Abstract
Background: Limited data exists regarding long-term morbidity of the offspring in women with psoriasis. The objective of this study was to assess long-term infectious morbidity of the offspring born to women with psoriasis.
Study Design: We conducted a population-based cohort study comparing the long-term infectious-related morbidity of offspring (up to the age of 18) born to mothers with and without psoriasis, between the years 1991-2021 in a regional tertiary medical center. A Kaplan-Meier survival curve was used to assess cumulative incidence of infectious-related morbidity of the offspring and a Cox regression model was performed to control for possible confounders.
Results: During the study period 356,356 deliveries met the inclusion criteria, of which 240 (0.07%) occurred in women with psoriasis. Children born to mothers with psoriasis had higher rates of ear-nose-throat (ENT) and respiratory infections, and the total infectious-related hospitalization rate was also higher for this group. The cumulative incidence of long-term infectious morbidity was higher among offspring born to women with psoriasis (Log-rank, p=0.050). The Cox regression model, controlling for birthweight, found that fetal exposure to maternal psoriasis was independently associated with long-term infectious morbidity of the offspring (adjusted HR=1.26, 95% CI 1.01-1.58; p=0.049).
Conclusion: Being born to mothers with psoriasis is an independent risk factor for long-term infectious morbidity of the offspring.
Keywords
Psoriasis, Infectious morbidity, Pregnancy outcomes, Pediatric hospitalization, Long-term follow up
Introduction
Psoriasis is a chronic, T cell mediated, inflammatory skin disease that affects millions of patients worldwide and impairs their quality of life. Psoriasis affects between 2-4% of the population worldwide and is twice as prevalent in Caucasians than in African Americans [1,2]. Psoriasis is evenly distributed between genders with a bimodal distribution of onset; an earlier peak at 15–39 years of age and a later peak at 50–60 years of age [3]. The early peak overlaps with reproductive age, thus women of childbearing potential are affected by the disease [4].
A reciprocal relationship exists between pregnancy and psoriasis. While the disease is expected to improve in 40-60% of patients during pregnancy, with most improvement during the late first and second trimesters [5,6], psoriasis may provoke pregnancy complications and adversely affect perinatal outcomes in psoriatic women.
Maternal psoriasis has been associated with obesity, diabetes, hypertension and depression [7]. Data on pregnancy outcomes of women with psoriasis are lacking and inconsistent. While some studies have demonstrated an association with various adverse pregnancy outcomes such as recurrent pregnancy loss, gestational diabetes, pregnancy-related hypertensive disorders, increased risk for caesarean delivery and low birth weight infants, other studies failed to demonstrate such an association [8-12].
Although early concepts of the pathogenesis of psoriasis focused primarily on keratinocyte hyper-proliferation, dysregulation of the immune system is now recognized as a critical event in this disease. Interactions between dendritic cells, T cells, keratinocytes, neutrophils, and the cytokines released from immune cells likely contribute to the initiation and perpetuation of the cutaneous inflammation that is characteristic of psoriasis [13,14]. Studies have associated maternal aberrant immune mechanism, especially abnormal shift in cellular immunity, with adverse pregnancy outcomes [15,16]. As maternal psoriasis is also associated with altered immunity and pro-inflammatory conditions, it can be speculated that the inflammatory environment supporting the developing fetus in pregnant mothers with psoriasis, may have a secondary effect for the infant, making them more prone to acquire infections later in life. The purpose of the current study is to examine a possible association between maternal psoriasis, perinatal outcomes and long-term infectious morbidity of the offspring.
Materials and Methods
This is a population-based retrospective cohort study of all infants born between the years 1991-2021 at the Soroka University Medical Center (SUMC), the sole tertiary hospital in the southern region of Israel. Perinatal outcomes and long-term infectious morbidity of infants exposed to maternal psoriasis during pregnancy were compared to unexposed infants, based on data from hospitalizations of offspring involving infectious morbidity up to the age of 18 years. Multiple gestations and fetuses with chromosomal or congenital anomalies were excluded from the study. Perinatal mortality cases (intra-uterine fetal demise, intrapartum or post-partum neonatal death up to 30 days) were excluded from the long-term analysis.
