Commentary
High fat high cholesterol containing Western-type diet (WD)-induced obesity remains one of the major causes for the development of metabolic syndrome and associated metabolic diseases such as Type 2 Diabetes (T2DM) and atherosclerosis (that leads to cardiovascular diseases including heart disease and stroke). In addition to changes in lipid metabolism and excessive lipid accumulation, recent studies have also described direct effects of WD on gut microbiome and attributed dysbiosis of gut flora to the observed metabolic effects. However, strong association between circulating gut bacteria-derived lipopolysaccharide (LPS) and metabolic diseases (such as T2DM and atherosclerosis) has shifted the focus from WD-induced changes in gut microbiota per se to release of gut bacteria-derived products (e.g., LPS) into circulation as the possible mechanism for the chronic inflammatory state underlying the development of these diseases.
Under physiological conditions, an intact intestinal barrier prevents the translocation of LPS underscoring the importance of detailed understanding of the direct effects of WD on intestinal barrier function. WD enhances local intestinal inflammation [1] and increases translocation of gut bacteria-derived LPS to systemic circulation by multiple mechanisms. Excess chylomicron formation and secretion during WD-feeding facilitates the translocation of LPS associated with chylomicrons [2]. WD, low in fiber, deprives intestinal bacteria of essential nutrients whereby luminal bacteria turn to alternate source of energy, namely, the carbohydrate-rich mucosal layer [3] enhancing direct contact between gut bacteria and intestinal epithelial layer promoting local inflammation and barrier dysfunction [4]. WD is also shown to reduce the expression of tight junction protein occludin thereby increasing paracellular transport across the intestinal epithelial cell layer [5]. Increased dietary fat from WD also increase luminal hydrophobic bile acids that compromise epithelial integrity and inflammation [6]. Collectively, these data establish several novel mechanisms by which WD directly affects intestinal barrier function.
