergy in platinum-based chemoresistance of head and neck squamous cell carcinoma. Br J Cancer. 2020 Mar;122(5):640–7.
108. Scarth JA, Patterson MR, Morgan EL, Macdonald A. The human papillomavirus oncoproteins: a review of the host pathways targeted on the road to transformation. J Gen Virol. 2021 Mar;102(3):001540.
109. Desrichard A, Kuo F, Chowell D, Lee KW, Riaz N, Wong RJ, et al. Tobacco Smoking-Associated Alterations in the Immune Microenvironment of Squamous Cell Carcinomas. J Natl Cancer Inst. 2018 Dec 1;110(12):1386–92.
110. Wahle BM, Zolkind P, Ramirez RJ, Skidmore ZL, Anderson SR, Mazul A, et al. Integrative genomic analysis reveals low T-cell infiltration as the primary feature of tobacco use in HPV-positive oropharyngeal cancer. iScience. 2022 Apr 6;25(5):104216.
111. Charap AJ, Enokida T, Brody R, Sfakianos J, Miles B, Bhardwaj N, et al. Landscape of natural killer cell activity in head and neck squamous cell carcinoma. J Immunother Cancer. 2020 Dec;8(2):e001523.
112. Sim RHZ, Voon PJ, Cheo SW, Lim DW; Asian Clinical Trials Network for Cancers Project—Head and Neck Cancer Group (ATLAS-HNCG). Novel Therapeutic Strategies for Squamous Cell Carcinoma of the Head and Neck: Beyond EGFR and Checkpoint Blockade. Biomedicines. 2025 Aug 14;13(8):1972.
113. Aboaid H, Khalid T, Hussain A, Myat YM, Nanda RK, Srinivasmurthy R, et al. Advances and challenges in immunotherapy in head and neck cancer. Front Immunol. 2025 Jun 6;16:1596583.
114. Bian L, Zhang H, Wang T, Zhang S, Song H, Xu M, et al. JS001, an anti-PD-1 mAb for advanced triple negative breast cancer patients after multi-line systemic therapy in a phase I trial. Ann Transl Med. 2019 Sep;7(18):435.
115. Liu Y, Hu Y, Xue J, Li J, Yi J, Bu J, et al. Advances in immunotherapy for triple-negative breast cancer. Mol Cancer. 2023 Sep 2;22(1):145.
116. Bräutigam K, Kabore-Wolff E, Hussain AF, Polack S, Rody A, Hanker L, et al. Inhibitors of PD-1/PD-L1 and ERK1/2 impede the proliferation of receptor positive and triple-negative breast cancer cell lines. J Cancer Res Clin Oncol. 2021 Oct;147(10):2923–33.
117. He Q, Peng Y, Sun J, Liu J. Platinum-Based Chemotherapy and Immunotherapy in Early Triple-Negative Breast Cancer: A Meta-Analysis and Indirect Treatment Comparison. Front Oncol. 2021 Jul 1;11:693542.
118. Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019 Aug 1;30(8):1279–88.
119. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al; KEYNOTE-522 Investigators. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020 Feb 27;382(9):810–21.
120. Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019 May 9;17(1):90.
121. Guo M, Wang SM. Genome Instability-Derived Genes Are Novel Prognostic Biomarkers for Triple-Negative Breast Cancer. Front Cell Dev Biol. 2021 Jul 12;9:701073.
122. Jain A, Barge A, Parris CN. Combination strategies with PARP inhibitors in BRCA-mutated triple-negative breast cancer: overcoming resistance mechanisms. Oncogene. 2025 Feb;44(4):193–207.
123. Yang M, Wang C, Chen G, Zhang H, Lin J. Efficacy and safety analysis of AKT inhibitor in triple-negative breast cancer: A meta-analysis and systematic review. Medicine (Baltimore). 2024 Aug 30;103(35):e39347.
124. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017 Aug 23;8:561.
125. Bader JE, Voss K, Rathmell JC. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol Cell. 2020 Jun 18;78(6):1019–33.
126. Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018 Oct 12;362(6411):eaar3593.
127. Haddad RI, Seiwert TY, Chow LQM, Gupta S, Weiss J, Gluck I, et al. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer. 2022 Feb;10(2):e003026.
128. Wang P, Chen Y, Wang C. Beyond Tumor Mutation Burden: Tumor Neoantigen Burden as a Biomarker for Immunotherapy and Other Types of Therapy. Front Oncol. 2021 Apr 29;11:672677.
129. Lauss M, Donia M, Harbst K, Andersen R, Mitra S, Rosengren F, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun. 2017 Nov 23;8(1):1738.
130. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017 Jul 13;547(7662):217–221.
131. Riaz N, Morris L, Havel JJ, Makarov V, Desrichard A, Chan TA. The role of neoantigens in response to immune checkpoint blockade. Int Immunol. 2016 Aug;28(8):411–9.
132. Nishizaki D, Kurzrock R, Adashek JJ, Kim KH, Lim HJ, Nikanjam M, et al. Gene- and immune-targeted therapy combinations using dual-matched biomarkers for patient selection. NPJ Precis Oncol. 2025 Jul 24;9(1):253.
133. Rodrigo JP, Sánchez-Canteli M, Otero-Rosales M, Martínez-Camblor P, Hermida-Prado F, García-Pedrero JM. Tumor mutational burden predictability in head and neck squamous cell carcinoma patients treated with immunotherapy: systematic review and meta-analysis. J Transl Med. 2024 Feb 4;22(1):135.
