Abstract
Inflammation is a key factor in retinal damage in response to diabetes. Sortilin represents a new regulator of retinal inflammation. Sortilin is involved in over 50 different signaling cascades. To investigate sortilin in the diabetic retina, we first measured protein levels in retinal lysates from diabetic humans and diabetic mice. We then inhibited sortilin using a small molecule inhibitor, AF38469, and evaluated retinal function using electroretinogram (ERG) and fluorescein angiography. We also measured key inflammatory and autophagic proteins. Data showed that sortilin levels were significantly increased in retinal lysates from diabetic patients and diabetic mice. Treatment of mice with AF38469 in their drinking water restored ERG and angiography to normal levels in diabetic mice. We found that AF38469 reduced high mobility group box 1 (HMGB1) and interleukin-1beta (IL-1β) levels. We also found that inhibiting sortilin led to reduced LAMP2 levels in diabetic mice. In conclusion, our data suggest that inhibition of sortilin may offer a new pathway to protect the diabetic retina.
Keywords
Diabetic Retinopathy, Sortilin, Retina, Therapeutics
Introduction
Despite decades of research, diabetic retinopathy remains the leading cause of blindness in working age adults. While several inciting factors are likely involved, research has shown increased levels of inflammatory mediators in the diabetic retina [1–4]. Sortilin has emerged as a novel protein that can regulate inflammatory mediators [5]. Sortilin is a member of the type-I transmembrane receptor family containing a Vps10p domain [6]. All 5 members of this family have the Vps10 domain in the N-terminal region and play key roles in signal transduction and protein trafficking [6]. Sortilin can be bound by pro-neurotrophins, pro-nerve growth factor (NGF) and pro-brain derived nerve growth factor (proBDNF), along with P75NTR, to activate inflammatory and apoptotic pathways [7]. To date, sortilin has been associated with cellular trafficking of over 50 molecules, including lipoproteins, cytokines, and enzymes [5]. One function of sortilin is as a co-receptor for proNGF with P75NTR to mediate neuronal apoptosis [8]. In addition to binding proNGF, sortilin binds proBDNF to mediate neuropathic pain, as shown in sortilin knockout mice [9]. Sortilin plays a role in cardiovascular disease through the regulation of LDL [5]. It serves as a receptor for lipids, cytokines, and enzymes to mediate pathological changes involved in atherosclerosis and inflammation [10]. Sortilin also mediates endothelial cell dysfunction, leading to arterial hypertension [11].
In addition to inflammation, sortilin has also been linked to other pathological pathways associated with diabetic retinopathy, including autophagy. In hippocampal neurons treated with amyloid β1-42, Sortilin contributed to elimination of clustrin protein through targeting into lysosomes [12]. The mannose-6-phosphate receptor pathway is a mechanism by which sortilin targets cathepsins D and H into lysosomes for degradation [13], specifically in rat epididymal cells [14]. Sortilin also regulates macrophage accumulation of cholesterol by lysosomal degradation [15].
Some research has been done on sortilin in the retina and the diabetic retina. In a model of retinal ischemia, sortilin was localized to Müller cells with reduced expression in RGCs [16]. In studies of patients with proliferative diabetic retinopathy, significantly increased levels of sortilin were observed in retinal lysates of the patients [17,18]. A recent study showed increased sortilin levels in the human diabetic retina and in the retina of STZ-treated diabetic mice, an increase which was associated with the actions of proNGF/P75NTR in mice that were diabetic for 8 weeks [19].
Recently, a small molecule inhibitor of sortilin, AF38469, was shown effective when given orally in breast cancer studies [20]. AF38469 reduced metastases into the skin. Because sortilin is linked to inflammation, we wanted to evaluate whether inhibition of sortilin with AF38469 would reduce inflammatory mediators in diabetic mice. We hypothesized that inhibition of sortilin with AF38469 given in the drinking water would reduce inflammatory mediators and improve retinal function in 8-week diabetic mice.
Materials and Methods
Human subjects
Dr. Mohamed Al-Shabrawey (Oakland University) provided postmortem retinal protein samples from 4 non-diabetic donors and 5 donors with diabetic retinopathy (both type 1 and type 2). The eyes were obtained from the Ever sight Eye Bank (Ann Arbor, MI). Approval for these samples was obtained from Oakland University stating “This study followed the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Oakland University (5/9/23; IRB-FY2023-292)”. Samples were deemed not human research on May 9, 2023 for the study entitled “Molecular and cellular mechanisms of diabetic retinopathy.” All diabetic patients had disease for 10+ years. These samples were processed for protein detection by Western blotting, as we have done in the past [21].
Diabetic mice for sortilin protein assessment
C57BL/6 male mice were purchased from Jackson Laboratories at 8 weeks of age. Diabetes was induced as we have done in the past [22]. Glucose levels >250 mg/dl were considered diabetic. At 2 months of diabetes, 5 control and 5 diabetic mice were sacrificed for analyses [23]. Mice were allowed free access to water and food and kept at a constant temperature for all experiments. Euthanasia was done via CO2 overdose and cervical dislocation. All mouse experiments were approved by the Wayne State University IACUC committee (IACUC 24-02-6569) and adhere to the rules provided by the Association for Research in Vision and Ophthalmology (ARVO).
Diabetic mice for AF38469 treatment
Additional 8-week-old male C57BL/6 mice were made diabetic with STZ (60 mg/kg over 5 days). Once blood glucose was >250 mg/dl, a subset of the diabetic mice was given oral AF38469 (10 ug/kg) [20] for 6 weeks in their drinking water. AF38469 was dissolved in saline to make a 10 ug/kg dose and then placed into drinking water for the mice. Water intake was not measured directly. All mice were given free access to water.