Intestinal barrier consists of multiple layers including: 1) luminal intestinal alkaline phosphatase (IAP) that dephosphorylates LPS to detoxify it; 2) the mucus layer that provides a physical barrier preventing interactions between gut bacteria and intestinal epithelial cells; 3) the tight junctions between the epithelial cells that limit the paracellular transport of bacteria and/or bacterial products to systemic circulation; and 4) the anti- bacterial proteins secreted by the intestinal epithelial cells. However, the identity of the component(s) of intestinal barrier affected by WD remains currently undefined and furthermore whether targeted improvement of only one layer of the intestinal barrier would be sufficient to prevent the translocation of bacterial-derived products to systemic circulation is not established.
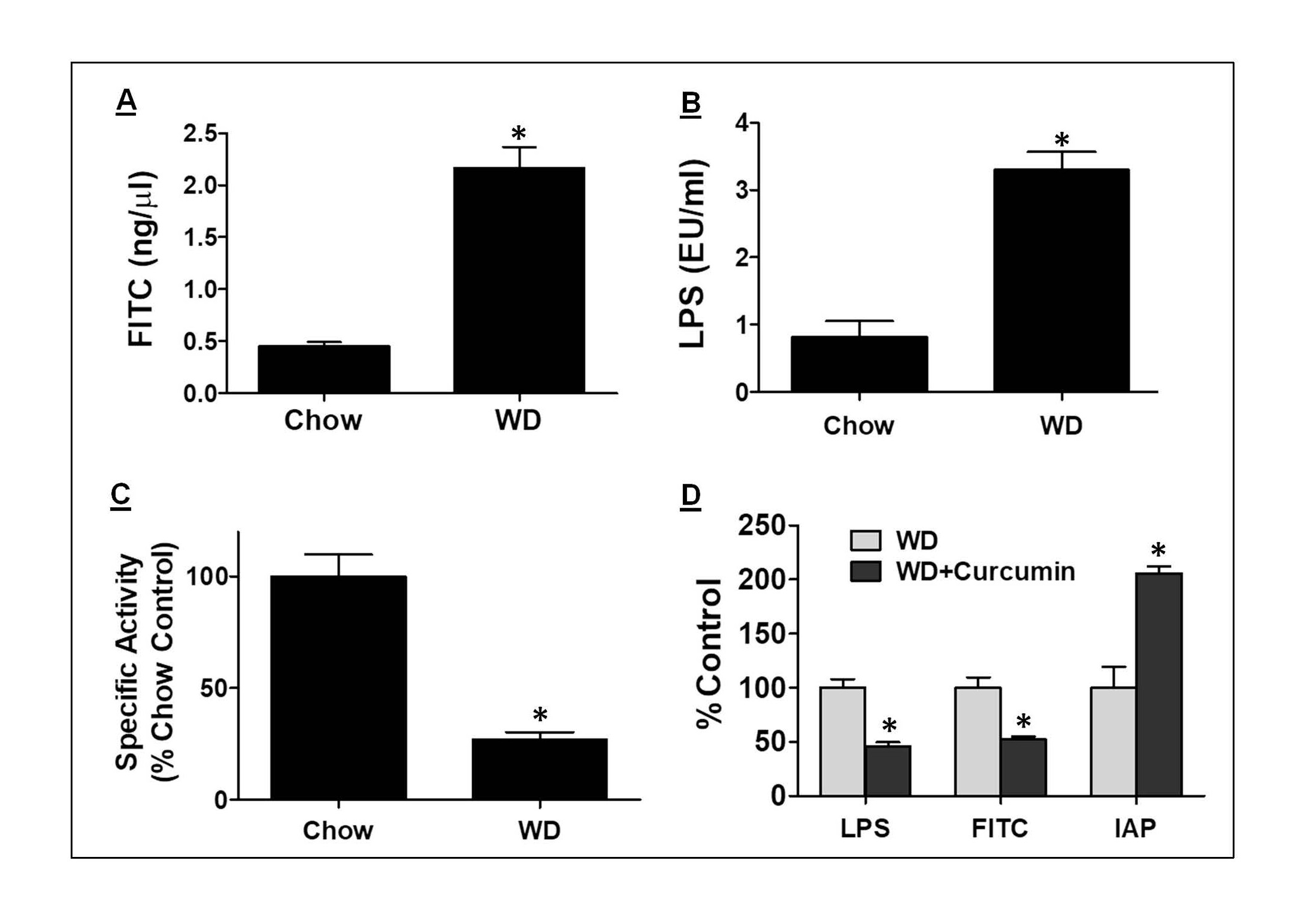
We earlier demonstrated disruption of intestinal barrier function by WD resulting in significant increase in paracellular transport and release of bacterial LPS into the circulation of WD-fed mice (Figure 1A and 1B, respectively). This WD-induced increase in intestinal barrier permeability was also associated with a significant reduction in intestinal IAP activity (Figure 1C) indicating disruption of this first layer of the intestinal barrier by WD. Consistently, oral supplementation with curcumin that significantly increased IAP activity also improved the intestinal barrier function in WD fed LDLR-/- mice resulting in decreased translocation of gut-derived LPS into systemic circulation (Figure 1D) [7]. This led to improved glucose tolerance and attenuated atherosclerosis without affecting plasma lipid profiles [7] providing direct evidence that IAP may represent the “first line of defense” against the metabolic consequences of WD and targeted modulation of IAP may represents a novel strategy to attenuate WD-induced metabolic diseases.
Figure 1. WD-mediated effects on intestinal barrier function and its modulation by oral supplementation with curcumin. Mice were fed a standard rodent chow or WD (TD88137) for 16 weeks. Panel A: Appearance of FITC in plasma following oral gavage with FITC-Dextran was monitored. Panel B: At the time of euthanasia, blood was collected, and plasma LPS levels were determined using LAL assay. Panel C: IAP enzyme activity was measured using ileal tissue homogenates and p-nitro phenyl phosphate as substrate. Panel D: Mice were fed WD and gavaged daily with either vehicle (WD) or curcumin (WD + Curcumin) for 16 weeks. Translocation of FITC-dextran and LPS to plasma as well as ileal IAP activity was determined. Data shown are Mean ± SD, n ≥ 6. *p<0.05.