The study is based on two computerized data sets: the first is the perinatal database of the obstetric and gynecologic department in SUMC, which includes demographic, obstetrical and perinatal data of women who delivered in SUMC during the 30 years of the study period. The data was documented by obstetricians at admission and immediately following delivery. The second database is a pool of computerized pediatric hospitalization records in SUMC, that includes demographic data and medical diagnosis of hospitalized children, that were classified by the international classification of disease (ICD-9) used in the pediatric departments in SUMC. The two databases were cross-linked and merged based on maternal and offspring ID numbers. Prior to data archiving, medical secretaries review patients’ files to ensure accuracy of the diagnoses given.
All infectious-related encounters with the hospital were analyzed so that multiple infectious diagnoses could be given on different occasions to a single child. However, only the first hospitalization with an infectious morbidity was counted as an event. Infectious morbidities that were analyzed included viral, bacterial and fungal infections such as urinary tract infections, gastroenteritis, meningitis, sepsis and pneumonia.
Statistical analysis
Univariable analysis was performed to compare pregnancy complications and perinatal outcomes between the two study groups. Generalized estimated equation (GEE) models were used for the association between maternal psoriasis and pregnancy complications and perinatal outcomes, in which mothers in the cohort were entered as clusters to account for dependence between siblings, and to control for possible confounding factors such as fertility treatments, diabetes mellitus and hypertensive disorders. Cumulative incidence rates of long-term infectious morbidity were compared between the groups using Kaplan-Meier survival curves, with the log-rank test to determine significant differences. Cox proportional hazards model was used to compare infectious-associated hospitalizations risk among offspring born to mothers with and without psoriasis, adjusting to length of follow up and potential confounders. All analysis were performed using SPSS package 23rd. ed. as well as the STATA software 12th ed.
Results
During the study period 356,356 deliveries met the inclusion criteria, of which 240 (0.07%) occurred in women with psoriasis. Table 1 presents maternal characteristics, pregnancy complications and perinatal outcome of women with and without psoriasis. Women with psoriasis were older and more likely to undergo fertility treatments, and had higher rates of hypertensive disorders and diabetes mellitus. Likewise, the rate of cesarean deliveries (CD) was higher in the psoriasis group. Using GEE models, controlling for the variables found significant in the univariate analysis, including fertility treatments, diabetes mellitus and hypertensive disorders, each of these complications was found to be independently associated with psoriasis (Table 2). In another GEE model, controlling for the same variables above and non-reassuring fetal heart rate monitoring, maternal psoriasis was found to be an independent risk factor for CD (Table 2). Gestational age at birth and birthweight were comparable between groups. Also, psoriasis was not associated with other adverse perinatal outcome, as Apgar scores and perinatal mortality rates were comparable between groups (Table 1).
