Abstract
The adaptive immune system depends on the efficient response of lymphocytes, protecting the organism from infection or malignant diseases. T-cell immunodeficiency, innate or acquired, put the patient at risk for developing opportunistic infections and cancers. We previously described a novel approach to overcome the major limitations of T-cell immunotherapy and hematopoietic stem cell transplant (HSCT) by transplanting human T-lymphoid progenitors (HTLP), with the aim to achieve shortened immune reconstitution and fully functional naïve T-cell repertoire in immunodeficient or immunocompromised patients. By complementing our DL4-based cell culture with TNFα we developed and scaledup clinical strategies involving hematopoietic stem and progenitor cells (HSPC) differentiated into T-lymphoid progenitors. We discuss here recent advances made in the characterization of different cell sources used as starting materials for T-lymphoid progenitors manufacturing, as well as gene modification of these cells, highlighting new perspectives for the development of therapeutical strategies.
Keywords
Clinical immunology, Immune reconstitution, Immunodeficiency disorders, Immunotherapy, Stem cell, T-cell progenitors, Thymus, Transplantation immunology
Introduction
Adaptive immunity relies on an efficient lymphocyte response, enabling an organism to defend itself against infections or malignancies [1]. Lymphocyte repertoire may be impacted by various factors, including conditions such as primary immune deficiencies or Acquired Immunodeficiency Syndrome (AIDS), or by treatments such as chemotherapy [2]. The development of therapeutic strategies to restore and boost adaptive immunity is imperative for patients suffering from immune deficiencies.
As described by Moirangthem and colleagues [3], immunotherapeutic human T-lymphoid progenitors (HTLP) have demonstrated efficient thymus engraftment in NOD.Cg- Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice and could play an important role in conferring a shortened immune recovery following adoptive transfer into immunodeficient patients. Only a rare subset of blood circulating T-lymphoid progenitors is capable of seeding the thymus, hampering their use as a cell therapy product source. Thus, their ex vivo generation at a large scale is of great interest. A 7-day feeder-cell free culture process allows their GMP-grade generation from hematopoietic stem and progenitor cells (HSPCs) [3].
First described for its key role in the inflammatory and antiviral responses, Tumor Necrosis Factor α (TNFα), has been linked with a significant effect on HSPCs when cultured in a Notch receptor-pressuring environment, both in terms of cell expansion and T-lymphoid differentiation [4]. By taking advantage of this behavior, we developed and scaledup clinical strategies involving HSPCs differentiated into T-lymphoid progenitors.
Such cell-based therapy could not only considerably improve allogeneic Hematopoietic Stem Cell Transplantation (allo- HSCT) prognosis [5], but also constitute an interesting platform of engineered cell products for cancer immunotherapy. Here, we propose a commentary on the use of different cell sources as starting materials for T-lymphoid progenitors manufacturing, namely CD34+ cells isolated from mobilized peripheral blood (mPB) or from Cord Blood (CB) units.
We lead a thorough analysis of cell therapy products obtained from both types of cell sources using a complementary approach relying on single-cell transcriptomic and on mass cytometry. Thus, we are able to highlight the similarity of T lymphoid progenitor cells’ phenotypes and underline some source-related specificities.
Based on the proof of concept realized by Moirangthem and colleagues to confirm the compatibility of T-lymphoid progenitors manufacturing and gene modification, we demonstrate here the possibility to express a CAR construct through lentiviral transduction, allowing the efficient production of high CAR-expressing human T-lymphoid progenitors (CAR-HTLP). Altogether, the present data pave the way for the development of new immunotherapies relevant not only for transplanted patients but also for oncology and beyond.
Clinical Interests of mPB cells and CB cells
Our TNFα-supplemented immobilized DL-4 culture system was shown to generate high numbers of HTLPs from CB or mPB HSPCs. Our interest was focused on the differences existing between HSPCs isolated from these two sources.