IAP catalyzes the removal of one of the two phosphate groups from the toxic lipid A moiety of LPS producing monophosphoryl-LPS that still binds to TLR4 but predominantly acts as an TLR4 antagonist [8]. Therefore, IAP is central in maintaining the critical homeostasis that exists between the host and the luminal microbial environment [9]. Consistently, Tuin et al. have reported a decrease in IAP in patients with inflammatory bowel disease [10]. In addition, during active fat absorption, IAP is selectively endocytosed from the enterocyte brush border leading to increase in circulating IAP levels [11]. This decrease in luminal IAP during increased fat absorption following WD-feeding is likely to result in barrier dysfunction and increased paracellular translocation of LPS in addition to increased chylomicron-associated translocation [2]. Accordingly, exogenous supplementation with IAP represents a viable strategy for decreasing the development of diseases linked to increase in intestinal permeability and systemic translocation of bacteria/ bacterial products. Instability and loss of activity of IAP at pH<5.0, precludes its oral administration without specific enteric formulation to circumvent gastric inactivation. Consistently, Tuin et al. reported attenuation of DSSinduced colitis in rats by supplementation with IAP given as enteric coated tablets that prevents dissolution in the stomach and inactivation of acid labile IAP [10]. To bypass gastric inactivation, Lukas et al. administered 30,000U of alkaline phosphatase intraduodenally and reported successful treatment of patients with severe ulcerative colitis [12]. Chimeric human alkaline phosphatase that displays greatly increased heat stability, increased Zn2+ binding affinityincreased transphosphorylation, a higher turnover number, narrower substrate specificityand selectivity for bacterial LPS, developed by Kiffer-Moreiraet al. is currently being evaluated as a protein therapeutic for gut dysbiosis, inflammatry bowel disease and acute kidney injury [13].
To obtain the direct in vivo “proof-of-concept” that increasing IAP will modulate WD-induced effects on intestinal barrier function and subsequent downstream effects such as development of glucose intolerance, Ghosh et al. reported the development of a novel mouse model with intestine-specific transgenic expression of human chimeric IAP (IAP transgenic mice, IAPTg) [14]. While the luminal bacterial load increases from duodenum to colon, endogenous expression of IAP declines along this tract resulting in less IAP activity at site of maximal LPS generation, i.e., colon. IAP transgenic (IAPTg) mice, in contrast, displayed a uniform expression of IAP along the entire length and improved intestinal barrier function as assessed by lumen to plasma translocation of FITC-Dextran that only occurs via para-cellular transport and fecal zonulin levels. In addition, WD-induced intestinal as well as systemic inflammation was also significantly reduced in IAPTg mice leading to improved glucose tolerance [14]. These studies not only provide direct evidence for targeted modulation of IAP as a novel strategy to improve intestinal barrier function and reduce WD-induced intestinal/ systemic inflammation but also underscore the importance of gut barrier homeostasis in WD-induced development of metabolic diseases such as diabetes or glucose intolerance.