|
Characteristic |
Psoriasis (n=240) |
No psoriasis (n=356,116) |
Odds ratio (OR) |
95% Confidence interval (CI) |
P value |
|
Maternal age (years ± SD) |
30.1±5.3 |
28.2±5.8 |
|
|
<0.001 |
|
Fertility treatments a |
10 (4.2%) |
5,925 (1.7%) |
2.57 |
1.36-4.84 |
0.002 |
|
Recurrent pregnancy loss |
12 (5.0%) |
14,223 (4.0%) |
1.26 |
0.70-2.26 |
0.426 |
|
Hypertensive disorders b |
24 (10.0%) |
16,652 (4.7%) |
2.26 |
1.48-3.45 |
<0.001 |
|
Diabetes mellitus c |
23 (9.6%) |
16,987 (4.8%) |
2.12 |
1.38-3.25 |
<0.001 |
|
Gestational age (weeks±SD) |
38.8±1.7 |
39.0±1.9 |
|
|
0.124 |
|
Preterm <37 weeks |
21 (8.8%) |
24,490 (6.9%) |
1.29 |
0.83-2.03 |
0.253 |
|
Cesarean delivery |
65 (27.1%) |
49,857 (14.0%) |
2.28 |
1.72-3.03 |
<0.001 |
|
Birthweight (grams±SD) |
3,160±521 |
3,200±519 |
|
|
0.230 |
|
Low Apgar at 5 min (<7) |
1 (0.4%) |
2,029 (0.6%) |
0.73 |
0.10-5.19 |
0.750 |
|
Perinatal mortality |
2 (0.8%) |
2,822 (0.8%) |
1.05 |
0.26-4.23 |
0.943 |
|
a Including ovulation induction and in vitro fertilization (IVF) b Including pre-gestational hypertension, gestational hypertension and preeclampsia c Including pre-gestational and gestational diabetes mellitus |
|||||
|
Characteristic |
Adjusted* odds ratio (aOR) |
95%CI |
p value |
|
Fertility treatments |
2.18 |
1.25-3.79 |
0.006 |
|
Recurrent pregnancy loss |
1.12 |
0.56-2.25 |
0.737 |
|
Hypertensive disorders |
1.70 |
1.08-2.67 |
0.020 |
|
Diabetes mellitus |
1.83 |
1.11-3.01 |
0.017 |
|
Cesarean delivery |
1.60 ** |
1.19-2.12 |
0.001 |
|
* Adjusted for fertility treatments, diabetes mellitus and hypertensive disorders ** Adjusted for fertility treatments, diabetes mellitus, hypertensive disorders, and non-reassuring fetal heart rate |
|||
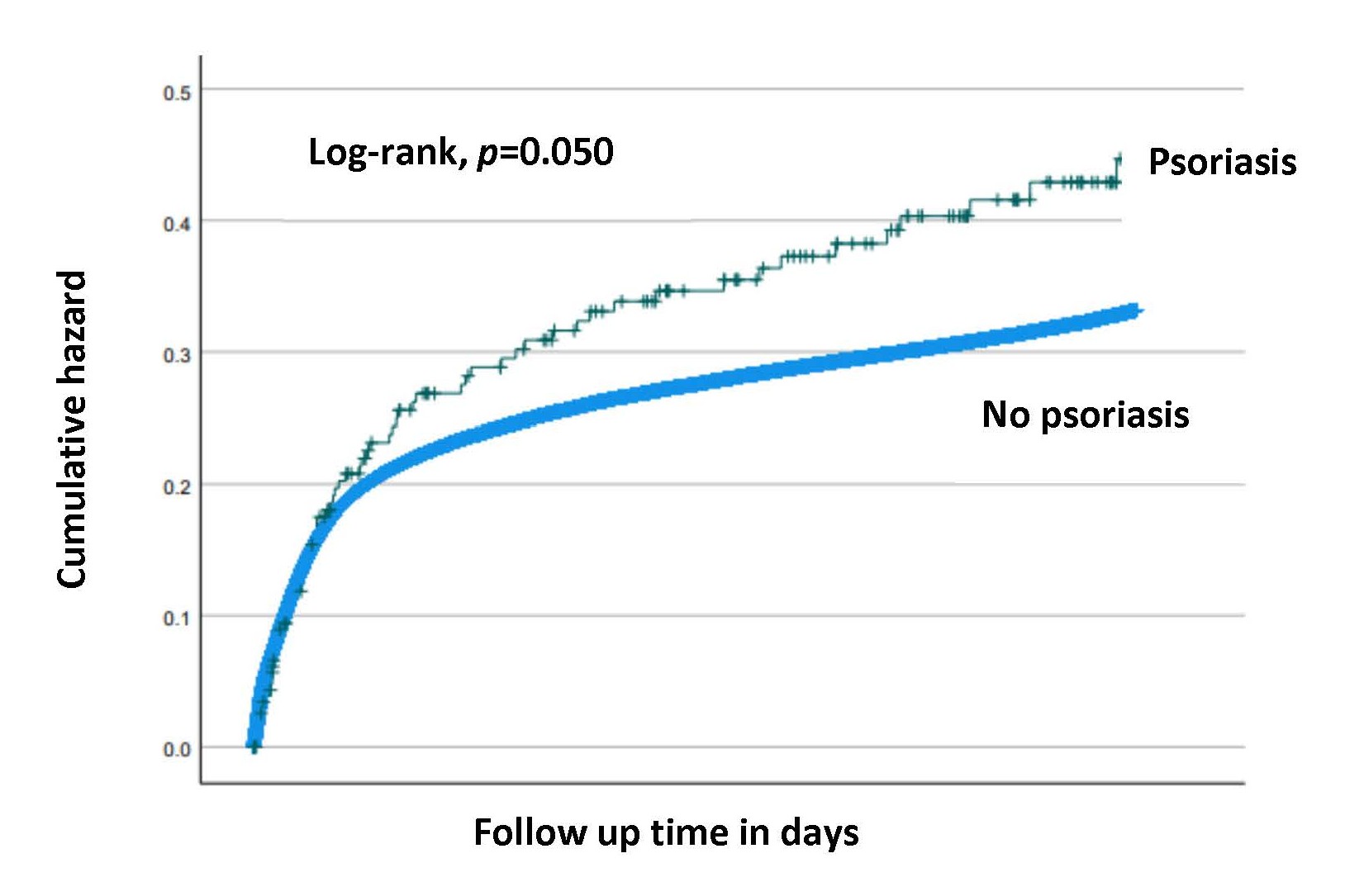