Electroretinogram (ERG)
ERG was done on mice after 6 weeks of AF38469 treatment. Mice were dark-adapted overnight. The following morning, mice were anesthetized with ketamine/xylazine. Anesthetized animals were given eye drops of topical anesthesia (proparacaine, Alcon, Fort Worth, Texas), and pupils dilated with topical drops of tropicamide 1% (Alcon Laboratories, Fort Worth, TX). DTL ERG thread electrodes (Diagnosys LLC.) were placed on the cornea of both eyes to allow simultaneous recording using a Celeris machine. A plastic contact lens custom-made using Aclar film (Ted Pella) was used to help hold the electrodes in place. Tests will be carried out sequentially at light intensities of 0, 10, 100 mcd •s/m2. A five-minute recovery period will be allowed between different intensities. Each response will be recorded for 250 ms. Responses to 25 light flashes will be averaged to produce an ERG recording at each intensity. Analysis of ERG graphs was carried out using ERGView software to extract values for the b-wave amplitudes, as well as oscillatory potential amplitudes.
Fluorescein angiography
After 6 weeks of diabetes and AF38469 treatment, some mice were subjected to fluorescein angiography. Briefly, the pupil of the mice was dilated with tropicamide ophthalmic solution. Fifteen minutes later, mice were anesthetized by ketamine and xylazine. After mice were deeply anesthetized without toe pinch reflex, 150 µl of AK-FLUOR (1% W/V, AKORN, INC, Lake Forest, IL) was injected intraperitoneally. Retinal vessel leakage was analyzed and photographed using the Micron IV (Phoenix Research Labs, Pleasanton, CA).
Western blotting
The Western blotting on the human and diabetic mouse retinal protein samples was done as we have previously published [24]. After blocking in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA, the membranes were treated overnight with primary antibodies against sortilin (ab263864, Abcam) or beta actin (sc-47778, Santa Cruz Biotechnology, Santa Cruz, CA) followed by incubation with secondary antibodies (anti-rabbit-HRP, Promega, Madison, WI) for 0.5–1 hour. Antigen-antibody complexes were detected with a chemiluminescence reagent kit (Thermo Scientific, Pittsburgh, PA) and data were acquired using an Azure C500 imager (Azure Biosystems, Dublin, CA). Western blot data were assessed with Image Studio Lite software.
For the western blots for Figures 2–6 on diabetic mouse samples treated with AF38469, we used Capillary Electrophoresis SDS (CE-SDS), also known as Simple Westerns. It is an automated assay performed by a machine, Abby (Biotechne) that allows for quantitative protein analysis. The total protein for each sample is determined before running and diluted to equal concentrations. Samples are then prepared to manufacture specifications and subjected to CE-SDS. Abby’s CE-SDS allows for the migration of proteins based on size within the capillary, which are then immobilized to the sides of the sample’s respective capillary. After the immobilization, the resolving matrix is removed, blocked, and treated with subsequent antibodies. Detection is done via chemiluminescent and images at varying predetermined time points. Light intensity is recorded and then quantified. After the first run, the capillaries can be stripped and re-probed for further proteins. Results can be normalized to either a housekeeping protein or a total protein. Due to the automated nature of the assay, consistency, reproducibility, and efficiency are greatly improved. It also removes problematic steps that can hinder data acquisition. Due to the combination of processes, samples can be treated as truly quantifiable and compared to conventional Western blots. Antibodies used for these studies were the same sortilin and beta actin antibodies, as well as HMGB1 (ab18256, Abcam), IL-1β, (2805, Invitrogen) proBDNF (ab68151, Abcam), and LAMP2 (66301-1-lg, Proteintech).
Statistics
All experiments were repeated a minimum of three times, and the data are presented as mean ± SEM. Data were analyzed by a non-parametric Kruskal-Wallis 1-way ANOVA, followed by a Dunn’s test or 2-tailed t-test with p values <0.05 considered statistically significant. A representative blot is shown.