134. Wildsmith S, Li W, Wu S, Stewart R, Morsli N, Raja R, et al. Tumor Mutational Burden as a Predictor of Survival with Durvalumab and/or Tremelimumab Treatment in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2023 Jun 1;29(11):2066–74.
135. Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013 Mar 15;73(6):1733–41.
136. Wu B, Zhang B, Li B, Wu H, Jiang M. Cold and hot tumors: from molecular mechanisms to targeted therapy. Signal Transduct Target Ther. 2024 Oct 18;9(1):274.
137. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015 Apr 3;348(6230):69–74.
138. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015 Apr 3;348(6230):124–8.
139. McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021 May;32(5):661–72.
140. Jou J, Harrington KJ, Zocca MB, Ehrnrooth E, Cohen EEW. The Changing Landscape of Therapeutic Cancer Vaccines-Novel Platforms and Neoantigen Identification. Clin Cancer Res. 2021 Feb 1;27(3):689-703.
141. Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016 Apr;22(4):433–8.
142. Li L, Li Y, Yin Z, Zhu J, Yan D, Lou H. Increased frequency of regulatory T cells in the peripheral blood of patients with endometrioid adenocarcinoma. Oncol Lett. 2019 Aug;18(2):1424–30.
143. Fritah H, Rovelli R, Chiang CL, Kandalaft LE. The current clinical landscape of personalized cancer vaccines. Cancer Treat Rev. 2022 May;106:102383.
144. Sellars MC, Wu CJ, Fritsch EF. Cancer vaccines: Building a bridge over troubled waters. Cell. 2022 Jul 21;185(15):2770–88.
145. Oliveira G, Stromhaug K, Cieri N, Iorgulescu JB, Klaeger S, Wolff JO, et al. Landscape of helper and regulatory antitumour CD4+ T cells in melanoma. Nature. 2022 May;605(7910):532–8.
146. Wakita D, Iwai T, Harada S, Suzuki M, Yamamoto K, Sugimoto M. Cisplatin Augments Antitumor T-Cell Responses Leading to a Potent Therapeutic Effect in Combination With PD-L1 Blockade. Anticancer Res. 2019 Apr;39(4):1749–60.
147. Principe N, Aston WJ, Hope DE, Tilsed CM, Fisher SA, Boon L, et al. Comprehensive Testing of Chemotherapy and Immune Checkpoint Blockade in Preclinical Cancer Models Identifies Additive Combinations. Front Immunol. 2022 May 11;13:872295.
148. Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015 Feb 15;21(4):712–20.
149. Wang E, Aifantis I. RNA Splicing and Cancer. Trends Cancer. 2020 Aug;6(8):631-644.
150. Hoyos LE, Abdel-Wahab O. Cancer-Specific Splicing Changes and the Potential for Splicing-Derived Neoantigens. Cancer Cell. 2018 Aug 13;34(2):181–3.
151. Kahles A, Lehmann KV, Toussaint NC, Hüser M, Stark SG, Sachsenberg T, et al. Comprehensive Analysis of Alternative Splicing Across Tumors from 8,705 Patients. Cancer Cell. 2018 Aug 13;34(2):211–24.e6.
152. Patskovsky Y, Natarajan A, Patskovska L, Nyovanie S, Joshi B, Morin B, et al. Molecular mechanism of phosphopeptide neoantigen immunogenicity. Nat Commun. 2023 Jun 23;14(1):3763.
153. Finnigan JP, Newman JH, Patskovsky Y, Patskovska L, Ishizuka AS, Lynn GM, et al. Structural Basis for Self-Discrimination by Neoantigen-Specific TCRs. Res Sq [Preprint]. 2023 Jan 31:rs.3.rs-2531184.
154. Apoorvi T, Yury P, Iryna V, Michelle K. Phosphopeptide Neoantigens as Emerging Targets in Cancer Immunotherapy. J Cancer Immunol (Wilmington). 2024;6(4):135–47.
155. Aparicio B, Theunissen P, Hervas-Stubbs S, Fortes P, Sarobe P. Relevance of mutation-derived neoantigens and non-classical antigens for anticancer therapies. Hum Vaccin Immunother. 2024 Dec 31;20(1):2303799.
156. Ward JP, Gubin MM, Schreiber RD. The Role of Neoantigens in Naturally Occurring and Therapeutically Induced Immune Responses to Cancer. Adv Immunol. 2016;130:25–74.
157. Paulson KG, Voillet V, McAfee MS, Hunter DS, Wagener FD, Perdicchio M, et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat Commun. 2018 Sep 24;9(1):3868.
158. Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017 Oct 26;8(1):1136.
159. Torrejon DY, Galvez M, Abril-Rodriguez G, Campbell KM, Medina E, Vega-Crespo A, et al. Antitumor Immune Responses in B2M-Deficient Cancers. Cancer Immunol Res. 2023 Dec 1;11(12):1642–55.
160. Song Z, Tao Y, You J. The potential applications of peptide-loading complex in cancer treatment. Front Immunol. 2025 Mar 3;16:1526137.
161. Lee JV, Housley F, Yau C, Nakagawa R, Winkler J, Anttila JM, et al. Combinatorial immunotherapies overcome MYC-driven immune evasion in triple negative breast cancer. Nat Commun. 2022 Jun 27;13(1):3671.