IAP represents one of the four “layers” of the intestinal barrier and evidence exists to demonstrate the roles of other layers in mediating detrimental effects of WD especially the mucus layer. A continuous and well-formed mucin layer is essential for prevention of direct interactions between luminal bacteria and epithelial cells and is considered as the second layer of the intestinal barrier. In addition to being rich in fat and cholesterol, WD is also a poor source of dietary fiber. Dietary fiber is probably best known for its ability to prevent or relieve constipation. However, deficiency of dietary fiber also has other serious consequences. Dietary fiber represents a major source of nutrients for gut bacteria which are unable to degrade fatty acids released from dietary acyl lipids by the action of lipases. In anaerobic gut environment, bacteria can only ferment glycerol, galactose, choline, and other non-fatty acid components released during hydrolysis of acyl lipids [15]. In the absence of adequate dietary fiber, colonic bacteria turn to the alternate energy source, the mucin-2 (MUC-2) glycoprotein-rich mucus layer. This phenomenon leads to mucus barrier erosion [16]. Low fiber containing WD is thought to impair the inner colonic mucus layer that presents a barrier between direct interactions of bacteria and the epithelial cell layer [17]. Supplementation of WD with dietary fiber is widely used to selectively affect the composition of gut bacteria (the prebiotic effect) with beneficial effects on obesity [18], glycemia [19] as well as cognitive and appetite dysfunction [20]. Recently, Ghosh et al tested the hypothesis that supplementation of WD with fiber (galacto-oligosaccharides, GOS) would attenuate the development of WD-induced metabolic diseases by restoring the mucin layer of the intestinal barrier. These studies directly demonstrate that targeted restoration of the intestinal mucin layer by simple dietary modulation, namely addition of 5% GOS to WD, improves intestinal barrier function and attenuates WD-induced development of glucose intolerance and atherosclerosis [21]. Collectively, these studies focused on increasing IAP either by oral curcumin supplementation or transgenic IAP expression or restoring mucin layer by fiber supplementation confirm that strategies for targeted improvement of intestinal barrier function by simple dietary modulations could provide novel therapeutic options for managing WDinduced metabolic disease development.
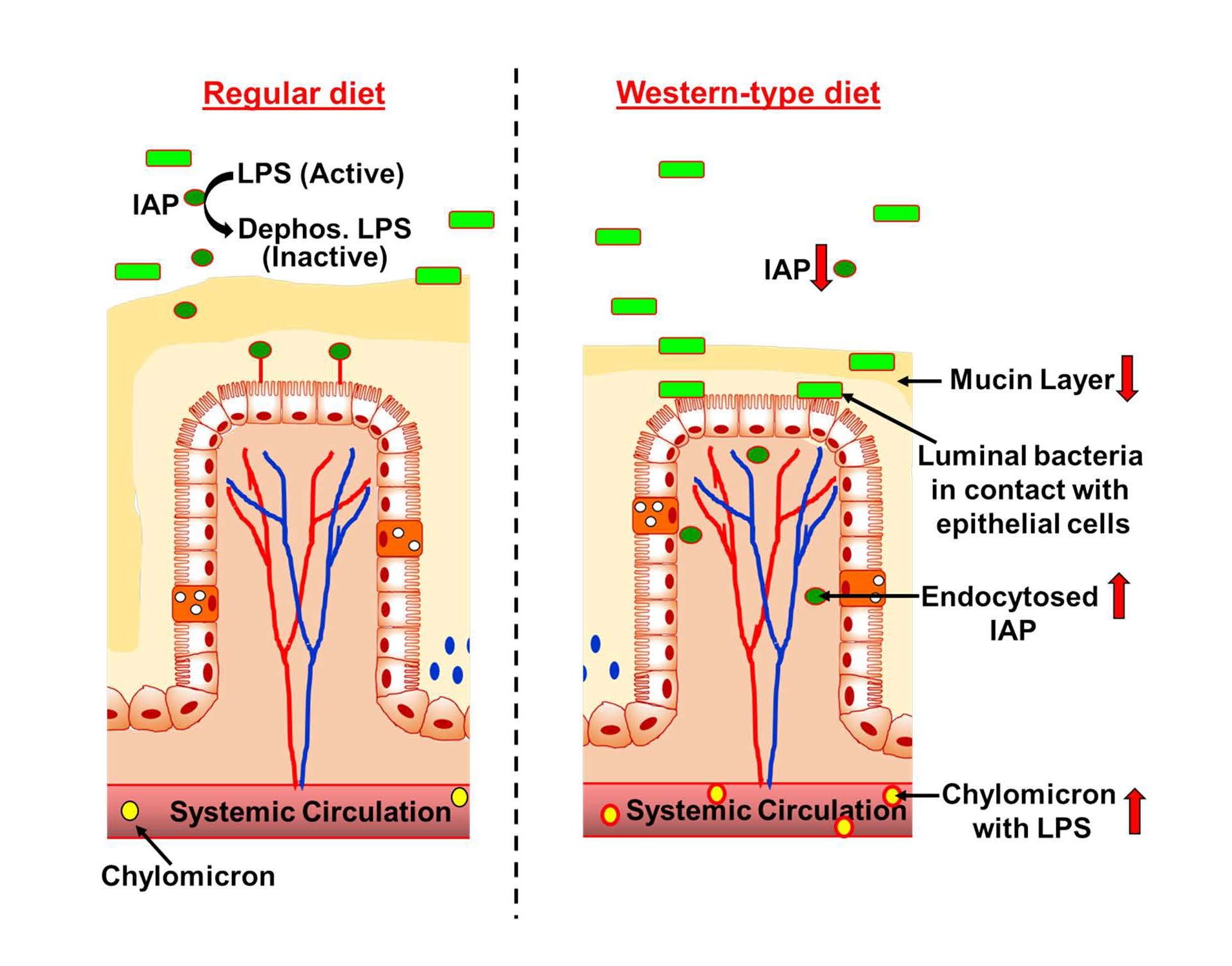
