Abstract
Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), known to cause Coronavirus disease 2019 (COVID-19), is a new strain of Coronavirus which was first reported in the Wuhan city of China in December 2019. COVID-19 has shown to increase the risk for thrombotic or thromboembolic events by altering the prothrombotic factor levels and direct endothelial cell invasion. Deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infarction (MI), and stroke have been more commonly reported, and incidence of these events is noted to be higher in patients with severe infection. Here we describe a case of a 38-year-old male with mild, self-limiting COVID-19 who developed an isolated renal infarction. He tested positive for Lupus Anticoagulant (LAC). The role of autoantibodies like LAC as a risk factor for thrombotic complications is a topic of debate and needs to be studied further. This uncommon case warrants identification of patients who might be at a higher risk for thrombotic events even with milder forms of COVID-19, and following and managing such patients closely.
Keywords
Coronavirus/COVID-19, Infarction, Kidney diseases, Lupus coagulation inhibitor, Thrombosis/thromboembolism
Essentials
• Covid-19 associated hypercoagulable state is predominantly observed with severe infections.
• Isolated arterial infarcts with Covid-19 are seldom reported.
• Risk factors for thrombophilia and its management in mild COVID-19 needs further studies.
• We report a case of an isolated kidney infarction in a young patient in mild Covid-19 with lupus anticoagulant positivity.
Introduction
The incidence of thromboembolic events in COVID-19 is reportedly higher in intensive care unit (ICU) patients (29.4%) as compared to non-ICU patients (11.5%) [1], while data on thromboembolic events in non-hospitalized, self-limiting COVID-19 is unavailable. Renal infarction with COVID-19 has only been reported in three patients who had severe infection, multiple comorbidities, and/or immuno-compromised state post kidney transplantation [2,3]. Since the onset of the SARS-CoV-2 pandemic, guidelines for the prophylaxis and treatment of COVID- 19-related hypercoagulability have only been focused on hospitalized patients. Concurrently, risk factors and management guidelines for thromboembolic events in mild COVID-19 have remained unidentified. In this report, we describe a case of a 38-year-old male–with no medical history pertinent to hypercoagulability–who developed an isolated renal infarction one week after being diagnosed with mild COVID-19.
Case
38-year-old male developed a mild, nonproductive cough, loose stools, and sore throat after he was exposed to a patient with COVID-19. He was afebrile and presented without dyspnea. Subsequent qualitative polymerase chain reaction test for SARS-CoV-2 was positive and he was discharged home with instructions for self-isolation and emergency follow-up. His medical history included nephrolithiasis and arthralgias. He did have equivocal antinuclear antibody (ANA) positivity (1:160) from 2 years ago. He did not have any diagnosed rheumatological disease or other conditions predisposing him to hypercoagulability, as well as no history of atrial fibrillation or significant atherosclerotic risk factors. One week following diagnosis of COVID-19, he presented with right flank pain radiating anteriorly. He did not have any fever, cough, dyspnea, nausea, or diarrhea. A non-contrast computed tomography (CT) scan showed non-obstructive bilateral kidney stones and a urinalysis was unremarkable, so he was discharged home from the emergency department (ED) with analgesics.
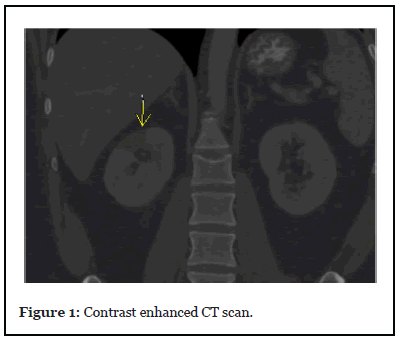
However, he returned later that day with the same complaints, at which point a contrast enhanced CT scan was done that showed a new, wedge-shaped region of hypoattenuation within the upper pole of the right kidney, thereby suggestive of an infarct (yellow arrow in the image). He was admitted for management of kidney infarction. His laboratory findings revealed hemoglobin of 14.5 g/dl, white blood cell counts 9600 UL, platelet count 258 K/UL, baseline renal function with a serum creatinine of 0.92 mg/dl, blood urea nitrogen 13.5 mg/ dl, and activated partial thromboplastin time (aPTT) 32.4 seconds, prothrombin time 12.0 seconds, International normalized ratio 1.1, D-Dimer <150 ng/ml (peaked to 250 ng/ml during the stay), lactate dehydrogenase (LDH) 299 U/L, ferritin 224 ng/ml, C-reactive protein 4.3 mg/dl (peaked to 11.9), procalcitonin level of 0.08 ng/mL. He was treated with analgesics and intravenous heparin. Renal function remained stable and the patient did not develop any respiratory symptoms throughout the admission. After 4 days, he was discharged in stable condition on apixaban, with instructions for follow-up with his primary care physician (PCP). His hypercoagulability workup was negative for Factor V Leiden mutation, Antithrombin III activity, Homocysteine levels, Protein C, and S activity and his repeat ANA was negative as well but he tested positive for Lupus Anticoagulant (LAC), with a dilute Russell viper venom time of 79 secs and negative for cardiolipin antibody IgM and IgG. His transthoracic echocardiogram was negative for thrombus, and his repeat ANA was <1:80.