Results
Sortilin is increased in the diabetic retina
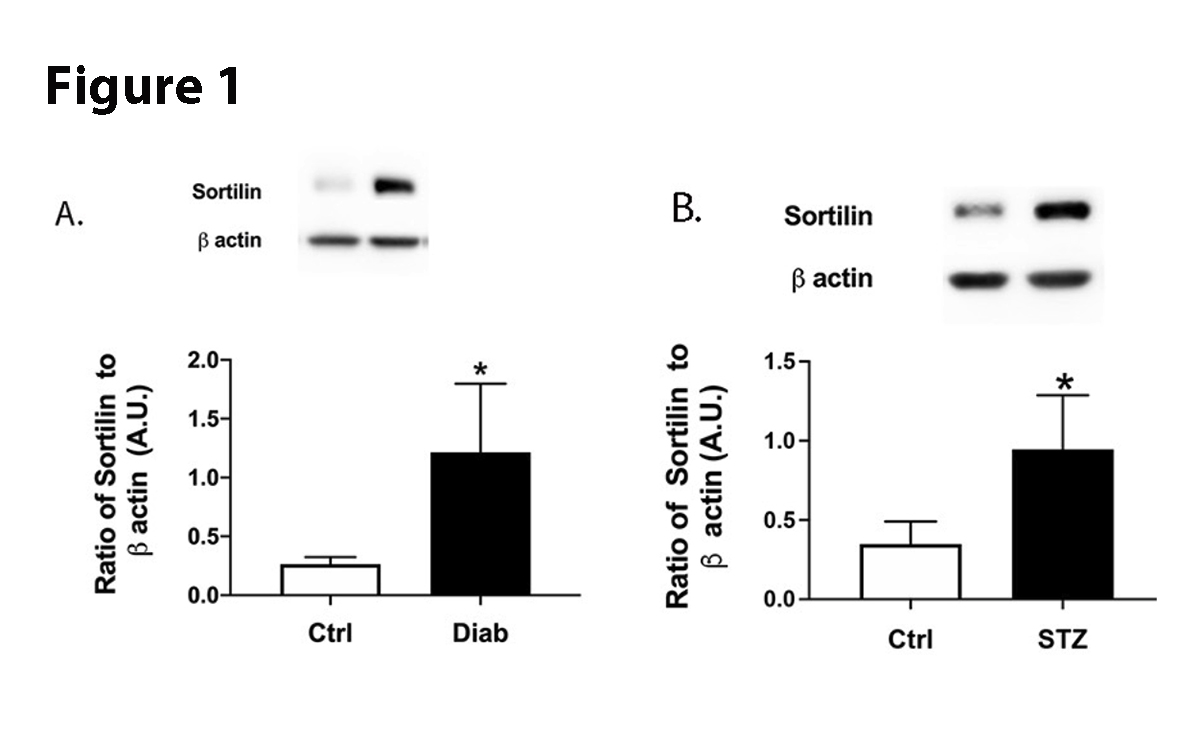
We used retinal protein samples from human diabetic patients and 2-month diabetic mice to measure protein levels. We found that diabetes significantly increased sortilin levels in both human and mouse retina (Figure 1). These findings suggest diabetes affects sortilin actions.
Figure 1. Protein levels of sortilin from retinal lysates from control (N=4) and diabetic (N=5) patients (A). Panel B is protein levels of sortilin in control and diabetic mice (N=5 for ctrl and STZ). Data are mean±SEM. *P<0.05 vs. Ctrl measured by two-tailed T test.
Sortilin inhibitor AF38469 significantly reduced sortilin levels
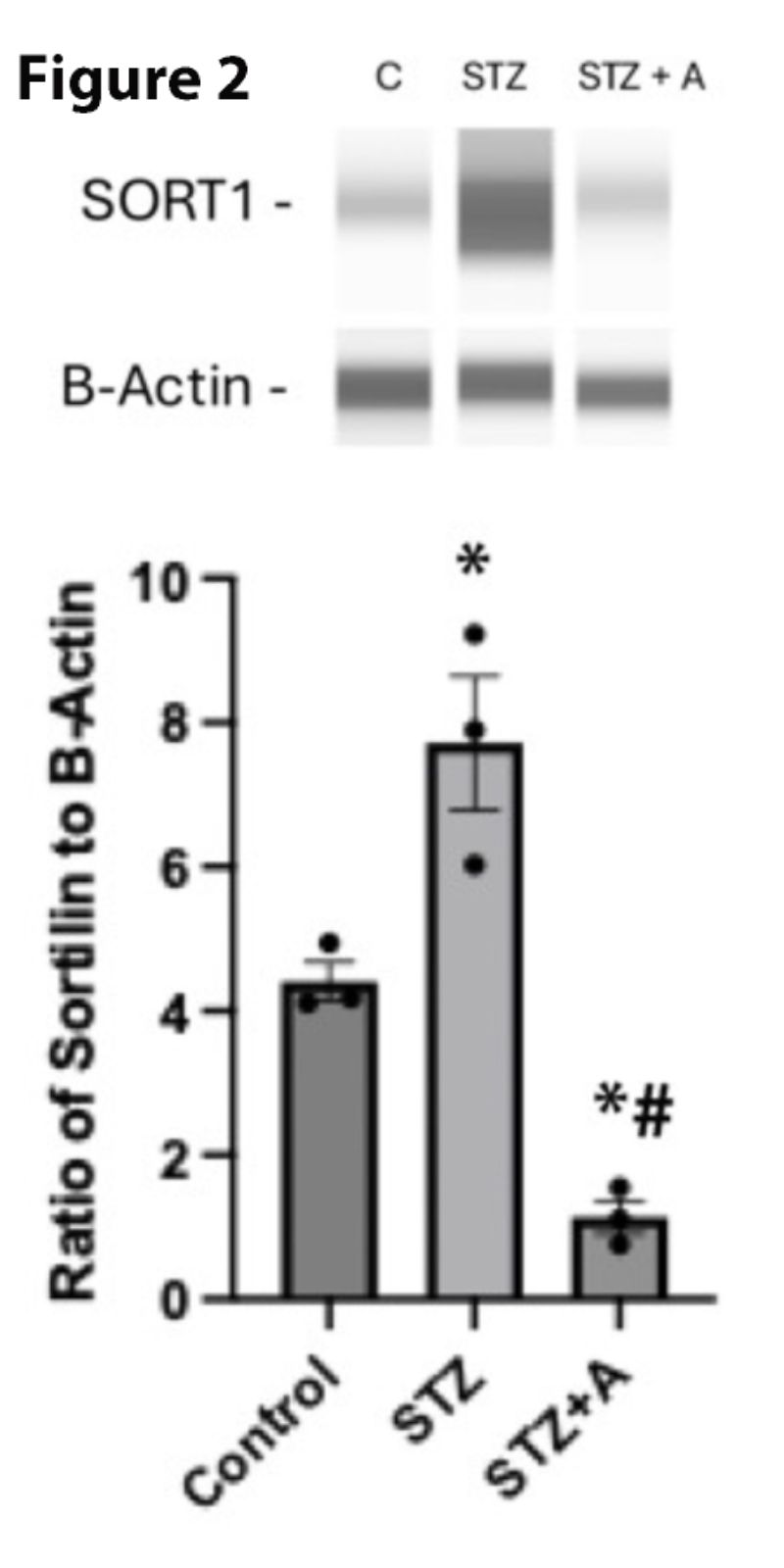
To determine whether AF38469, a small molecule inhibitor for sortilin, could reduce sortilin levels in whole retina from diabetic mice, we made mice diabetic and then gave a subset of mice the AF38469 dissolved in their drinking water for 8 weeks. Figure 2 shows that diabetes increased sortilin levels, which was significantly decreased by oral delivery of AF38469, even relative to untreated mice.
