Abstract
Hodgkin lymphoma (HL) is a lymphoid malignancy of germinal center B cell origin. Conventional chemotherapy with or without radiation induces high cure rates but these treatments can have relevant long-term toxic side effects. As a result, there remains a lot of debate about the optimal management of these patients, especially for limited-stage disease. The last two decades have resulted in a greater understanding of the underlying biology of HL, including the presence of an aberrant phenotype on the tumor cells and the necessity of immune escape. Based on these insights, novel therapeutic approaches have been introduced, including the CD30-directed antibody drug conjugate brentuximab vedotin (BV) and the immune checkpoint inhibitors (ICI), nivolumab and pembrolizumab. After demonstrating to be effective and well tolerated in patients with relapsed/refractory (R/R) disease, these agents are currently being investigated as part of front-line regimens in clinical trials for both limited and advanced stage HL. Results are encouraging and the expectation is that these immunotherapies will be incorporated for the treatment of all stages of the disease. Not only is the hope these agents will further improve outcomes, but it will also reduce our dependence on chemotherapy and radiation in these settings, thereby mitigating the risk of long-term toxicity. This review will provide an overview of the molecular biology of HL, discuss the current standard of care for all stages of the disease, and highlight how immunotherapies are changing the current treatment landscape.
Keywords
Hodgkin Lymphoma, Brentuximab vedotin, Immune checkpoint inhibitor, Nivolumab, Frontline
Introduction
Hodgkin Lymphoma (HL) is an aggressive malignancy of B cell lymphocytes accounting for approximately 11% of lymphomas diagnosed world-wide [1]. Though HL had previously been subdivided into classical HL and nodular lymphocyte-predominant HL, this latter entity was renamed in 2022 as nodular lymphocyte-predominant B-cell lymphoma because of distinct pathological, biological and clinical differences [2,3]. Treatment advances over the last five decades, have significantly improved the outcome of patients diagnosed with HL, which is reflected by a 5-year survival rate of nearly 89% [4]. The goal of treatment for most patients is cure, including for patients with advanced and relapsed disease. With further improvements in multi-modal treatment approaches, the goal has been to maximize cure rates and minimize long-term toxicity. The latter is crucial as a significant number of patients are diagnosed with HL in the third decade of their life. Novel therapeutic approaches have been introduced that specifically target abnormalities unique to the disease as a result of a greater understanding of the underlying biology. Among these novel therapeutic approaches are the CD30-directed antibody-drug conjugate brentuximab vedotin (BV), and the immune checkpoint inhibitors (ICI), nivolumab and pembrolizumab. Both BV as well as pembrolizumab and nivolumab have been shown to be highly efficacious in clinical trials of HL and have been approved for use in the advanced and relapsed and refractory (R/R) settings. Moreover, it is the expectation that these immunotherapy agents will be increasingly used in the frontline setting, not only because of their efficacy but also because of their favorable safety profile. Here, we will review the current knowledge about the molecular biology of HL and summarize the important clinical advances in management of HL for both the frontline as well as the R/R settings.