In conclusion, although dysbiosis attributed to consumption of WD is extensively examined as the likely mechanism underlying WD-induced increase in metabolic disease development, the mechanisms by which this dysbiosis “communicates” with the host to modify host physiology are poorly defined. WD-induced intestinal barrier dysfunction leading to translocation of bacteria/bacterial products from intestinal lumen to systemic circulation is increasing being recognized to play a causative role in the development of metabolic endotoxemia and eventual development of WD-induced metabolic diseases. Figure 2 summarizes the multiple ways by which WD disrupts the individual layers of the intestinal barrier.
Figure 2. WD-induced changes in the intestinal barrier. Consumption of WD increases endocytosis of IAP decreasing the luminal IAP available for dephosphorylation/detoxification of LPS. Low fiber content of WD enhances bacterial erosion of the mucin layer facilitating increased contact between luminal bacteria and the epithelial cell monolayer resulting in intestinal inflammation. Increased lipid absorption during WD consumption also increases translocation of LPS bound to chylomicrons. Collectively, these WD-induced changes in intestinal barrier increase metabolic endotoxemia leading to intestinal and systemic inflammation and resulting in the development of metabolic diseases such as diabetes and atherosclerosis.
Increased lipid absorption during WD feeding enhances IAP endocytosis decreasing the availability of IAP for luminal dephosphorylation/detoxification of LPS and also increases translocation of chylomicron-bound LPS. Due to the low fiber content, WD also enhances erosion of mucin layer increasing intestinal permeability. Simple modulations of WD such as curcumin or fiber supplementation, by virtue of either increasing IAP activity or maintaining the integrity of the mucin layer, significantly restore the intestinal barrier function and attenuate the development of glucose intolerance and atherosclerosis. These developments provide an innovative approach for attenuating metabolic diseases based on targeted improvement of individual layers of the intestinal barrier. Future studies are likely to identify additional nutritional supplements or develop novel therapeutic agents to restore intestinal barrier function and reduce the burden of metabolic diseases.
References
2. Ghoshal S, Witta J, Zhong J, De Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. Journal of Lipid Research. 2009 Jan 1;50(1):90-7.
3. Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016 Nov 17;167(5):1339-53.