Figure 2. Sortilin protein levels in control, diabetic (STZ), and diabetic mice treated with AF38469 (STZ+A). *P<0.05 vs. control, #P<0.05 vs, STZ assessed using 1-way ANOVA. N=3.
AF38469 improves retinal electrical activity
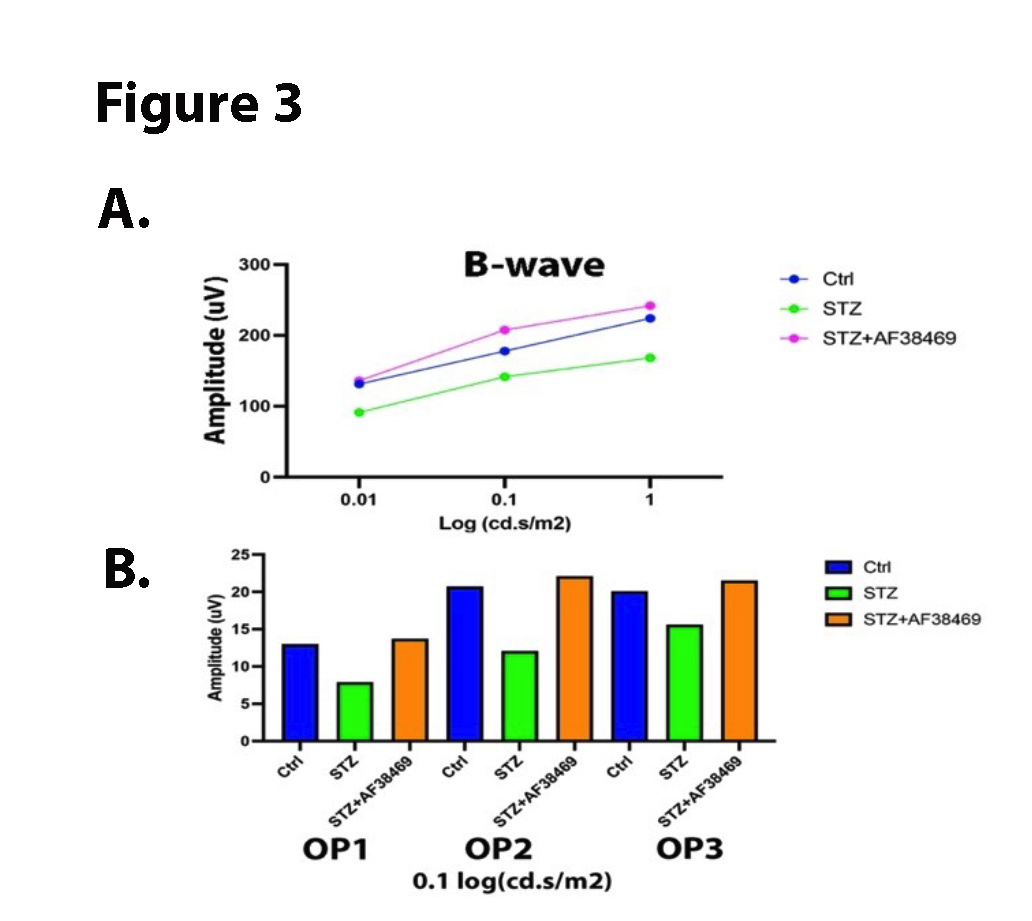
To determine whether inhibition of sortilin could protect the diabetic retina, we measured the electroretinogram (ERG) from control, diabetic, and diabetic+ oral AF38469 mice. We found that diabetes decreased the B-wave and OPs in the retina, which has been reported by others [25]. Figure 3 shows that, diabetic mice treated with oral AF38469 had improved ERG waveforms compared to diabetes alone.
Figure 3. Data show ERG findings for control (Ctrl), diabetic (STZ), and diabetic +oral AF38469. Top Panel is B-wave amplitude, and bottom panel is oscillatory potentials. Data represents a mean amplitude of 5 mice.