162. Kumar S, Chatterjee M, Ghosh P, Ganguly KK, Basu M, Ghosh MK. Targeting PD-1/PD-L1 in cancer immunotherapy: An effective strategy for treatment of triple-negative breast cancer (TNBC) patients. Genes Dis. 2022 Aug 24;10(4):1318–50.
163. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016 Sep 1;375(9):819–29.
164. Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017 Feb;7(2):188–201.
165. Balasubramanian A, John T, Asselin-Labat ML. Regulation of the antigen presentation machinery in cancer and its implication for immune surveillance. Biochem Soc Trans. 2022 Apr 29;50(2):825–37.
166. Dixon-Douglas J, Loi S. Immunotherapy in Early-Stage Triple-Negative Breast Cancer: Where Are We Now and Where Are We Headed? Curr Treat Options Oncol. 2023 Aug;24(8):1004–20.
167. Alspach E, Lussier DM, Schreiber RD. Interferon γ and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb Perspect Biol. 2019 Mar 1;11(3):a028480.
168. Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 Expression in Cancer. Mol Cell. 2019 Nov 7;76(3):359–70.
169. Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016 Oct 6;167(2):397–404.e9.
170. Soltani M, Abbaszadeh M, Fouladseresht H, Sullman MJM, Eskandari N. PD-L1 importance in malignancies comprehensive insights into the role of PD-L1 in malignancies: from molecular mechanisms to therapeutic opportunities. Clin Exp Med. 2025 Apr 3;25(1):106.
171. de la Iglesia JV, Slebos RJC, Martin-Gomez L, Wang X, Teer JK, Tan AC, et al. Effects of Tobacco Smoking on the Tumor Immune Microenvironment in Head and Neck Squamous Cell Carcinoma. Clin Cancer Res. 2020 Mar 15;26(6):1474–85.
172. He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020 Aug;30(8):660–9.
173. Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017 Jan 10;8(2):2171–86.
174. Grossman JE, Vasudevan D, Joyce CE, Hildago M. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene. 2021 Feb;40(8):1393–5.
175. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019 Oct 26;7(1):278.
176. Aygün MİŞ, Yalçın Ö. LAG-3 and TIM-3 expression in melanoma and histopathological correlation: a single-center study. Clin Transl Oncol. 2025 Jul;27(7):3084–99.
177. Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023 Sep 5;16(1):101.
178. Freed-Pastor WA, Lambert LJ, Ely ZA, Pattada NB, Bhutkar A, Eng G, et al. The CD155/TIGIT axis promotes and maintains immune evasion in neoantigen-expressing pancreatic cancer. Cancer Cell. 2021 Oct 11;39(10):1342-60.e14.
179. Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020 May;200(2):108–19.
180. Tang W, Chen J, Ji T, Cong X. TIGIT, a novel immune checkpoint therapy for melanoma. Cell Death Dis. 2023 Jul 26;14(7):466.
181. Mortezaee K, Majidpoor J. Alternative immune checkpoints in immunoregulatory profile of cancer stem cells. Heliyon. 2023 Dec 2;9(12):e23171.
182. Mao L, Xiao Y, Yang QC, Yang SC, Yang LL, Sun ZJ. TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells. Oral Oncol. 2021 Oct;121:105472.
183. Pandey AK, Chauvin JM, Brufsky A, Pagliano O, Ka M, Menna C, et al. Abstract P5-04-28: Targeting TIGIT and PD-1 in triple negative breast cancer. Cancer Res. 2020 Feb 15;80(4_Supplement):P5–04.
184. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018 Aug;560(7718):382–6.
185. Janku F, Wheler JJ, Naing A, Falchook GS, Hong DS, Stepanek VM, et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res. 2013 Jan 1;73(1):276–84.
186. Collins NB, Al Abosy R, Miller BC, Bi K, Zhao Q, Quigley M, et al. PI3K activation allows immune evasion by promoting an inhibitory myeloid tumor microenvironment. J Immunother Cancer. 2022 Mar;10(3):e003402.
187. Li X, Xiang Y, Li F, Yin C, Li B, Ke X. WNT/β-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front Immunol. 2019 Sep 26;10:2293.
188. Reschke R, Olson DJ. Leveraging STING, Batf3 Dendritic Cells, CXCR3 Ligands, and Other Components Related to Innate Immunity to Induce A "Hot" Tumor Microenvironment That Is Responsive to Immunotherapy. Cancers (Basel). 2022 May 16;14(10):2458.
189. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014 Feb;13(2):140–56.
190. Zhong Z, Virshup DM. Wnt Signaling and Drug Resistance in Cancer. Mol Pharmacol. 2020 Feb;97(2):72–89.
191. Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol Cancer Ther. 2014 Nov;13(11):2477–88.
192. Castellani G, Buccarelli M, Arasi MB, Rossi S, Pisanu ME, Bellenghi M, et al. BRAF Mutations in Melanoma: Biological Aspects, Therapeutic Implications, and Circulating Biomarkers. Cancers (Basel). 2023 Aug 8;15(16):4026.
193. Harper TA, Bacot SM, Fennell CJ, Matthews RL, Zhu C, Yue P, et al. IL-10 Signaling Elicited by Nivolumab-Induced Activation of the MAP Kinase Pathway Does Not Fully Contribute to Nivolumab-Modulated Heterogeneous T Cell Responses. Int J Mol Sci. 2021 Oct 31;22(21):11848.
194. Zhang HP, Jiang RY, Zhu JY, Sun KN, Huang Y, Zhou HH, et al. PI3K/AKT/mTOR signaling pathway: an important driver and therapeutic target in triple-negative breast cancer. Breast Cancer. 2024 Jul;31(4):539–51.