Clinical Presentation and Staging
HL has a bimodal age distribution with a first peak seen among adults 20-30 years and a second peak in patients after 60 years of age [5,6]. However, incidence patterns vary among socio-economic status, gender and race. Most patients present with painless supradiaphragmatic nodal enlargement, often located cervical and supraclavicular [1,7,8]. Approximately 50-60% of patients have mediastinal lymphadenopathy at time of diagnosis, which can be bulky. Although the definition of “bulky” disease has changed over the last decades, this designation is generally given if a single nodal mass is over 10 cm in diameter, or if a mediastinal mass has a mass to mediastinal ratio (MMR) greater than 1/3 the maximum diameter of the thorax [9]. This distinction is important, because the presence of bulky disease has long been considered to be a poor prognostic factor. Symptoms can arise as a result of local compression by this growing mediastinal mass [10]. Other sites of nodal involvement include axillary, splenic, abdominal, and inguinal. Bone marrow involvement is rare at the time of diagnosis. About 30% of patients exhibit constitutional symptoms (“B” symptoms), which include (cyclical) fever, drenching night sweats and >10% unintended weight loss. Although less frequent, other symptoms could include pruritic, alcohol-related pain at nodal sites, as well as fatigue and decreased appetite. Excisional lymph node biopsy should be obtained to establish a diagnosis. Although a core needle biopsy is less ideal, this can be pursued in cases that have no easily accessible disease. Fluorodeoxyglucose (FDG) positron emission tomography (PET) – computed tomography (CT) has high sensitivity and specificity in HL and is recommend for clinical staging. In the absence of FDG uptake on PET/CT in the bone marrow, a bone marrow biopsy does not have to be performed [11]. Radiographic staging is based on the Lugano classification, which is derived from the Ann Arbor staging system (Table 1) [12]. Treatment is based on classifying patients as having either limited stage (stage I or II) disease, also sometimes referred to as early-stage disease, or advanced disease (stage III or IV). Limited stage disease is further classified as either “favorable” of “unfavorable” disease based on the presence of baseline risk factors including age, number of nodal sites affected, extranidal status, presence of constitutional symptoms, erythrocyte sedimentation rate, and the presence of bulky disease [13]. The internal prognostic score (IPS) is the most widely used clinical prediction model to predict progression-free survival (PFS) and overall survival (OS) for advanced stage HL [14].
|
Stage |
Involvement |
Extranodal (E) Status |
|
Limited |
||
|
I |
Involvement of one node or a group of adjacent nodes (one region) (I) |
Single extra-lymphatic site in the absence of nodal involvement (IE) |
|
II |
Involvement of two or more nodal groups on the same side of the diaphragm (II) |
Stage I or stage II by nodal involvement with limited contiguous extranidal involvement (IIE) |
|
Advanced |
||
|
III |
Involvement of nodes on both sides of the diaphragm; nodes above the diaphragm with splenic involvement |
Not applicable |
|
IV |
Involvement of non-contiguous extranidal sites (bone marrow, liver, lungs) |
Not applicable |
|
||
Molecular Biology of Hodgkin Lymphoma
For years, the cellular origins of HL remained unknown due to its unique morphology and immunophenotype [15]. The malignant cells are collectively termed Hodgkin and Reed-Sternberg (HRS) cells and consist of the mono-nuclear Hodgkin and the Patho gnomic multi-nucleated Reed-Sternberg (RS) cells [16]. Classical RS cells have a large-cell morphology with abundant basophilic cytoplasm and often a bilobed nucleus containing eosinophilic nucleoli. Most studies support that RS cells are continuously being generated as a result of incomplete cytokinesis and re-fusion of Hodgkin cells [17]. Despite being neoplastic, the presence of HRS cells in tumor tissue is rare, and their limited number is surrounded by a complex inflammatory microenvironment composed of various elements, including reactive T cells, granulocytes, plasma cells, fibroblasts and endothelial cells [18]. Based on the composition of this tumor micro-environment (TME), HL is further divided into nodular sclerosing (80%), mixed cellularity (15%), lymphocyte-rich (5%) and lymphocyte depleted (<1%) subtypes. The presence of HRS cells surrounded by resetting CD4+ T cells is not an uncommon finding and highlights the dependance of HRS cells on the surrounding TME.