Sortilin inhibition improves retinal leakage
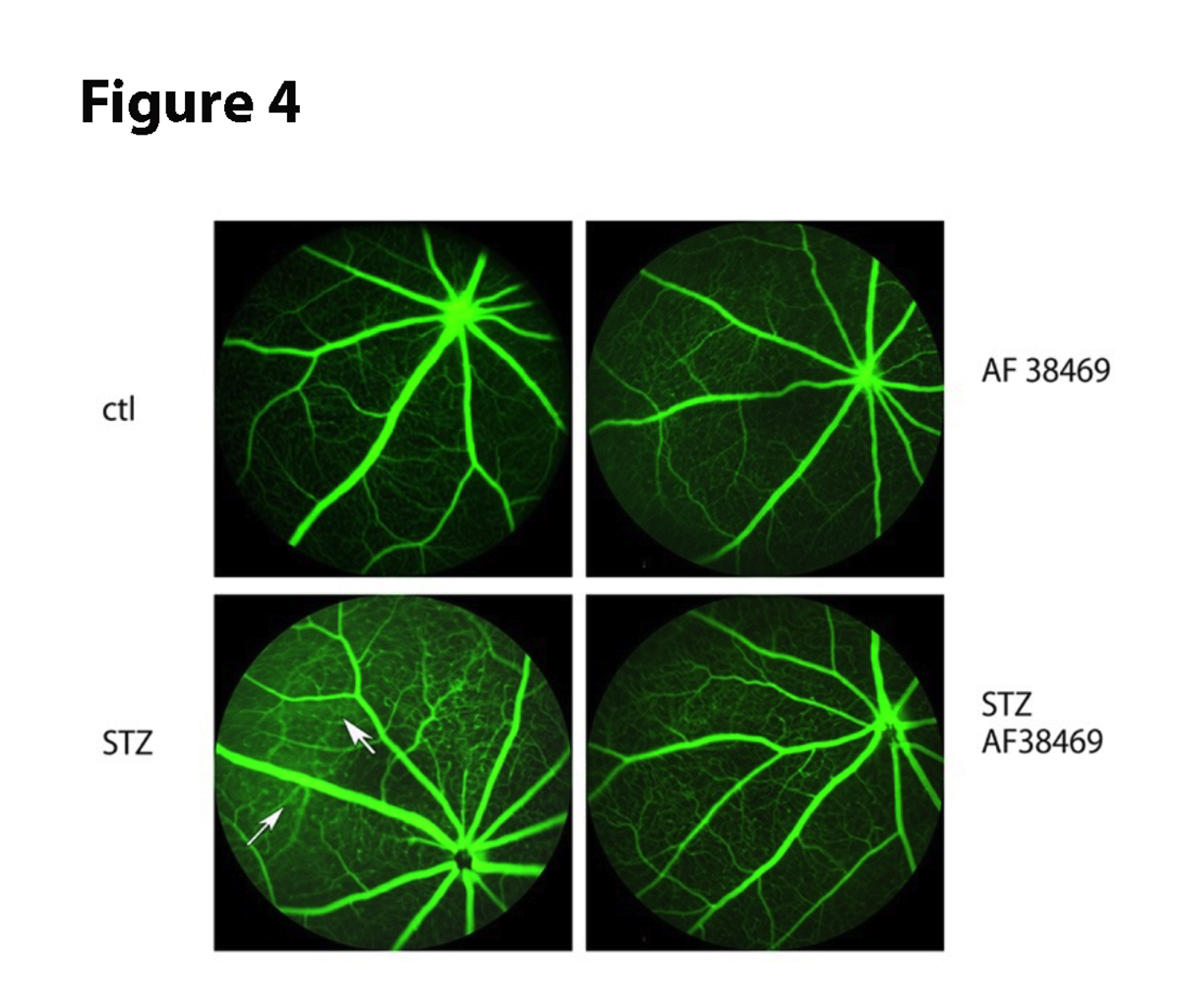
Since diabetes increases retinal permeability [26], we wanted to determine if inhibition of sortilin could reduce vascular leakage. Figure 4 shows that diabetes has increased permeability (arrows), which is substantially reduced in diabetic mice that received oral AF38469.
Figure 4. Fluorescein angiography for control (Ctl) and 6-week diabetic mice (STZ, left panels) and control +AF38469 (top right) and STZ+AF38469 (bottom right). Arrows show areas of leakage in STZ.
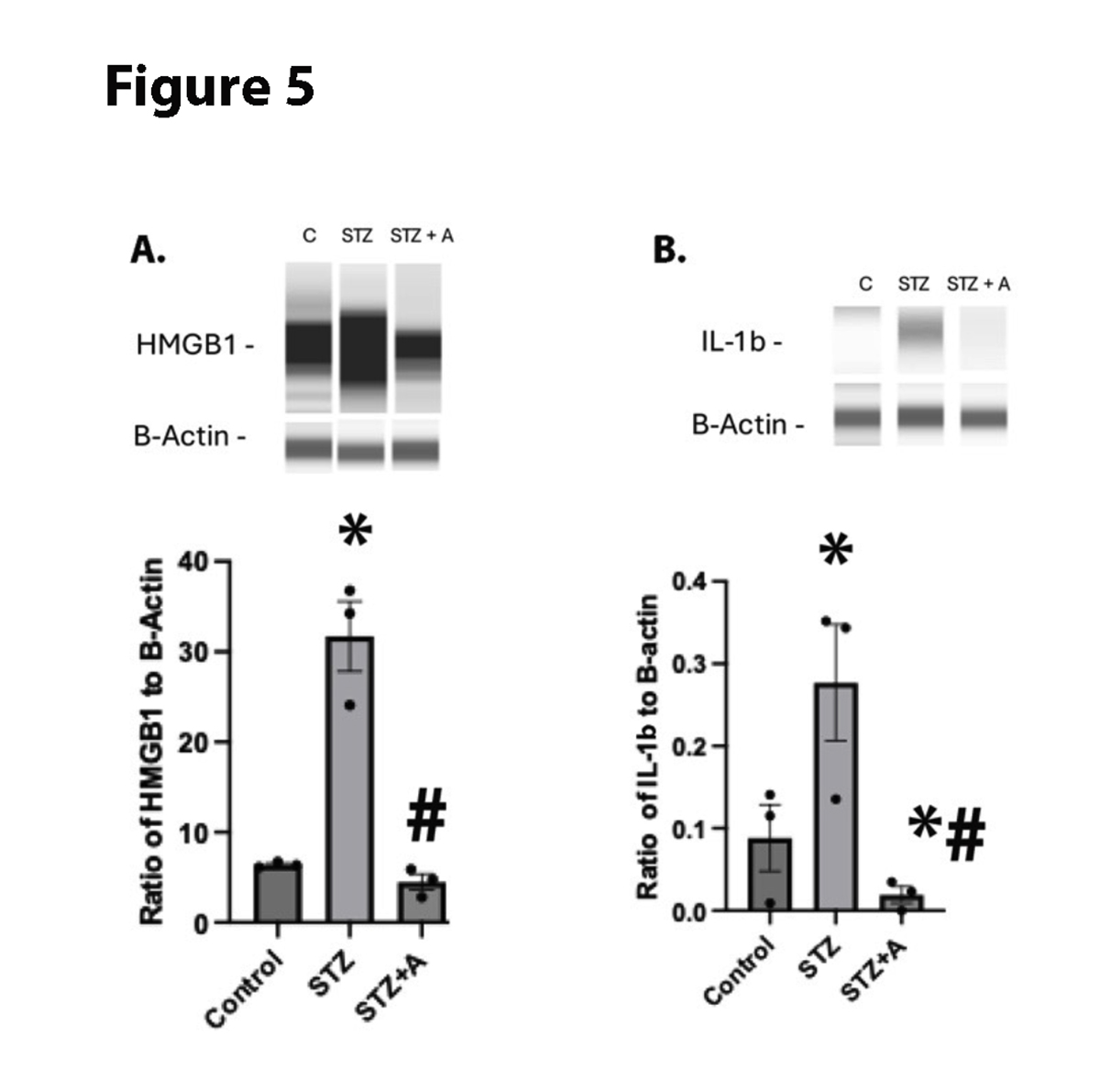
AF38469 reduced inflammatory mediators in diabetic mice
We have previously reported that diabetes significantly increased inflammatory mediators [22]. We found that diabetes increased HMGB1 and IL-1β levels in the mice. Figure 5 shows that oral AF38469 significantly reduced protein levels of both HMGB1 and IL-1β.