Table 3 summarizes selected long-term infectious morbidities that were investigated in this study. There was a higher rate of ear-nose-throat (ENT) and respiratory infections in children born to mothers with psoriasis as compared with unexposed children (9.6% vs 6.4%, p=0.046 and 18.3% vs 13.8%, p=0.043 respectively). The total long-term infectious-related hospitalization rate was higher for offspring to mothers with psoriasis as compared with unexposed offspring (30.4% vs 24.1%, p=0.022). Likewise, the Kaplan-Meier survival curve demonstrated higher cumulative incidence of long-term infectious morbidity among offspring born to women with psoriasis (Log-rank, p=0.050), (Figure 1). In a Cox proportional hazards model, controlling for birthweight, maternal psoriasis was independently associated with long-term infectious morbidity of the offspring (adjusted HR=1.26, 95% CI 1.01-1.58; p=0.049).
|
Offspring long-term infectious morbidity |
Psoriasis (n= 240) |
No psoriasis (n= 356,116) |
P value |
|
Ear, nose, throat (ENT) |
23 (9.6%) |
22,875 (6.4%) |
0.046 |
|
Gastrointestinal (GI) |
6 (2.5%) |
10,521 (3.0%) |
0.678 |
|
Respiratory |
44 (18.3%) |
49,205 (13.8%) |
0.043 |
|
Skin infections |
7 (2.9%) |
8,261 (2.3%) |
0.539 |
|
Viral infections |
11 (4.6%) |
11,485 (3.2%) |
0.234 |
|
Bacterial infection |
2 (0.8%) |
5,189 (1.5%) |
0.420 |
|
Total infectious hospitalizations |
73 (30.4%) |
85,719 (24.1%) |
0.022 |
Figure 1. Kaplan-Meier survival curve for the cumulative incidence of long-term infectious morbidity of the offspring born to mothers with and without psoriasis (Log-rank, p=0.050).
Discussion
In this population-based cohort study, we investigated the association between maternal psoriasis and a spectrum of perinatal outcomes and long-term infectious morbidity of the offspring. While our results regarding pregnancy and perinatal outcomes of women with psoriasis are consistent with the literature, to the best of our knowledge, this is the first study to demonstrate a long-term health effect for their offspring. Our study revealed an increased risk of long-term infectious morbidity for the offspring of mothers with psoriasis.