195. Jiang Q, Xiao J, Hsieh YC, Kumar NL, Han L, Zou Y, et al. The Role of the PI3K/Akt/mTOR Axis in Head and Neck Squamous Cell Carcinoma. Biomedicines. 2024 Jul 19;12(7):1610.
196. Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023 Aug 18;22(1):138.
197. Li H, Prever L, Hirsch E, Gulluni F. Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers (Basel). 2021 Jul 14;13(14):3517.
198. Garg P, Ramisetty S, Nair M, Kulkarni P, Horne D, Salgia R, et al. Strategic advancements in targeting the PI3K/AKT/mTOR pathway for Breast cancer therapy. Biochem Pharmacol. 2025 Jun;236:116850.
199. Su YC, Lee WC, Wang CC, Yeh SA, Chen WH, Chen PJ. Targeting PI3K/AKT/mTOR Signaling Pathway as a Radiosensitization in Head and Neck Squamous Cell Carcinomas. Int J Mol Sci. 2022 Dec 12;23(24):15749.
200. Zhang Z, Richmond A, Yan C. Immunomodulatory Properties of PI3K/AKT/mTOR and MAPK/MEK/ERK Inhibition Augment Response to Immune Checkpoint Blockade in Melanoma and Triple-Negative Breast Cancer. Int J Mol Sci. 2022 Jul 1;23(13):7353.
201. Mallick S, Duttaroy AK, Dutta S. The PIK3CA gene and its pivotal role in tumor tropism of triple-negative breast cancer. Transl Oncol. 2024 Dec;50:102140.
202. Shao Z, Bao Q, Jiang F, Qian H, Fang Q, Hu X. VS-5584, a Novel PI3K-mTOR Dual Inhibitor, Inhibits Melanoma Cell Growth In Vitro and In Vivo. PLoS One. 2015 Jul 23;10(7):e0132655.
203. Parkman GL, Turapov T, Kircher DA, Burnett WJ, Stehn CM, O'Toole K, et al. Genetic Silencing of AKT Induces Melanoma Cell Death via mTOR Suppression. Mol Cancer Ther. 2024 Mar 4;23(3):301–15.
204. Wu X, Que H, Li Q, Wei X. Wnt/β-catenin mediated signaling pathways in cancer: recent advances, and applications in cancer therapy. Mol Cancer. 2025 Jun 10;24(1):171.
205. Jiang W, Guan B, Sun H, Mi Y, Cai S, Wan R, et al. WNT11 Promotes immune evasion and resistance to Anti-PD-1 therapy in liver metastasis. Nat Commun. 2025 Feb 7;16(1):1429.
206. Hong Y, Manoharan I, Suryawanshi A, Majumdar T, Angus-Hill ML, Koni PA, et al. β-catenin promotes regulatory T-cell responses in tumors by inducing vitamin A metabolism in dendritic cells. Cancer Res. 2015 Feb 15;75(4):656–65.
207. Jung KH, LoRusso P, Burris H, Gordon M, Bang YJ, Hellmann MD, et al. Phase I Study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin Cancer Res. 2019 Jun 1;25(11):3220–8.
208. Ngan HL, Liu Y, Fong AY, Poon PHY, Yeung CK, Chan SSM, et al. MAPK pathway mutations in head and neck cancer affect immune microenvironments and ErbB3 signaling. Life Sci Alliance. 2020 May 7;3(6):e201900545.
209. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014 Sep 4;6(10):a016295.
210. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020 Jan;20(1):25–39.
211. Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P, et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin Cancer Res. 2016 Mar 15;22(6):1499–509.
212. Bayati F, Mohammadi M, Valadi M, Jamshidi S, Foma AM, Sharif-Paghaleh E. The Therapeutic Potential of Regulatory T Cells: Challenges and Opportunities. Front Immunol. 2021 Jan 15;11:585819.
213. Heintzman DR, Fisher EL, Rathmell JC. Microenvironmental influences on T cell immunity in cancer and inflammation. Cell Mol Immunol. 2022 Mar;19(3):316–26.
214. Habib S, Osborn G, Willsmore Z, Chew MW, Jakubow S, Fitzpatrick A, et al. Tumor associated macrophages as key contributors and targets in current and future therapies for melanoma. Expert Rev Clin Immunol. 2024 Aug;20(8):895–911.
215. Ibrahim A, Mohamady Farouk Abdalsalam N, Liang Z, Kashaf Tariq H, Li R, O Afolabi L, et al. MDSC checkpoint blockade therapy: a new breakthrough point overcoming immunosuppression in cancer immunotherapy. Cancer Gene Ther. 2025 Apr;32(4):371–92.
216. Sun Y, Yinwang E, Wang S, Wang Z, Wang F, Xue Y, et al. Phenotypic and spatial heterogeneity of CD8+ tumour infiltrating lymphocytes. Mol Cancer. 2024 Sep 9;23(1):193.
217. Dos Santos LV, Abrahão CM, William WN Jr. Overcoming Resistance to Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinomas. Front Oncol. 2021 Mar 5;11:596290.
218. Galizia D, Minei S, Maldi E, Chilà G, Polidori A, Merlano MC. How Risk Factors Affect Head and Neck Squamous Cell Carcinoma (HNSCC) Tumor Immune Microenvironment (TIME): Their Influence on Immune Escape Mechanisms and Immunotherapy Strategy. Biomedicines. 2022 Oct 7;10(10):2498.