Figure 5. HMGB1 (A) and IL-1B (B) levels in whole retinal lysates from control, diabetic (STZ), or diabetic+AF38469 (STZ+A). *P<0.05 vs. Control, #P<0.05 vs STZ assessed by 1 way ANOVA. N=3.
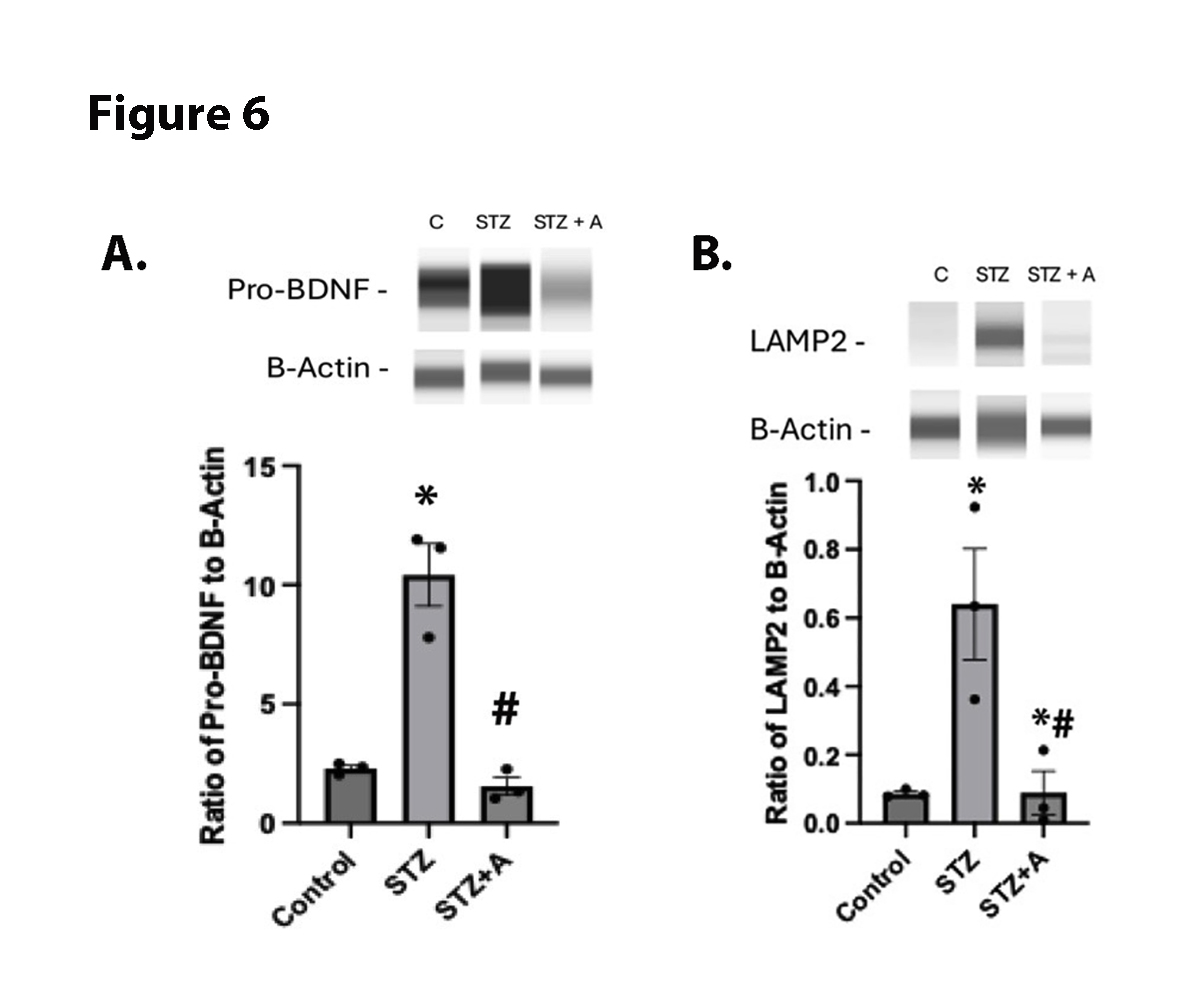
Sortilin regulates proBDNF and LAMP2 Levels
Literature suggests that sortilin should regulate proBDNF and autophagic proteins [15,27]. To investigate this in the retina, we measured protein levels of proBDNF and LAMP2 in the diabetic retina. Figure 6 shows that diabetic mice have significantly increased proBDNF and LAMP2 levels, which were significantly reduced by oral AF38469. These data suggest that inhibition of sortilin may offer a newer therapeutic option for diabetic retinopathy.