The insight that HRS cells represent transformed B cells, came from studies that showed that HRS cells carried clonal Ig V gene rearrangements [19]. Subsequent sequence analysis of rearranged V genes in HRS cells revealed presence of somatic hypermutation suggesting that the cells were descendants of germinal center (GC) B cells [20]. Importantly, 25% of HRS cells carried non-sense mutations rendering originally functioning gene rearrangements non-functional [21]. Because this usually results in GC B cells undergoing apoptosis, the thought is that HRS are derived from pre-apoptotic GC B cells. Although the exact mechanism through which these GC B cells are able to escape apoptosis remains unclear, Ebstein-Barr Virus (EBV), appears to have a lymphoma-initiating role [22]. Latent EBV is identified in HRS cells in up to 20-40% of cases of HL, preferentially in mixed cellularity and lymphocyte depleted subtypes, and in most cases of patients infected with HIV. In EBV positive cases, viral proteins LMP1 and LMP2 are expressed [23]. Studies have shown that these two membrane proteins can mimic CD40 and BCR signaling, respectively, and thereby prevent apoptosis of transformed GC B cells with non-functional BCR signaling. Although most tumor cells retain phenotypic features of the cells from which they originate, HRS cells, strikingly, have lost the expression of most B-cell associated antigens [24]. While prominent expression for CD30 is almost universally observed, HRS cells are also often positive for CD15, MUM1 and the B cell transcription factor PAX5 (dim/weak) [25]. HRS cells are usually negative for CD3, CD20, CD45, and CD79a. The exact mechanism that underlies this reprogramming is not well understood, but studies have revealed several contributing factors including downregulation of B cell transcription factors (OCT2, BOB1, PU.1), epigenetic silencing of B cell genes (CD19, IgH) and deregulated expression of inhibitors of B cell molecules (ID2, ABF1, and NOTCH1). Increased expression of the latter results in inhibition of early B cell factor 1 (EBF1) as well as E2A, which are necessary transcription factors for B cell development [24,26-28].
The observation that HRS cells are able to escape apoptosis resulted in the recognition that these cells must rely on alternative signaling pathways for their survival and growth. Moreover, considering the extensive TME, HRS cells must be able to evade anti-tumor mechanisms from the cells residing in it. Constitutive activation of both the canonical and non-canonical NF-κB pathways is a contributor to the anti-apoptotic phenotype of HRS cells [29,30]. Similarly, gains and amplifications in positive regulators of NF-κB (REL, NIK, and BCL3), as well as deletions and inactivating mutations in negative regulators (TNFAIP3, NFKBIA, NFKBIE, CYLD and TRAF3) have been identified over the last two decades with often presence of multiple abnormalities in HRS cells at the same time. In addition to constitutive activation of NF-κB, overactivation of the JAK/STAT pathway is an almost universal finding in HL and crucial for promoting tumor inflammation and suppressing antitumor immunity [31,32]. While copy number gain of 9p24.1, where the JAK2 gene is located, is a recurrent abnormality and observed in over 95% of HRS cells, activating mutations in STAT3 and STAT6, as well as inactivating mutations in negative regulators, PTPN1 and SOCS1, are commonly seen as well [33]. In addition to JAK2, the 9p24.1 locus carries the PD-1 ligand genes, PD-L1 and PD-L2, and co-amplification is commonly observed [34]. Furthermore, both JAK2 expression as well as EBV infection are able to further induce PD-L1 expression [35]. Overexpression of PD-L1 and PD-L2 is able to induce immune evasion by directly engaging with PD-1 immune effector cells in the TME and suppressing their anti-tumor immune response [36]. Interestingly, despite PD-L1 expression, there only appears to be a limited role for true immune cell exhaustion in HL, and instead PD-L1 appears to have several roles in HRS survival, which all result in shaping the TME as a biological niche. While other genes and pathways have been implicated in Hodgkin lymphomagenesis, including PI3K/Akt (GNA13, ITPKB), MAPK/ERK (DUSP2), export signaling (XPO1), immune evasion (B2M, MHC2TA), and epigenetic regulation (ARID1A, JMJD2C), all ultimately support the growth and survival of HRS cells in the TME [37,38].