219. Wei J, Li W, Zhang P, Guo F, Liu M. Current trends in sensitizing immune checkpoint inhibitors for cancer treatment. Mol Cancer. 2024 Dec 26;23(1):279.
220. Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, et al. Noncanonical NF-κB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 2014 Sep 1;193(5):2574–86.
221. Smith AD, Lu C, Payne D, Paschall AV, Klement JD, Redd PS, et al. Autocrine IL6-Mediated Activation of the STAT3-DNMT Axis Silences the TNFα-RIP1 Necroptosis Pathway to Sustain Survival and Accumulation of Myeloid-Derived Suppressor Cells. Cancer Res. 2020 Aug 1;80(15):3145–56.
222. Chen AY, Wolchok JD, Bass AR. TNF in the era of immune checkpoint inhibitors: friend or foe? Nat Rev Rheumatol. 2021 Apr;17(4):213–23.
223. Angioni R, Sánchez-Rodríguez R, Viola A, Molon B. TGF-β in Cancer: Metabolic Driver of the Tolerogenic Crosstalk in the Tumor Microenvironment. Cancers (Basel). 2021 Jan 22;13(3):401.
224. Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, et al. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front Immunol. 2018 Jun 11;9:1310.
225. Wang CW, Biswas PK, Islam A, Chen MK, Chueh PJ. The Use of Immune Regulation in Treating Head and Neck Squamous Cell Carcinoma (HNSCC). Cells. 2024 Feb 27;13(5):413.
226. Bruni S, Mercogliano MF, Mauro FL, Cordo Russo RI, Schillaci R. Cancer immune exclusion: breaking the barricade for a successful immunotherapy. Front Oncol. 2023 May 22;13:1135456.
227. Kather JN, Suarez-Carmona M, Charoentong P, Weis CA, Hirsch D, Bankhead P, et al. Topography of cancer-associated immune cells in human solid tumors. Elife. 2018 Sep 4;7:e36967.
228. Zheng S, Wang W, Shen L, Yao Y, Xia W, Ni C. Tumor battlefield within inflamed, excluded or desert immune phenotypes: the mechanisms and strategies. Exp Hematol Oncol. 2024 Aug 6;13(1):80.
229. Li C, Wang X, Xing L, Chen T, Li W, Li X, et al. Huaier-induced suppression of cancer-associated fibroblasts confers immunotherapeutic sensitivity in triple-negative breast cancer. Phytomedicine. 2024 Dec;135:156051.
230. Zhao X, Zhu Y, He Y, Gu W, Zhou Q, Jin Bet al. Unraveling the immune evasion mechanisms in the tumor microenvironment of head and neck squamous cell carcinoma. Front Immunol. 2025 May 14;16:1597202.
231. Indini A, Grossi F, Mandalà M, Taverna D, Audrito V. Metabolic Interplay between the Immune System and Melanoma Cells: Therapeutic Implications. Biomedicines. 2021 May 26;9(6):607.
232. Zhou Y, Tao L, Qiu J, Xu J, Yang X, Zhang Y, et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther. 2024 May 20;9(1):132.
233. Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020 Sep;20(9):516–31.
234. Qiang W, Dai Y, Xing X, Sun X. Identification of a metabolic reprogramming-related signature associated with prognosis and immune microenvironment of head and neck squamous cell carcinoma by in silico analysis. Cancer Med. 2022 Aug;11(16):3168–81.
235. Li X, Escoffier H, Sauter T, Tavassoli M. Targeting Fibroblast-Derived Interleukin 6: A Strategy to Overcome Epithelial-Mesenchymal Transition and Radioresistance in Head and Neck Cancer. Cancers (Basel). 2025 Jan 15;17(2):267.
236. Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011 Dec;9(12):1658–67.
237. Sebestyén A, Kopper L, Dankó T, Tímár J. Hypoxia Signaling in Cancer: From Basics to Clinical Practice. Pathol Oncol Res. 2021 May 3;27:1609802.
238. Cheu JW, Chiu DK, Kwan KK, Yang C, Yuen VW, Goh CC, et al. Hypoxia-inducible factor orchestrates adenosine metabolism to promote liver cancer development. Sci Adv. 2023 May 5;9(18):eade5111.
239. Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–7.
240. Chen Z, Han F, Du Y, Shi H, Zhou W. Hypoxic microenvironment in cancer: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023 Feb 17;8(1):70.
241. Gong Y, Ji P, Yang YS, Xie S, Yu TJ, Xiao Y, et al. Metabolic-Pathway-Based Subtyping of Triple-Negative Breast Cancer Reveals Potential Therapeutic Targets. Cell Metab. 2021 Jan 5;33(1):51–64.e9.
242. Dratkiewicz E, Simiczyjew A, Mazurkiewicz J, Ziętek M, Matkowski R, Nowak D. Hypoxia and Extracellular Acidification as Drivers of Melanoma Progression and Drug Resistance. Cells. 2021 Apr 9;10(4):862.
243. Liu L, Bai J, Hu L, Jiang D. Hypoxia-mediated activation of hypoxia-inducible factor-1α in triple-negative breast cancer: A review. Medicine (Baltimore). 2023 Oct 27;102(43):e35493.
244. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016 Nov 8;24(5):657–71.
245. Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022 Jun 3;15(1):77.
246. Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020 Jan;17(1):1–12.