Figure 6. Panel A is ProBDNF levels in whole retinal lysates from control, diabetic (STZ), or diabetic+AF38469 (STZ+A). Panel B is LAMP2 levels in in whole retinal lysates from control, diabetic (STZ), or diabetic+AF38469 (STZ+A). For both panels, *P<0.05 vs. Control, #P<0.05 vs STZ assessed by 1 way ANOVA. N=3.
Discussion
Our findings of increased sortilin in the diabetic retina of human patients and mice agree with the literature [18,19]. Jakobsen et al., showed improved neuronal responses using a nerve growth factor (NGF)-binding antibody [19]. For our studies we chose to use the small molecule inhibitor of sortilin (AF38469), which had been shown effective in reducing metastases in breast cancer models [20]. Using the same dose as in the breast cancer model, we treated some diabetic mice with AF38469 in the drinking water. After mice consumed the water for 6 weeks, we measured ERG and performed angiography on the mice. We and others have previously reported that diabetes causes a decrease in the B-wave and oscillatory potentials in the ERG [28–30]. These studies confirmed a loss of B-wave amplitude, which was significantly improved by oral AF38469. Additionally, diabetes leads to increased retinal leakage [31]. Oral AF38469 reduced vascular leakage in diabetic mice.
While we saw improvement in retinal function in diabetic mice treated with oral AF38469, we also wanted to investigate potential pathways that are often associated with diabetes. We have spent the past decade investigating inflammatory pathways in the diabetic retina [21,22,32]. These studies showed increased HMGB1 and IL-1β levels in the diabetic retina that was significantly reduced by oral AF38469. Our findings of a role for sortilin in the retina follows literature in other models [5,11]. In addition to inflammatory mediators, we found that oral AF38469 reduced pro-BDNF and LAMP2 levels. These findings also match literature for sortilin actions in other models [12,27].
While these studies demonstrate the potential for AF38469 as a therapeutic for diabetic retinopathy, additional studies need to be completed for the chronic effects of AF38469 treatment. Side effects of oral drug delivery need to be further studies. We also need to measure local retinal levels of sortilin and the AF38469 inhibitor. We also acknowledge that the human samples were post-mortem, and the collection times/disease duration could be different. Control mice will also need to be studied for toxicology of AF38469 alone. Future studies will be designed to address these concerns.
Conclusion
In conclusion, these early studies show that sortilin is significantly increased in the diabetic retina. This was associated with increased lysosomal proteins and inflammatory mediators. Delivery of an oral sortilin antagonist, AF38469, significantly improved the ERG and angiography measures in diabetic mice. These mice also had reduced inflammatory mediators and LAMP2 protein levels.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
LL did all mouse work; YJ performed the Western blotting work; MA provided the human samples; JJS designed the experiments and wrote the text.
Acknowledgements
These studies were funded by R01EY030284 (JJS), P30EY04068 Core grant (LDH, PI of Core grant), an unrestricted grant from Research to Prevent Blindness, and R01EY030054 (MA).
References
2. Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Mol Vis. 2009 Jul 25;15:1418–28.
3. Liu L, Jiang Y, Steinle J. Epac1 regulates TLR4 signaling in the diabetic retinal vasculature. Cytokine. 2021 Aug;144:155576.
4. Liu L, Jiang Y, Steinle JJ. Glycyrrhizin Protects the Diabetic Retina against Permeability, Neuronal, and Vascular Damage through Anti-Inflammatory Mechanisms. J Clin Med. 2019 Jul 2;8(7):957.
5. Mitok KA, Keller MP, Attie AD. Sorting through the extensive and confusing roles of sortilin in metabolic disease. J Lipid Res. 2022 Aug;63(8):100243.
6. Hermey G. The Vps10p-domain receptor family. Cell Mol Life Sci. 2009 Aug;66(16):2677–89.
7. Mysona BA, Shanab AY, Elshaer SL, El-Remessy AB. Nerve growth factor in diabetic retinopathy: beyond neurons. Expert Rev Ophthalmol. 2014 Apr;9(2):99–107.
8. Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004 Feb 26;427(6977):843–8.
9. Richner M, Pallesen LT, Ulrichsen M, Poulsen ET, Holm TH, Login H, et al. Sortilin gates neurotensin and BDNF signaling to control peripheral neuropathic pain. Sci Adv. 2019 Jun 19;5(6):eaav9946.