For HSCT, mPB stem cells have progressively replaced bone marrow as a cell source as they don’t require invasive aspiration and enable a more rapid neutrophil and platelet recovery as well as faster immune reconstitution [6]. Peripheral blood represented 70% of autologous transplants in 2018 [7]. Collection of peripheral blood HSPCs is still time-consuming and expensive, requiring several days of mobilization, followed by often multiple apheresis sessions. Innovations in HSPC harvest tend to reduce the side effects of these protocols. G-CSF mobilization is progressively complemented or switched with other mobilization protocols, such as CXCR4 antagonists or VLA4 antagonists [8,9].
HSPCs isolated from CB have the major advantage of being rapidly available and requiring a markedly reduced stringency of HLA-matching compared with adult unrelated PBMC- or BM-isolated cells [10]. The use of CB has dramatically extended allograft access to racial and ethnic minorities [11]. Although CB unit use has been initially limited for the treatment of adult patients because of the low number of cells for transplantation, the combination of double CB units has enabled an increase in cell dose [12,13]. Recently, Yabe et al. demonstrated that even a low number of CB isolated CD34+ cells, as low as 75,000 cells/ kg, could lead to satisfactory engraftment [14].
Treatments relying on the use of CD34+ HSPCs should explore differences existing in these two cell sources and their impact on cell cultures to produce cell and gene therapies.
Molecular and Cellular Comparison of mPB and CB HSPCs
Based on phenotypical characterization led by several teams [12-15], Wisniewski and colleagues carried out a comparison of expressed hematopoietic cell surface antigens by HSPCs isolated from CB and mPB [19]. Focusing on Lin-CD34+CD38- CD90+CD45RA-, they demonstrated that CB cells more frequently express HLA-DR compared with mPB cells (75 ± 3.6% vs 40 ± 18%), and similarly for expression of KIT (50 ± 7% vs 10 ± 4%). Conversely, mPB cells more frequently express IL- 3R compared with CB cells (43 ± 14% vs 19 ± 4%). Furthermore, CB HSPCs were shown to contain more lymphoid progenitors than their adult counterparts, which exhibited a myeloid bias. To further evaluate differences in cell behavior, Forte and colleagues demonstrated the specific impact of different proinflammatory cytokines on 14 samples of mPB HSPCs and 14 samples of CB HSPCs, suggesting that CD34+ cells from CB were more actively responsive to inflammatory cues than their mPB counterparts [20].