4. Schroeder BO. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterology Report. 2019 Feb;7(1):3-12.
5. Sellmann C, Baumann A, Brandt A, Jin CJ, Nier A, Bergheim I. Oral supplementation of glutamine attenuates the progression of nonalcoholic steatohepatitis in C57BL/6J mice. The Journal of Nutrition. 2017 Nov 1;147(11):2041-9.
6. Stenman LK, Holma R, Eggert A, Korpela R. A novel mechanism for gut barrier dysfunction by dietary fat: epithelial disruption by hydrophobic bile acids. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2013 Feb 1;304(3):G227-34.
7. Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice–role of intestinal permeability and macrophage activation. PloS one. 2014 Sep 24;9(9):e108577.
8. Bentala H, Verweij WR, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, et al. Removal of phosphate from lipid A as a strategy to detoxify lipopolysaccharide. Shock. 2002 Dec 1;18(6):561-6.
9. Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host & Microbe. 2007 Dec 13;2(6):371- 82.
10. Tuin A, Poelstra K, de Jager-Krikken A, Bok L, Raaben W, et al. Role of alkaline phosphatase in colitis in man and rats. Gut. 2009 Mar 1;58(3):379-87.
11. Hansen GH, Niels-Christiansen LL, Immerdal L, Nystrom BT, Danielson EM. Intestinal alkaline phosphatase: selective endocytosis from the enterocyte brush border during fat absorption. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007 Dec;293(6):G1325-32.
12. Lukas M, Drastich P, Konecny M, Gionchetti P, Urban O, Cantoni F, et al. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflammatory Bowel Diseases. 2010 Jul 1;16(7):1180-6.
13. Kiffer-Moreira T, Sheen CR, Gasque KC, Bolean M, Ciancaglini P, et al. Catalytic signature of a heat-stable, chimeric human alkaline phosphatase with therapeutic potential. PLoS One. 2014 Feb 24;9(2):e89374.
14. Ghosh SS, He H, Wang J, Korzun W, Yannie PJ, Ghosh S. Intestine-specific expression of human chimeric intestinal alkaline phosphatase attenuates Western dietinduced barrier dysfunction and glucose intolerance. Physiological Reports. 2018 Jul;6(14):e13790.
15. Mackie RI, White BA, Bryant MP. Lipid metabolism in anaerobic ecosystems. Critical Reviews in Microbiology. 1991 Jan 1;17(6):449-79.
16. Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell. 2016 Nov 17;167(5):1339-53.
17. Birchenough G, Schroeder BO, Bäckhed F, Hansson GC. Dietary destabilisation of the balance between the microbiota and the colonic mucus barrier. Gut Microbes. 2019 Mar 4;10(2):246-50.
18. Wang H, Zhang X, Wang S, Li H, Lu Z, Shi J, Xu Z. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food & Function. 2018;9(7):3916-29.
19. Hashemi Z, Fouhse J, Im HS, Chan CB, Willing BP. Dietary pea fiber supplementation improves glycemia and induces changes in the composition of gut microbiota, serum short chain fatty acid profile and expression of mucins in glucose intolerant rats. Nutrients. 2017 Nov;9(11):1236.
20. Yu Y, Patch C, Weston-Green K, Zhou Y, Zheng K, Huang XF. Dietary Galacto-Oligosaccharides and Resistant Starch Protect Against Altered CB1 and 5-HT1A and 2A Receptor Densities in Rat Brain: Implications for Preventing Cognitive and Appetite Dysfunction During a High-Fat Diet. Molecular Nutrition & Food Research. 2018 Nov;62(21):1800422
21. Ghosh SS, Wang J, Yannie PJ, Sandhu YK, Korzun WJ, Ghosh S. Dietary supplementation with galactooligosaccharides attenuates high-fat, highcholesterol diet–induced glucose intolerance and disruption of colonic mucin layer in C57BL/6 mice and reduces atherosclerosis in Ldlr–/–mice. The Journal of Nutrition. 2020 Feb 1;150(2):285-93.