10. Xu J, Shen CJ, Ooi JD, Tang YS, Xiao Z, Yuan QJ, et al. Serum Sortilin Is Associated with Coronary Artery Calcification and Cardiovascular and Cerebrovascular Events in Maintenance Hemodialysis Patients. Kidney Dis (Basel). 2021 Jul 15;7(6):503–13.
11. Di Pietro P, Carrizzo A, Sommella E, Oliveti M, Iacoviello L, Di Castelnuovo A, et al. Targeting the ASMase/S1P pathway protects from sortilin-evoked vascular damage in hypertension. J Clin Invest. 2022 Feb 1;132(3):e146343.
12. Wang Y, Qin X, Paudel HK. Amyloid β peptide promotes lysosomal degradation of clusterin via sortilin in hippocampal primary neurons. Neurobiol Dis. 2017 Jul;103:78–88.
13. Canuel M, Korkidakis A, Konnyu K, Morales CR. Sortilin mediates the lysosomal targeting of cathepsins D and H. Biochem Biophys Res Commun. 2008 Aug 22;373(2):292–7.
14. Aguilera AC, Leiva N, Alvarez PA, Pulcini G, Pereyra LL, Morales CR, et al. Sortilin knock-down alters the expression and distribution of cathepsin D and prosaposin and up-regulates the cation-dependent mannose-6-phosphate receptor in rat epididymal cells. Sci Rep. 2023 Mar 1;13(1):3461.
15. Lv Y, Yang J, Gao A, Sun S, Zheng X, Chen X, et al. Sortilin promotes macrophage cholesterol accumulation and aortic atherosclerosis through lysosomal degradation of ATP-binding cassette transporter A1 protein. Acta Biochim Biophys Sin (Shanghai). 2019 May 23;51(5):471–83.
16. Xu F, Wei Y, Lu Q, Zheng D, Zhang F, Gao E, et al. Immunohistochemical localization of sortilin and p75(NTR) in normal and ischemic rat retina. Neurosci Lett. 2009 Apr 17;454(1):81–5.
17. Abu El-Asrar AM, Mohammad G, De Hertogh G, Nawaz MI, Van Den Eynde K, Siddiquei MM, et al. Neurotrophins and neurotrophin receptors in proliferative diabetic retinopathy. PLoS One. 2013 Jun 7;8(6):e65472.
18. Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, et al. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients With Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017 Oct 1;58(12):5594–603.
19. Jakobsen TS, Østergaard JA, Kjolby M, Birch EL, Bek T, Nykjaer A, et al. Sortilin Inhibition Protects Neurons From Degeneration in the Diabetic Retina. Invest Ophthalmol Vis Sci. 2023 Jun 1;64(7):8.
20. Rhost S, Hughes É, Harrison H, Rafnsdottir S, Jacobsson H, Gregersson P, et al. Sortilin inhibition limits secretion-induced progranulin-dependent breast cancer progression and cancer stem cell expansion. Breast Cancer Res. 2018 Nov 20;20(1):137.
21. Liu L, Jiang Y, Steinle JJ. PKA and Epac1 Reduce Nek7 to Block the NLRP3 Inflammasome Proteins in the Retinal Vasculature. Invest Ophthalmol Vis Sci. 2022 Jan 3;63(1):14.
22. Liu L, Jiang Y, Steinle JJ. Epac1 and Glycyrrhizin Both Inhibit HMGB1 Levels to Reduce Diabetes-Induced Neuronal and Vascular Damage in the Mouse Retina. J Clin Med. 2019 May 31;8(6):772.
23. Seidel A, Liu L, Jiang Y, Steinle JJ. Loss of TLR4 in endothelial cells but not Müller cells protects the diabetic retina. Exp Eye Res. 2021 May;206:108557.
24. Jiang Y, Liu L, Steinle JJ. Compound 49b Regulates ZO-1 and Occludin Levels in Human Retinal Endothelial Cells and in Mouse Retinal Vasculature. Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):185–9.
25. Ramsey DJ, Ripps H, Qian H. An electrophysiological study of retinal function in the diabetic female rat. Invest Ophthalmol Vis Sci. 2006 Nov;47(11):5116–24.
26. Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC Jr. Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002 Feb;80(4):667–77.
27. Li Q, Hu YZ, Gao S, Wang PF, Hu ZL, Dai RP. ProBDNF and its receptors in immune-mediated inflammatory diseases: novel insights into the regulation of metabolism and mitochondria. Front Immunol. 2023 Apr 18;14:1155333.
28. Yang L, Yao Y, Zheng W, Zheng X, Xie M, Huang L. Nitric oxide mediates negative feedback on the TXNIP/NLRP3 inflammasome pathway to prevent retinal neurovascular unit dysfunction in early diabetic retinopathy. Free Radic Biol Med. 2025 Jun;233:279–91.
29. Imdad L, Xu S, Meng Y, Bao K, Dong W, Yin X, et al. Txnip/Trx Is a Potential Element in Regulating O-GlcNAc Modification in Photoreceptors to Alleviate Diabetic Retinopathy. Int J Mol Sci. 2025 Jun 4;26(11):5369.
30. Zhang Q, Guy K, Pagadala J, Jiang Y, Walker RJ, Liu L, et al. Compound 49b prevents diabetes-induced apoptosis through increased IGFBP-3 levels. Invest Ophthalmol Vis Sci. 2012 May 17;53(6):3004–13.
31. Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004 Jul;36(7):1206–37.
32. Curtiss E, Liu L, Steinle JJ. miR15a regulates NLRP3 inflammasome proteins in the retinal vasculature. Exp Eye Res. 2018 Nov;176:98–102.