Progress made in single-cell transcriptomics created new insights into the comparison between CB and mPB HSPCs. In particular, Dong and colleagues performed scRNAseq of fresh and cultured CD34+ cells from CB and mPB, enabling them to delineate distinct stemness-related genes associated with cell sources and culture conditions for both sources [21].
Generation of T Lymphoid Progenitors from mPB and CB HSPCs
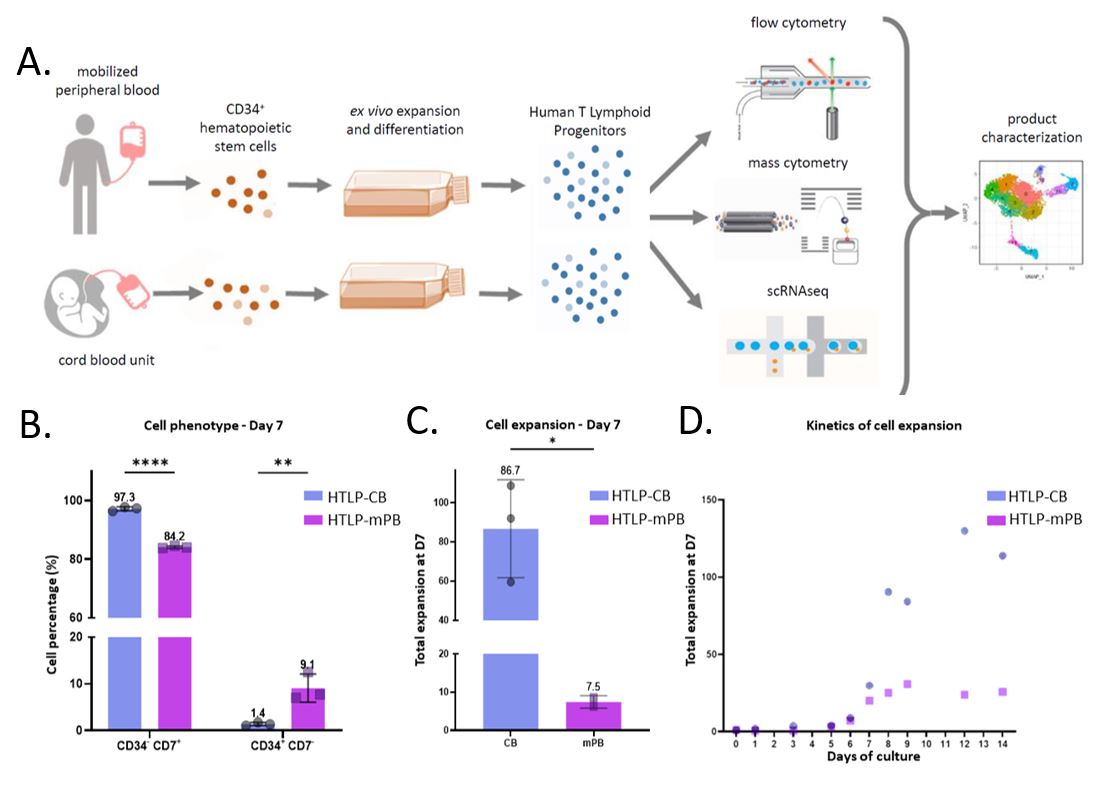
To further evaluate the possible impact of the HSPC source as starting material of Human T lymphoid progenitors’ generation, we initiated a comparative study based on 3 samples obtained from differentiated CB cells (HTLP-CB) and 3 other samples obtained from differentiated mPB cells (HTLPmPB). All samples were analyzed through a complementary approach involving mass cytometry and scRNAseq, in addition to classical flow cytometry (Figure 1A).
Extending the observation of specific cell expansion patterns by [3], HTLP obtained from CB CD34+ HSPCs were associated with a higher CD7+ subset percentage (Figure 1B). The remaining CD34+CD7- fraction was larger in samples originating from mPB. We confirmed superior CB cell expansion over mPB in the DL- 4-based culture supplemented with TNFα. CB CD34+ HSPCs demonstrated on average an 11.5-fold higher expansion than the mPB counterparts (Figure 1C). One CD34+ cell from CB was able to give rise to 86.7 CD7+ cells (vs 7.5 CD7+ cells for mPB CD34+ cells). Superior cell expansion of CB HSPCs compared with mPB HSPCs was also confirmed through culture extension up to 14 days after seeding (Figure 1D).
Single Cell Transcriptomic Analysis of HTLP Obtained from mPB and CB HSPCs
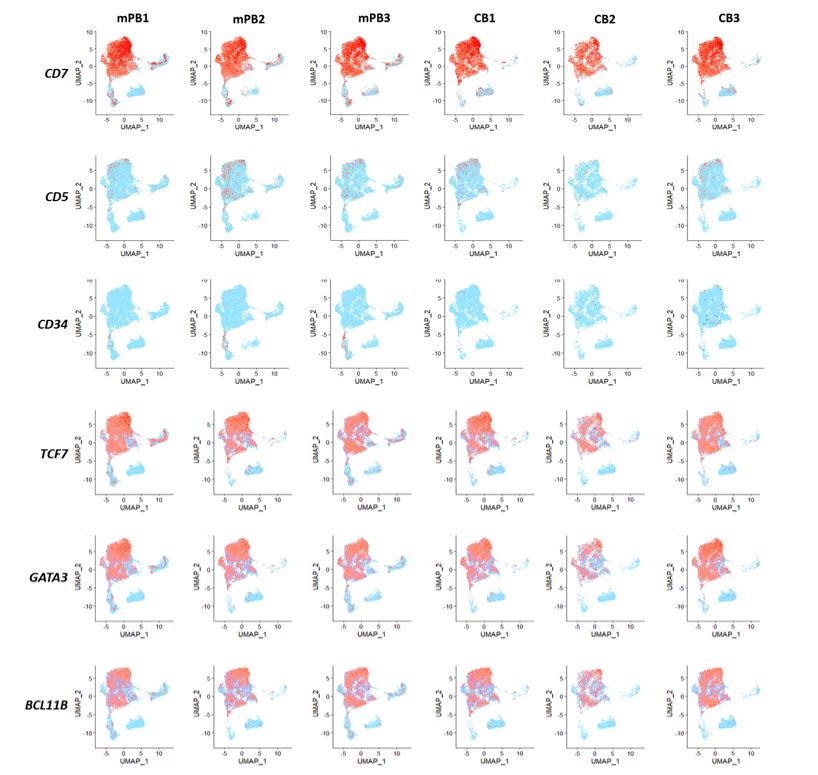
The vast majority of HTLP were associated with the expression of transcription factors that are usually identified as transcriptional drivers of the T-cell lineage program, GATA3, TCF7, and BCL11B (Figure 2). TCF7 is a direct Notch target [22], consistent with sustained activation dependent on DL-4 of NOTCH1 activation exercised during cell culture. Although our analysis demonstrated overall homogeneity of cell populations, specifically for T cell lineage markers, we highlighted some specificities of HTLP-CB and HTLP-mPB.