Discussion
Renal infarction is rare with an incidence rate of 0.007% [4,5]. Its most common etiologies are cardioembolic disease (55.7%), renal artery injury (7.5%), hypercoagulable state (6.6%), and idiopathic (30.1%), and commonly presents with acute flank or abdominal pain, fever, nausea and vomiting. Elevated LDH, leukocytosis, elevated creatinine, and microscopic hematuria are also common findings [6]. Renal infarction in COVID-19 patients has been reported three times. These patients had hypoxemia requiring supplemental oxygen at least once during their illness. One of these three patients required mechanical ventilation and continuous venovenous hemofiltration which was complicated with frequent filter failure due to clotting, and developed extensive small bowel ischemia and skin mottling besides renal infarction [3]. The other two patients were immunocompromised post kidney transplantation and had other comorbidities [2,3].
COVID-19 related thrombotic and thromboembolic events commonly involve DVT (3.9%), PE (3.2%), stroke (1.6%), myocardial infarction (8.9%) and other thromboembolic events 1% (which comprise of acute limb ischemia, upper extremity thrombosis, renal and splenic infarct, and portal vein thrombosis altogether) [1]. Incidence of thrombotic events in COVID-19 positively correlates with the severity of infection and elevation in D-Dimer levels. Table 1 summarizes the D-Dimer levels noted in different studies involving COVID-19 patients. A study conducted at New York City Health System (n=3334), noted a total of 533 thrombotic events including both ICU and non-ICU patients. It was found that thrombotic events occurred in 29.4% of ICU patients as compared to 11.5% of non-ICU patients [1]. Another study conducted in Milan, Italy, which included 388 patients, reported thrombotic events in 16.7% of the ICU patients as compared to the 6.4% seen in patients on general ward [7]. At the time of literature review, none of the studies for thrombotic events with COVID-19 included non-hospitalized patients with selflimiting infection.
| New York City Health System[1] | Patients with thrombotic complication: Initial- 628 Maximum-3952 Patients without thrombotic complication: Intial-361 Maximum- 657 |
| Venous and arterial thrombotic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy [7] | Survivors (ICU/ General) ward: - Day 1-3: 615/329 Day 7-9: 3137/472 Non-survivors (ICU/ General ward): - Day 1-3: 1022/868 Day 7-9: 7746/1093 |
| Tang et al. [8] | Survivors- 610 Non-survivors- 2120 |
| Huang et al. [9] | ICU patients: 2400 Non-ICU patient: 500 |
| Zhou et al. [10] | >1000 in: Non-survivors: 44/54 (81%) Survivors: 28/118 (24%) |
| Wang et al. [11] (reference range 0-500 mg/L) | ICU patients: 414 (n=36) Non-ICU patients: 136 (n=102) |
Table 1: Average D-Dimer levels (ng/ml) observed in different studies which correlates with the severity of illness.