247. Zolkind P, Uppaluri R. Checkpoint immunotherapy in head and neck cancers. Cancer Metastasis Rev. 2017 Sep;36(3):475–89.
248. Sauer N, Janicka N, Szlasa W, Skinderowicz B, Kołodzińska K, Dwernicka W, et al. TIM-3 as a promising target for cancer immunotherapy in a wide range of tumors. Cancer Immunol Immunother. 2023 Nov;72(11):3405–25.
249. Yan Z, Wang C, Wu J, Wang J, Ma T. TIM-3 teams up with PD-1 in cancer immunotherapy: mechanisms and perspectives. Mol Biomed. 2025 May 7;6(1):27.
250. Jiang Y, Li Y, Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015 Jun 18;6(6):e1792.
251. Joller N, Anderson AC, Kuchroo VK. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity. 2024 Feb 13;57(2):206–22.
252. Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014 Apr 1;74(7):1924–32.
253. Yamac MA, Ayozger LEO, Ipek V. Immunohistochemical profiling of VISTA expression in canine mammary tumors: A pilot study for immunotherapeutic targeting. Res Vet Sci. 2025 Sep;193:105807.
254. Diaz N, Juarez M, Cancrini C, Heeg M, Soler-Palacín P, Payne A, et al. Seletalisib for Activated PI3Kδ Syndromes: Open-Label Phase 1b and Extension Studies. J Immunol. 2020 Dec 1;205(11):2979–87.
255. Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019 Jul 16;51(1):27–41.
256. McQuade JL, Hammers H, Furberg H, Engert A, André T, Blumenschein G Jr, et al. Association of Body Mass Index With the Safety Profile of Nivolumab With or Without Ipilimumab. JAMA Oncol. 2023 Jan 1;9(1):102–11.
257. Alsaafeen BH, Ali BR, Elkord E. Resistance mechanisms to immune checkpoint inhibitors: updated insights. Mol Cancer. 2025 Jan 15;24(1):20.
258. Denaro N, Bareggi C, Galassi B, Beltramini G, Wekking D, Proh M, et al. Nutrition in HNSCC: is it a matter for oncologists? The role of multidisciplinary team-a narrative literature review. Front Oncol. 2024 Jul 3;14:1430845.
259. Mazurek M, Mlak R, Kot A, Rahnama-Hezavah M, Małecka-Massalska T. Does Human Papillomavirus Infection Influence the Frequency and Severity of Nutritional Disorders in Head and Neck Cancer? Nutrients. 2022 Oct 27;14(21):4528.
260. Anghel R, Bîlteanu L, Folea AR, Marinescu ȘA, Pisoschi AM, Alexandrescu MF, et al. Assessing the Impact of Nutritional Status on the Quality of Life in Head and Neck Cancer Patients-The Need for Comprehensive Digital Tools. Cancers (Basel). 2025 Mar 27;17(7):1128.
261. Zhang K, Chen L, Zheng H, Zeng Y. Cytokines secreted from adipose tissues mediate tumor proliferation and metastasis in triple negative breast cancer. BMC Cancer. 2022 Aug 13;22(1):886.
262. Savulescu-Fiedler I, Mihalcea R, Dragosloveanu S, Scheau C, Baz RO, Caruntu A, et al. The Interplay between Obesity and Inflammation. Life (Basel). 2024 Jul 8;14(7):856.
263. Salvadori G, Zanardi F, Iannelli F, Lobefaro R, Vernieri C, Longo VD. Fasting-mimicking diet blocks triple-negative breast cancer and cancer stem cell escape. Cell Metab. 2021 Nov 2;33(11):2247–59.e6.
264. Corleto KA, Strandmo JL, Giles ED. Metformin and Breast Cancer: Current Findings and Future Perspectives from Preclinical and Clinical Studies. Pharmaceuticals (Basel). 2024 Mar 19;17(3):396.
265. Dorobisz K, Dorobisz T, Zatoński T. The Microbiome's Influence on Head and Neck Cancers. Curr Oncol Rep. 2023 Mar;25(3):163–71.
266. Zhang S, Lv K, Liu Z, Zhao R, Li F. Fatty acid metabolism of immune cells: a new target of tumour immunotherapy. Cell Death Discov. 2024 Jan 20;10(1):39.
267. Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, et al. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. 2022 Apr 29;15(1):47.
268. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021 Feb 5;371(6529):595–602.
269. Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021 Feb 5;371(6529):602–9.
270. Routy B, Lenehan JG, Miller WH Jr, Jamal R, Messaoudene M, Daisley BA, et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat Med. 2023 Aug;29(8):2121–32.
271. Ahmad S, Jayamanne D, Bergamin S, Lawless A, Guminski A, Lee A, et al. Oral Microbiome as a Biomarker and Therapeutic Target in Head and Neck Cancer: Current Insights and Future Directions. Cancers (Basel). 2025 Aug 15;17(16):2667.
272. Kwak S, Wang C, Usyk M, Wu F, Freedman ND, Huang WY, et al. Oral Microbiome and Subsequent Risk of Head and Neck Squamous Cell Cancer. JAMA Oncol. 2024 Nov 1;10(11):1537–47.
273. Luo YC, Huang XT, Wang R, Lin YJ, Sun JX, Li KF, et al. Advancements in understanding tumor-resident bacteria and their application in cancer therapy. Mil Med Res. 2025 Jul 25;12(1):38.
274. Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018 Jan 5;359(6371):104–8.