Interestingly, IGF2BP1 and IGF2BP3, coding for insulin-like growth factor 2 mRNA-binding proteins putatively associated with survival and proliferation [23], appeared to be signatures of CB samples (51.2% (CB) vs 0.6% (mPB) and 24.5% (CB) vs 0.1% (mPB) of total sample respectively). Further investigation is required to link the expression of these growth factors with superior cell expansion from CB HSPCs.
Single Cell Mass Cytometry Analysis of HTLP Obtained from mPB and CB HSPCs
To complete the transcriptomic approach for comparison of the two cell products, we employed mass cytometry to delineate specific cell populations and associated markers, confronting specific transcript expressions detailed earlier.
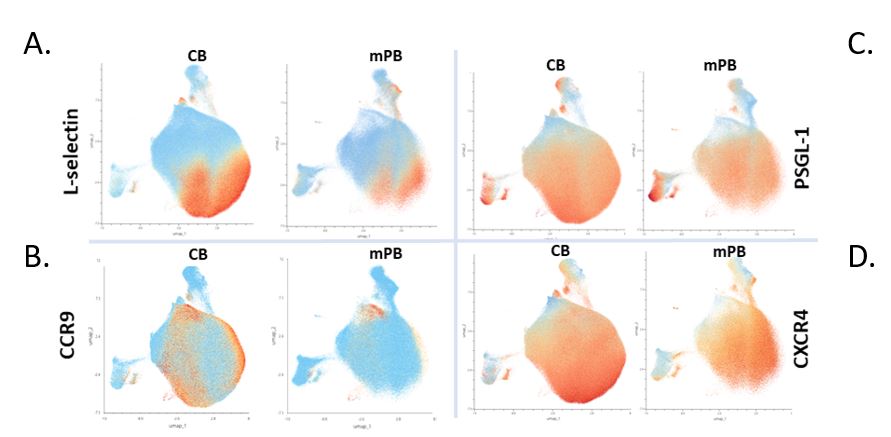
Mass cytometry analysis highlighted the overall homogeneity of markers expressed at the cell membrane of both CB and mPB-derived cells. CCR9 expression was found to be higher in HTLP-CB (43.5% (CB) vs 9.4% (mPB) of total sample) (Figure 3B).
In cell products obtained from both sources, we also highlight the high expression levels of the adhesion molecule PSGL-1 and the chemokine receptor CXCR4 (Figure 3C-3D), alongside a significant portion of cells expressing L-selectin (CD62L) (Figure 3A). These proteins may balance the impact of distinct levels of CCR9 expression among cell products. In the murine model, Calderon and Boehm demonstrated the cooperative use of multiple chemokine receptors enabling homing of hematopoietic progenitors into the embryonic thymus [24]. Among those, CXCR4 was found in the majority of HTLP differentiated from both CB and mPB HSPCs. Furthermore, the expression of PSGL-1, as a complement of L-selectin, could be involved in the efficient thymus homing of HTLP cells [25,26]. Mechanisms of thymus homing by lymphoid progenitors are still to be clarified in humans and thus far have been predicted from results obtained in murine models. Identification of which “facilitating” factors among cell phenotypes are associated with a better outcome for transplanted patients in clinical trials may shed light on unknown elements involved in lymphoid progenitors’ behaviors in the organism.