Here we have described a case of a 38-year-old male patient, who developed an acute renal infarction with COVID-19. Unpredictably, he did not have severe respiratory symptoms or as high D-Dimer levels as seen in patients with thrombotic complications from COVID-19. Thrombotic events–both venous and arterial– are uncommon in self-limited COVID-19 illness. Further, isolated arterial thrombotic events are least reported in the setting of COVID-19, and in general, isolated renal infarction is an uncommon occurrence. Patient tested positive for LAC. The data about LAC positivity in COVID-19 is contrasting and needs to be further studied. Whether LAC is present universally in COVID-19 patients, or in COVID-19 with thrombotic complications, or if it is unrelated, remains a topic of debate. Harzallah et al., Helms et al., and Bowles et al. are in favor of this relation while Tang et al. suggests otherwise [12-15]. Zhang et al. describes three patients who had thrombotic complications with COVID-19 and were found to be positive for anticardiolipin IgA antibodies and anti-beta 2 glycoprotein I IgA and IgG antibodies, and tested negative for LAC [16]. An association between presence of anticardiolipin antibodies and/or LAC with viral infections like hepatitis C, human immunodeficiency virus, cytomegalovirus, varicella zoster, Epstein-Barr virus, adenovirus, and parvovirus B has been described previously, with the presence of these antibodies in the aforementioned infections being associated with increased incidence of thrombosis [17].
Conclusion
Thrombotic or thromboembolic complications are commonly seen with severe COVID-19 but rarely involve isolated arterial infarctions. Current guidelines focus on the prophylaxis and treatment of such events in hospitalized or critically-ill patients. The case described above warrants identification of risk factors which might render certain patients vulnerable to thrombotic events with even mild forms of COVID-19. It also necessitates the need for close follow up and education following COVID-19 diagnosis in the outpatient setting. LAC may not be universally present in COVID-19, but it’s possible presence and known risk towards thrombosis suggests that larger studies looking into its incidence are needed.
Addendum
J. Mittal made substantial contributions to the conception and design; acquisition, analysis and interpretation of data; drafting of the manuscript; and revising the manuscript critically for important intellectual content. A. Sidhu and P.G. Misra revised the manuscript critically for important intellectual content. G. Vashishta served as the primary consultant in the management of the patient; he, with the other authors, gave final approval of the version to be published.
Disclosure of Conflict of Interests
The authors of this manuscript have no conflicts of interest to disclose.
References
2. Xu JJ, Samaha D, Mondhe S, Massicotte-Azarniouch D, Knoll G, Ruzicka M. Renal Infarct in a COVID-19 Positive Kidney-Pancreas Transplant Recipient. American Journal of Transplantation. 2020 Jun 1. 10.1111/ajt.16089
3. Post A, den Deurwaarder ES, Bakker SJ, de Haas RJ, van Meurs M, Gansevoort RT, et al. Kidney Infarction in Patients With COVID-19. American Journal of Kidney Diseases. 2020 May 29.
4. Korzets ZE, Plotkin E, Bernheim J, Zissin R. The clinical spectrum of acute renal infarction. IMAJ-RAMAT GAN-. 2002 Oct 1;4(10):781-4.
5. Domanovits H, Paulis M, Nikfardjam M, Meron G, Kürkciyan I, Bankier AA, et al. Acute renal infarction: clinical characteristics of 17 patients. Medicine. 1999 Nov 1;78(6):386-94.
6. Oh YK, Yang CW, Kim YL, Kang SW, Park CW, Kim YS, et al. Clinical characteristics and outcomes of renal infarction. American Journal of Kidney Diseases. 2016 Feb 1;67(2):243-50.
7. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research. 2020 Apr 23; 191:9-14.
8. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. Journal of Thrombosis and Haemostasis. 2020 Apr;18(4):844-7.
9. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020 Feb 15;395(10223):497-506.
10. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020 Mar 11;395(10229):1054-1062.
11. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020 Mar 17;323(11):1061-9.
12. Tang N. Response to “Lupus anticoagulant is frequent in patients with Covid-19”. Journal of Thrombosis and Haemostasis. 2020 May 7.
13. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Medicine. 2020 Jun;46(6):1089-1098
14. Bowles L, Platton S, Yartey N, Dave M, Lee K, Hart DP, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. New England Journal of Medicine. 2020 Jul 16;383(3):288-290.
15. Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19: Response to Reply. Journal of Thrombosis and Haemostasis. 2020 Aug;18(8):2064-2065.
16. Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. New England Journal of Medicine. 2020 Apr 23;382(17): e38.
17. Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. InSeminars in Arthritis and Rheumatism 2002 Feb 1 (Vol. 31, No. 4, pp. 256-263).