275. Ullern A, Holm K, Røssevold AH, Andresen NK, Bang C, Lingjærde OC, et al. Gut microbiota diversity is prognostic and associated with benefit from chemo-immunotherapy in metastatic triple-negative breast cancer. Mol Oncol. 2025 Apr;19(4):1229–43.
276. Perl M, Fante MA, Herfeld K, Scherer JN, Poeck H, Thiele Orberg E. Microbiota-derived metabolites: Key modulators of cancer immunotherapies. Med. 2025 Aug 8;6(8):100773.
277. Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021 Oct 14;184(21):5309–37.
278. Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018 Jan 11;378(2):158–68.
279. Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020 Aug;17(8):504–15.
280. Luke JJ, Patel MR, Blumenschein GR, Hamilton E, Chmielowski B, Ulahannan SV, et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: a phase 1 trial. Nat Med. 2023 Nov;29(11):2814–24.
281. Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018 May 24;7(8):e1466769.
282. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022 Jan 21;21(1):28.
283. Nederlof I, Isaeva OI, de Graaf M, Gielen RCAM, Bakker NAM, Rolfes AL, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in early-stage triple-negative breast cancer: a phase 2 adaptive trial. Nat Med. 2024 Nov;30(11):3223–35.
284. Ribas A, Medina T, Kirkwood JM, Zakharia Y, Gonzalez R, Davar D, et al. Overcoming PD-1 Blockade Resistance with CpG-A Toll-Like Receptor 9 Agonist Vidutolimod in Patients with Metastatic Melanoma. Cancer Discov. 2021 Dec 1;11(12):2998–3007.
285. Moser JC, Opyrchal M, Rodriguez Rivera I, Curti BD, Puzanov I, Sosman JA, et al. A phase 1/1b study of the IL-2 prodrug WTX-124 in patients with locally advanced or metastatic solid tumors after checkpoint inhibitor therapy: Initial results of the combination dose escalation with pembrolizumab. J Clin Oncol 42, 2024 May 29, 2623.
286. Mullard A. FDA approves first tumour-infiltrating lymphocyte (TIL) therapy, bolstering hopes for cell therapies in solid cancers. Nat Rev Drug Discov. 2024 Apr;23(4):238.
287. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J Clin Oncol. 2022 Jan 10;40(2):127–37.
288. Pao SC, Chu MT, Hung SI. Therapeutic Vaccines Targeting Neoantigens to Induce T-Cell Immunity against Cancers. Pharmaceutics. 2022 Apr 15;14(4):867.
289. Rugo HS, Bardia A, Marmé F, Cortés J, Schmid P, Loirat D, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023 Oct 21;402(10411):1423–33.
290. Qin H, Zhou Z, Shi R, Mai Y, Xu Y, Peng F, et al. Insights into next-generation immunotherapy designs and tools: molecular mechanisms and therapeutic prospects. J Hematol Oncol. 2025 Jun 7;18(1):62.
291. Zhang S, Zheng M, Nie D, Xu L, Tian H, Wang M, et al. Efficacy of cetuximab plus PD-1 inhibitor differs by HPV status in head and neck squamous cell carcinoma: a systematic review and meta-analysis. J Immunother Cancer. 2022 Oct;10(10):e005158.
292. Nishina T, Fujita T, Yoshizuka N, Sugibayashi K, Murayama K, Kuboki Y. Safety, tolerability, pharmacokinetics and preliminary antitumour activity of an antisense oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in combination with durvalumab in Japanese patients with advanced solid malignancies: a phase 1 study. BMJ Open. 2022 Oct 21;12(10):e055718.
293. Cohen EE, Harrington KJ, Hong DS, Mesia R, Brana I, Segura PP, et al. A phase Ib/II study (SCORES) of durvalumab (D) plus danvatirsen (DAN; AZD9150) or AZD5069 (CX2i) in advanced solid malignancies and recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC): Updated results. Ann Oncol. 2018 Oct 1;29:viii372.
294. Galot R, Le Tourneau C, Licitra L, Guigay J, Kong A, Tinhofer I, et al. A phase II study of monalizumab and durvalumab in patients with recurrent/metastatic squamous cell carcinoma of the head and neck: results of the I2 cohort of the EORTC-HNCG-1559 trial (UPSTREAM). ESMO Open. 2025 May;10(5):104554.
295. Chen TH, Chang PM, Yang MH. Combination of pembrolizumab and lenvatinib is a potential treatment option for heavily pretreated recurrent and metastatic head and neck cancer. J Chin Med Assoc. 2021 Apr 1;84(4):361–7.
296. Licitra L, Tahara M, Harrington K, de Mendoza MO, Guo Y, Aksoy S, et al. Pembrolizumab with or without lenvatinib as first-line therapy for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): phase 3 LEAP-010 study. Int J Radiat Oncol Biol Phys. 2024 Apr 1;118(5):e2–3.
297. Davis EJ, Martin-Liberal J, Kristeleit R, Cho DC, Blagden SP, Berthold D, et al. First-in-human phase I/II, open-label study of the anti-OX40 agonist INCAGN01949 in patients with advanced solid tumors. J Immunother Cancer. 2022 Oct;10(10):e004235.
298. Carneiro A, Hahn A, Ellmark P, Enell Smith K, Schultz L, Ambarkhane S, et al. First-in-human, multicenter, open-label, phase I study of ATOR-1017 (evunzekibart), a 4-1BB antibody, in patients with advanced solid malignancies. J Immunother Cancer. 2025 Jan 22;13(1):e010113.