Gene Modification of Human T Lymphoid Progenitors and Clinical Perspectives
CD19 CAR-T cells have proven to be highly effective for treating several types of B-cell malignancies such as acute B- lymphoblastic leukemia (B-ALL) and B- Non-Hodgkin Lymphoma (B-NHL). However, disease relapse remains still an issue with progression-free survival rates currently below 50% [24-26], and event-free survival 3 years post-treatment at only 16.1% [30] for relapsed/refractory B cell malignancies. In addition, side effects such as cytokine release syndrome (CRS) and immune-effector cell-associated neurotoxicity syndrome (ICANS), still need to be considered without impacting the efficacy of CAR-T cells [31,32].
Several reasons account for the failure to achieve durable complete remissions among which early loss, failure of expansion or exhaustion of CAR-T cells, as well as hampered reconstitution of the naïve T cell compartment, are important factors [33]. The delayed CD4+ T cell recovery with less than 50% of patients having >200 cells per μL CD4+ T cells at 1 year following CAR-T cell therapy [34-36] represents another significant challenge, as immunodeficiency not only increases the chances of relapse but also leads to a significant rate of infectious complications [30,34-36].
Finally, the cost and complexity of manufacturing CAR-T cells are significant barriers to their general use [37]. In this context, an allogeneic CAR-HTLP strategy could bypass some of those main challenges, such as immediate availability, efficient expansion, and sustained anti-tumor response. This strategy could accelerate the reconstitution of a polyclonal and naïve, host-thymus-educated and tolerized, mature T cell compartment, thus providing durable and efficient antitumor responses without the risk for graft versus host disease (GvHD) [38]. In addition, naïve status and activation at the physiological level of HTLP-derived T-cells may impact cell fitness and potentially reduce the incidence of CRS and ICANS [39].
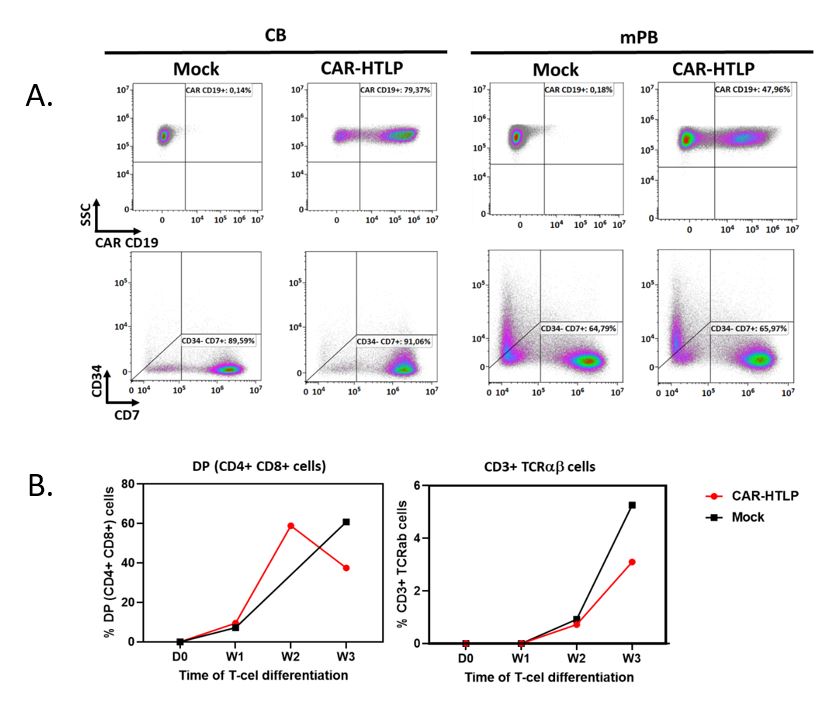
Recently we showed the feasibility of gene modification of our HTLPs [3]. Here we further show that our HTLP production platform is compatible with using a lentiviral vector expressing a CD19 CAR molecule (19BBz). CB or mPBderived HSPCs were subjected to a 7-day HTLP production process with an additional step of lentiviral transduction. The day-7 product was analyzed by flow cytometry for the expression of CD19 CAR molecule at cell surface. The result showed a high expression level of the CAR molecule in both CB and mPB derived products without altering the differentiation of stem cells toward HTLP (CD7+CD34-) (Figure 4A). Further differentiation of the day-7 CB-derived CAR-HTLP on the OP9-hDLL1 stromal cells demonstrated their efficient differentiation into double positive (CD4+CD8+) and CD3+ mature T-cells as compared to non-transduced HTLP (Figure 4B). This observation, as well as the high efficacy of our HTLP production platform to manufacture a large number of genemodified cells, offers a new opportunity to develop new immunotherapy cell products with the potential of improving the therapeutic index of current CAR-T cell therapies.