Psoriasis, a chronic autoimmune skin disorder, affects not only the skin but also has systemic implications, involving multiple systems [2]. The chronic systemic inflammatory state associated with psoriasis supposedly creates an undesirable physiological milieu that may have a determinantal effect on pregnancy, leading to complications that are associated with inflammatory conditions, such as reduced fecundability and the need for fertility treatments [17]. In our study, we also found that women with psoriasis were more likely to undergo fertility treatments. The observed increase in fertility treatments among women with psoriasis may also be associated to the underlying mechanisms of autoimmunity in this condition. Previous studies have demonstrated an association between the immune system and fertility, suggesting that autoimmune conditions negatively impact fertility and also contribute to adverse pregnancy outcomes [18]. Psoriasis as a chronic autoimmune disorder, involves dysregulation of the immune system, leading to an inflammatory response that may extend to reproductive processes, influencing fertility, and in pregnancy increasing the risk for diabetes mellitus and hypertensive disorders. In our population we found similar results, that strengthen those of previous studies, providing more evidence for the increased risk for these complications in women with psoriasis. The observed associations between psoriasis and hypertensive disorders, as well as diabetes mellitus, may be rooted in a combination of shared inflammatory pathways, genetic predispositions, and lifestyle factors. Some of these factors are difficult to control in retrospective studies, so we constructed a GEE model to account for dependence between siblings and neutralize some of the genetic and environmental effect. Using this model, we found that infertility, diabetes mellitus and hypertensive disorders were all independently associated with psoriasis.
In the conceptualization of this study, we hypothesized that the systemic inflammatory state of women with psoriasis during pregnancy may also lead to an altered immunological intra-uterine environment for the growing fetus, that later in life may render these children to be more susceptible to acquire infections. We found that some pediatric infectious morbidities such as ENT and respiratory infections were associated with maternal psoriasis and the total infectious-related hospitalization rate was also higher for these children. We also showed that when taking into account the length of follow-up, an increased cumulative risk for infectious morbidity was observed among the exposed children.
We believe the major strength of our study lies within our ability to have a long-term follow up on children born in our hospital, enabling us to cross-link information from the pregnancy course of the mothers to the long-term infectious morbidity of their offspring. SUMC is the sole tertiary hospital in the southern region of Israel, that provides health care to all the population of the area. This area is also characterized with positive migration rate, so most children born here are also expected to be hospitalized in SUMC if needed. This gives us a unique opportunity to follow these children through childhood and beyond. Unfortunately, this is also an important limitation of the study, as it only accounts for cases requiring hospitalization, and milder infectious cases that are treated in an ambulatory setting are not be accounted for. However, the increased risk for infectious morbidity seen in hospitalized children, that supposedly represents only more severe cases, may actually be an underestimation of the true infectious risk in exposed children.
The retrospective nature of this study, with the inherited limitations, also lacks information regarding the severity of the psoriatic disease or whether the affected women were treated for the condition. However, most women of reproductive age with psoriasis usually have a mild disease that, if treated, usually requires only topical treatments which are safe in pregnancy and probably has negligible, if any, effect on the growing fetus [19]. Those with a moderate or severe disease are also expected to encounter pregnancy complications [12] that may expose the fetus to additional risk. In this population-based, non-selective cohort, we assume a normal distribution of disease severity within the affected group of women.
In conclusion, our study allowed us to focus on a new perspective regarding reproductive age women with psoriasis, as it highlights long-term consequences of maternal psoriasis on offspring health, and demonstrates a higher cumulative risk for infectious morbidity in exposed children. Additional research on the long-term consequences of maternal psoriasis for the offspring should be encouraged and expanding our knowledge whether disease severity and treatments during pregnancy impact offspring health is warranted. This information can assist obstetricians and healthcare providers to better counsel pregnant women with psoriasis.