299. Northfelt DW, Ramanathan RK, Cohen PA, Von Hoff DD, Weiss GJ, Dietsch GN, et al. A phase I dose-finding study of the novel Toll-like receptor 8 agonist VTX-2337 in adult subjects with advanced solid tumors or lymphoma. Clin Cancer Res. 2014 Jul 15;20(14):3683–91.
300. Meric-Bernstam F, Sweis RF, Kasper S, Hamid O, Bhatia S, Dummer R, et al. Combination of the STING Agonist MIW815 (ADU-S100) and PD-1 Inhibitor Spartalizumab in Advanced/Metastatic Solid Tumors or Lymphomas: An Open-Label, Multicenter, Phase Ib Study. Clin Cancer Res. 2023 Jan 4;29(1):110–21.
301. Hu-Lieskovan S, Patnaik A, Eisenberg P, Sachdev J, Weise A, Kaufman DR, et al. Phase 1/2a study of double immune suppression blockade by combining a CSF1R inhibitor (pexidartinib/PLX3397) with an anti PD-1 antibody (pembrolizumab) to treat advanced melanoma and other solid tumors. Ann Oncol. 2015 Nov 1;26(8):viii5.
302. Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017 Sep 7;170(6):1109–19.e10.
303. Chesney JA, Ribas A, Long GV, Kirkwood JM, Dummer R, Puzanov I, et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined With Pembrolizumab for Advanced Melanoma. J Clin Oncol. 2023 Jan 20;41(3):528–40.
304. Shilpa P, Kaul R, Bhat S, Sultana N, Pandeshwar P. Oncolytic viruses in head and neck cancer: a new ray of hope in the management protocol. Ann Med Health Sci Res. 2014 Sep;4(Suppl 3):S178–84.
305. Sriramulu S, Thoidingjam S, Speers C, Nyati S. Present and Future of Immunotherapy for Triple-Negative Breast Cancer. Cancers (Basel). 2024 Sep 24;16(19):3250.
306. Zhang Z, Wang G, Li Y, Lei D, Xiang J, Ouyang L, et al. Recent progress in DNA methyltransferase inhibitors as anticancer agents. Front Pharmacol. 2022 Dec 16;13:1072651.
307. Antrobus J, Mackinnon B, Melia E, Hughes JR, Parsons JL. HDAC Inhibitors Can Enhance Radiosensitivity of Head and Neck Cancer Cells Through Suppressing DNA Repair. Cancers (Basel). 2024 Dec 7;16(23):4108.
308. Topper MJ, Vaz M, Marrone KA, Brahmer JR, Baylin SB. The emerging role of epigenetic therapeutics in immuno-oncology. Nat Rev Clin Oncol. 2020 Feb;17(2):75–90.
309. Huang W, Zhu Q, Shi Z, Tu Y, Li Q, Zheng W, et al. Dual inhibitors of DNMT and HDAC induce viral mimicry to induce antitumour immunity in breast cancer. Cell Death Discov. 2024 Mar 15;10(1):143.
310. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, et al; OlympiA Clinical Trial Steering Committee and Investigators. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N Engl J Med. 2021 Jun 24;384(25):2394–405.
311. Chesney J, Lewis KD, Kluger H, Hamid O, Whitman E, Thomas S, et al. Efficacy and safety of lifileucel, a one-time autologous tumor-infiltrating lymphocyte (TIL) cell therapy, in patients with advanced melanoma after progression on immune checkpoint inhibitors and targeted therapies: pooled analysis of consecutive cohorts of the C-144-01 study. J Immunother Cancer. 2022 Dec;10(12):e005755.
312. Kim YN, Park B, Kim JW, Kim BG, Kim SW, Kim HS, et al. Triplet maintenance therapy of olaparib, pembrolizumab and bevacizumab in women with BRCA wild-type, platinum-sensitive recurrent ovarian cancer: the multicenter, single-arm phase II study OPEB-01/APGOT-OV4. Nat Commun. 2023 Sep 6;14(1):5476.
313. Zhou L, Wan Y, Zhang L, Meng H, Yuan L, Zhou S, et al. Beyond monotherapy: An era ushering in combinations of PARP inhibitors with immune checkpoint inhibitors for solid tumors. Biomed Pharmacother. 2024 Jun;175:116733.
314. Xia L, Zhu X, Wang Y, Lu S. The gut microbiota improves the efficacy of immune-checkpoint inhibitor immunotherapy against tumors: From association to cause and effect. Cancer Lett. 2024 Aug 28;598:217123.
315. Mafe AN, Büsselberg D. Microbiome Integrity Enhances the Efficacy and Safety of Anticancer Drug. Biomedicines. 2025 Feb 10;13(2):422.
316. Farbehi N, Alinejad-Rokny H, Chtanova T, Rnjak-Kovacina J. Spatial and single-cell transcriptomics unravel the complex interplay between the body and medical implants. Cell Biomater. 2025 May 27;1(6): 100099
317. Ge S, Sun S, Xu H, Cheng Q, Ren Z. Deep learning in single-cell and spatial transcriptomics data analysis: advances and challenges from a data science perspective. Brief Bioinform. 2025 Mar 4;26(2):bbaf136.
318. Shao Y, Lv X, Ying S, Guo Q. Artificial intelligence-driven precision medicine: multi-omics and spatial multi-omics approaches in Diffuse Large B-Cell Lymphoma (DLBCL). Front Biosci (Landmark Ed). 2024 Nov 28;29(12):404.