Concluding Remarks
Altogether, our work indicates that a T-lymphoid-based cell product has the strong potential not only in the field of immune reconstitution after transplant but also in the oncoimmunotherapy field, especially in an allogeneic setting. Compatible with both CB and mPB-derived HSPCs with high production efficacy, this platform can ensure widespread therapy accessibility. A pool of naïve, polyclonal, thymuseducated, and thus tolerant products can overcome certain hurdles of T-cell-based and HSPC-based cell and gene therapy as discussed above for leukemia. In particular, the homogeneity of the final cell product despite the different initial material sources, as we demonstrated, can reduce although not eliminate patient-to-patient variability, allowing for better comparison of outcomes in different clinical trials.
Besides these advantages, the possibility of producing a large number of gene-modified cells offers an opportunity to develop new immunotherapy cell products with the potential of improving the safety and efficacy of current CAR-T cell therapies. However, it remains to be investigated, in preclinical settings, whether the CAR-HTLP product is superior to approved mature CAR-T cell products.
Material and Methods
Transduction and differentiation of CD34+ HSPCs, as well as OP9-hDL1 culture and flow cytometry, were performed according to methods described by Moirangthem et al. [3]. ScRNAseq libraries were generated with Chromium Next GEM Single Cell 3’ GEM, Library & Gel Bead Kit v3.1, following the manufacturer’s instructions. Transcriptomic datasets were analyzed using R [40]. Mass cytometry analysis was performed on the Helios mass cytometer and CyTOF software version 6.7.1014 (Fluidigm, Inc Canada) at the Cytometry Platform of La Pitié-Salpétrière Hospital (CyPS). Mass cytometry standard file analysis was done using OMIQ data analysis software (www.omiq.ai). Statistical analysis of results was conducted using GraphPad Prism 9.4.0.673.
Acknowledgment
We thank Jérôme Larghero, Audrey Cras, and Cord Blood Bank of Saint Louis Hospital, Assistance-Publique Hôpitaux de Paris, for providing CB samples. We thank Aurélien Corneau and La Pitié-Salpêtrière Cytometry Platform (CyPS) for Mass Cytometry analysis. We thank Ghent University Flow Cytometry Core facility and the Vlaams Instituut Voor Biotechnologie single cell core facility, Mickael Menager, Imagine Institute’s bioinformatic platform, and the LabTech Singel-CEll@Imagine for taking part in the discussion for single-cell transcriptomic analysis.
Author Contributions
PG and TSS wrote the manuscript. PG, RDM, PR, and HS conducted cell culture experiments and analyzed the data. FC and ML took part to the scRNAseq experiments and their analysis. TT discussed the data and reviewed the manuscript for critical content. TSS, IA, and ON designed and supervised the overall research and reviewed the manuscript for critical content.
Funding Statement
This work was supported by the French Institut National de la Santé et de la Recherche Médicale (INSERM), the European Union Seventh Framework Program under grant agreement No. 269037 and No. 261387, the European Union’s Horizon 2020 research and innovation program under grant agreement No. 666908 and No. 811171, and state funding from the Agence Nationale de la Recherche under the “Investissement d’avenir” program (ANR-10-IAHU01), the Paris Île-de-France Region under the “DIM Thérapie génique” initiative, and French Ministry of Health under the 2015 hospital clinical research program. Assistance Publique-Hôpitaux de Paris is sponsoring two clinical trials (NCT04707300 and NCT03879876); Smart Immune is sponsoring the NCT04959903 clinical trial. The Human Lymphohematopoiesis Laboratory and Smart Immune have contracted an Industrial Agreement of Training through Research (CIFRE #2019/0690) granted by French National Association for Research and Technology (ANRT).