Disclosures
Conflict of interest
The authors report no conflict of interest.
Funding
This study was not funded.
Financial disclosures
No financial disclosures.
Dr. Moran Shahar wrote the first draft of the manuscript. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
References
2. Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. Global Psoriasis Atlas. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020 May 28;369:m1590.
3. Swanbeck G, Inerot A, Martinsson T, Enerbäck C, Enlund F, Samuelsson L, et al. Genetic counselling in psoriasis: empirical data on psoriasis among first-degree relatives of 3095 psoriatic probands. Br J Dermatol. 1997 Dec;137(6):939-42
4. Levine D, Gottlieb A. Evaluation and management of psoriasis: an internist's guide. Med Clin North Am. 2009 Nov;93(6):1291-303.
5. Tauscher AE, Fleischer AB Jr, Phelps KC, Feldman SR. Psoriasis and pregnancy. J Cutan Med Surg. 2002 Nov-Dec;6(6):561-70.
6. Yang YW, Chen CS, Chen YH, Lin HC. Psoriasis and pregnancy outcomes: a nationwide population-based study. J Am Acad Dermatol. 2011 Jan;64(1):71-7.
7. Bröms G, Haerskjold A, Granath F, Kieler H, Pedersen L, Berglind IA. Effect of Maternal Psoriasis on Pregnancy and Birth Outcomes: A Population-based Cohort Study from Denmark and Sweden. Acta Derm Venereol. 2018 Aug 29;98(8):728-34.
8. Lambe M, Bergstrom AV, Johansson ALV, Weibull CE. Reproductive patterns and maternal and pregnancy outcomes in women with psoriasis-A population-based study. J Am Acad Dermatol. 2020 May;82(5):1109-16.
9. Johansen CB, Egeberg A, Jimenez-Solem E, Skov L, Thomsen SF. Psoriasis and adverse pregnancy outcomes: A nationwide case-control study in 491,274 women in Denmark. JAAD Int. 2022 Apr 19;7:146-55.
10. Horn EJ, Chambers CD, Menter A, Kimball AB; International Psoriasis Council. Pregnancy outcomes in psoriasis: why do we know so little? J Am Acad Dermatol. 2009 Aug;61(2):e5-8.
11. Bobotsis R, Gulliver WP, Monaghan K, Lynde C, Fleming P. Psoriasis and adverse pregnancy outcomes: a systematic review of observational studies. Br J Dermatol. 2016 Sep;175(3):464-72.
12. Ben-David G, Sheiner E, Hallak M, Levy A. Pregnancy outcome in women with psoriasis. J Reprod Med. 2008 Mar;53(3):183-7.
13. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL-23/IL-17 Signaling Pathway and the Treatment of Psoriasis. J Immunol. 2018 Sep 15;201(6):1605-13.
14. Yan D, Gudjonsson JE, Le S, Maverakis E, Plazyo O, Ritchlin C, et al. New Frontiers in Psoriatic Disease Research, Part I: Genetics, Environmental Triggers, Immunology, Pathophysiology, and Precision Medicine. J Invest Dermatol. 2021 Sep;141(9):2112-22.e3.
15. Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010 Aug;116(2 Pt 1):393-401.
16. Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011 Mar;1221(1):80-7.
17. Bucur Ș, Savu AP, Stănescu AMA, Șerban ED, Nicolescu AC, Constantin T, et al. Oversight and Management of Women with Psoriasis in Childbearing Age. Medicina (Kaunas). 2022 Jun 9;58(6):780.
18. De Simone C, Caldarola G, Moretta G, Piscitelli L, Ricceri F, Prignano F. Moderate-to-severe psoriasis and pregnancy: impact on fertility, pregnancy outcome and treatment perspectives. G Ital Dermatol Venereol. 2019 Jun;154(3):305-14.
19. Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA. 2020 May 19;323(19):1945-60.