Conflict of Interest
FC, ML and TT did not declare any conflict of interest. PG, PR, HS, ON, and TSS work at Smart Immune. IA is a co-founder and holds equity in Smart Immune. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
2. Edgar JD. T cell immunodeficiency. Journal of Clinical Pathology. 2008 Sep 1;61(9):988-93.
3. Moirangthem RD, Ma K, Lizot S, Cordesse A, Olivré J, de Chappedelaine C, et al. A DL-4-and TNFα-based culture system to generate high numbers of nonmodified or genetically modified immunotherapeutic human T-lymphoid progenitors. Cellular & Molecular Immunology. 2021 Jul;18(7):1662-76.
4. dos Santos Schiavinato JL, Oliveira LH, Araujo AG, Orellana MD, de Palma PV, Covas DT, et al. TNF-alpha and Notch signaling regulates the expression of HOXB4 and GATA3 during early T lymphopoiesis. In Vitro Cellular & Developmental Biology-Animal. 2016 Oct;52(9):920- 34.
5. Gaudeaux P, Moirangthem RD, Bauquet A, Simons L, Joshi A, Cavazzana M, et al. T-Cell Progenitors As A New Immunotherapy to Bypass Hurdles of Allogeneic Hematopoietic Stem Cell Transplantation. Frontiers in Immunology. 2022;13:10.
6. Arai S, Klingemann HG. Hematopoietic stem cell transplantation: bone marrow vs. mobilized peripheral blood. Archives of Medical Research. 2003 Nov 1;34(6):545-53.
7. D’Souza A, Fretham C. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2018. Center for International Blood and Marrow Transplant Research (CIBMTR). 2019 Aug 25;30:2019.
8. Karpova D, Rettig MP, DiPersio JF. Mobilized peripheral blood: an updated perspective. F1000Research. 2019 Dec 20;8:F1000 Faculty Rev-2125.
9. Kraft DL, Walck ER, Carrasco A, Crocker MD, Song L, Long MG, et al. The MarrowMiner: a novel minimally invasive and effective device for the harvest of bone marrow. Biology of Blood and Marrow Transplantation. 2020 Feb 1;26(2):219-29.
10. Dehn J, Spellman S, Hurley CK, Shaw BE, Barker JN, Burns LJ, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/ CIBMTR. Blood, The Journal of the American Society of Hematology. 2019 Sep 19;134(12):924-34.
11. Barker JN, Byam CE, Kernan NA, Lee SS, Hawke RM, Doshi KA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biology of Blood and Marrow Transplantation. 2010 Nov 1;16(11):1541-8.
12. Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005 Feb 1;105(3):1343-7.
13. Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood, The Journal of the American Society of Hematology. 2013 Jan 31;121(5):752-8.
14. Yabe H, Tabuchi K, Uchida N, Takahashi S, Onishi Y, Aotsuka N, et al. Could the minimum number of haematopoietic stem cells to obtain engraftment exist in unrelated, single cord blood transplantation?. British Journal of Haematology. 2020 Apr;189(2):e56-60.
15. Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. The Journal of Experimental Medicine. 2005 Apr 18;201(8):1307-18.
16. Broxmeyer HE, Srour E, Orschell C, Ingram DA, Cooper S, Plett PA, et al. Cord blood stem and progenitor cells. Methods in Enzymology. 2006 Jan 1;419:439-73.
17. Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. The American Journal of Pathology. 2006 Aug 1;169(2):338-46.
18. Majeti R, Park CY, Weissman IL. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007 Dec 13;1(6):635-45.
19. Wisniewski D, Affer M, Willshire J, Clarkson B. Further phenotypic characterization of the primitive lineage− CD34+ CD38− CD90+ CD45RA− hematopoietic stem cell/progenitor cell sub-population isolated from cord blood, mobilized peripheral blood and patients with chronic myelogenous leukemia. Blood Cancer Journal. 2011 Sep;1(9):e36.
20. Forte D, Sollazzo D, Barone M, Allegri M, di Martella Orsi A, Romano M,. Mobilized Peripheral Blood versus Cord Blood: Insight into the Distinct Role of Proinflammatory Cytokines on Survival, Clonogenic Ability, and Migration of CD34+ Cells. Mediators of inflammation. 2018 Oct;2018. Jul 4;2018:e5974613.
21. Dong G, Xu X, Li Y, Ouyang W, Zhao W, Gu Y, Li J, Liu T, Zeng X, Zou H, Wang S. Functional stemness-related genes revealed by single-cell profiling of naïve and stimulated human CD34+ cells from CB and mPB. BioRxiv. 2022 Jan 1.
22. Weber BN, Chi AW, Chavez A, Yashiro-Ohtani Y, Yang Q, Shestova O, et al. A critical role for TCF-1 in T-lineage specification and differentiation. Nature. 2011 Aug;476(7358):63-68.
23. Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004 Apr 1;103(7):2513-21.
24. Calderón L, Boehm T. Three chemokine receptors cooperatively regulate homing of hematopoietic progenitors to the embryonic mouse thymus. Proceedings of the National Academy of Sciences. 2011 May 3;108(18):7517-22.
25. Rossi F, Corbel SY, Merzaban JS, Carlow DA, Gossens K, Duenas J, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nature Immunology. 2005 Jun;6(6):626-34.
26. Kohn LA, Hao QL, Sasidharan R, Parekh C, Ge S, Zhu Y, et al. Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of L-selectin. Nature Immunology. 2012 Oct;13(10):963-71.
27. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. New England Journal of Medicine. 2017 Dec 28;377(26):2531-44.
28. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New England Journal of Medicine. 2018 Feb 1;378(5):439-48.
29. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a singlearm, multicentre, phase 1–2 trial. The Lancet Oncology. 2019 Jan 1;20(1):31-42.
30. Wudhikarn K, Flynn JR, Rivière I, Gönen M, Wang X, Senechal B, et al. Interventions and outcomes of adult patients with B-ALL progressing after CD19 chimeric antigen receptor T-cell therapy. Blood. 2021 Aug 19;138(7):531-43.
31. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Reviews. 2019 Mar 1;34:45-55.
32. Hirayama AV, Turtle CJ. Toxicities of CD19 CAR‐T cell immunotherapy. American Journal of Hematology. 2019 May;94(S1):S42-9.
33. Byrne M, Oluwole OO, Savani B, Majhail NS, Hill BT, Locke FL. Understanding and managing large B cell lymphoma relapses after chimeric antigen receptor T cell therapy. Biology of Blood and Marrow Transplantation. 2019 Nov 1;25(11):e344-51.
34. Baird JH, Epstein DJ, Tamaresis JS, Ehlinger Z, Spiegel JY, Craig J, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Advances. 2021 Jan 12;5(1):143-55.
35. Logue JM, Zucchetti E, Bachmeier CA, Krivenko GS, Larson V, Ninh D, et al. Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. Haematologica. 2021 Apr 1;106(4):978-86.
36. Strati P, Varma A, Adkins S, Nastoupil LJ, Westin JR, Hagemeister FB, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica. 2021 Oct 10;106(10):2667-72.
37. Lopes AG, Noel R, Sinclair A. Cost analysis of vein-to-vein CAR T-cell therapy: automated manufacturing and supply chain. Cell and Gene Therapy Insights. 2020;6(3):487-510.
38. Zakrzewski JL, Goldberg GL, Smith OM, Van den Brink MR. Enhancing T cell reconstitution after hematopoietic stem cell transplantation: a brief update of the latest trends. Blood Cells, Molecules, and Diseases. 2008 Jan 1;40(1):44-7.
39. Pietrobon V, Todd LA, Goswami A, Stefanson O, Yang Z, Marincola F. Improving CAR T-Cell Persistence. International Journal of Molecular Sciences. 2021 Oct 7;22(19):10828.
40. Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www. R-project. org/. 2013 Jun.