Limited-Stage Hodgkin Lymphoma
For decades, controversies have existed about the optimal treatment of patients with limited-stage HL (stage I-II). Although the majority of patients can be cured with combination chemotherapy alone, the role of radiation remains an issue of debate. HL is uniquely sensitive to radiation and the use of it results in improved disease control and increased PFS. However, delayed toxicities manifesting as increased cardiovascular complications and therapy-related malignancies have been observed even many decades after treatment completion. Due to high cure rates of this malignancy in this relatively young population of patients, attempts have been made to minimize radiation therapy (RT) as part of curative therapy. However, even by limiting radiation fields, the risk remains. The introduction of MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) back in the 1970’s was a major advancement in advanced HL yielding a 10-year overall survival (10-OS) of 66% without the need for radiation [39]. Further improvement came with the development of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine), which showed a survival benefit over MOPP [40]. Importantly, ABVD was also found to significantly lower the risk of secondary malignancies, as a result of omitting alkylating agents. The phase III HD10 trial further evaluated the number of cycles of ABVD and the dose of involved-field radiation therapy (IFRT) for patients with limited-stage HL with a favorable prognosis [41,42]. Favorable prognosis was defined as having absence of unfavorable risk factors, including the presence of a large mediastinal mass (mediastinal mass ratio MMR > 1/3), extra-nodal disease, involvement of ≥ 3 nodal areas, or elevated ESR (≥ 50 mm/hr for stage IA, IIA; ≥ 30 mm/hr for stage IB, IIB). This trial showed that two cycles of ABVD followed by 20 Gy is as effective, and less toxic, than four cycles of ABVD with 30 Gy for patients with limited-stage disease and no risk factors. 10-year PFS (10-PFS) was 87% each (HR 1.0; 95% CI 0.6-1.5) and 10-OS 94% each (HR 0.9; 95% CI 0.5-1.6), respectively. Based on the results of this trial, a new standard of care was established. However, no chemotherapy-only arm was included, and the rate of cardiovascular events and secondary malignancies had almost doubled at a median follow-up of 7.5 years. The HD.6 and RAPID trials further evaluated ABVD with or without radiotherapy for non-bulky, limited-stage HL [43,44]. Both studies highlighted that patients with non-bulky, limited-stage HL have excellent outcomes if they are treated with chemotherapy alone. Subsequent trials further improved our understanding by assessing the value of an interim PET/CT to guide early treatment adaptation and to identify patients who would benefit from additional cycles of chemotherapy and/or IFRT. In addition, trials tried to improve outcomes for patients with unfavorable disease characteristics, despite world-wide differences in classification of prognostic risk factors [13]. HD11 attempted to improve outcomes for patients with limited-stage, unfavorable HL. Four cycles of ABVD with either 20 or 30 Gy IFRT was compared to four cycles of the more intensified BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) regimen with 20 or 30 Gy IFRT [45]. At 5-year follow-up, four cycles of ABVD + 20 Gy IFRT were found to be non-inferior to the other three arms with similar 5-OS (BEACOPP + 20Gy vs ABVD + 20Gy; 95.1 vs 93.8%).
After successful introduction of BV and ICI for the treatment of HL in the advanced-stage as well as R/R setting, several studies have started to evaluate the efficacy of these agents for the frontline treatment of limited-stage HL (Table 2). A single-arm, pilot phase II trial evaluated efficacy of four cycles of BV-AVD followed by 30 Gy involved-site radiotherapy (ISRT) in 30 patients with untreated, limited-stage HL with unfavorable risk features as defined by German Hodgkin Study Group (GHSG) criterion [46]. 47% of patients had bulky disease and 57% had an MMR >1/3. After two and four cycles of BV-AVD, 90% and 93% of patients achieved PET/CT negativity, respectively. Two patients had primary refractory disease, and one patient discontinued treatment due to intolerable toxicity. All patients who completed BV-AVD + ISRT obtained a CR. 1-PFS was 93.9% (95% CI 84-102). Overall, the treatment was well tolerated with no evidence of significant pulmonary toxicity. A subsequent multicenter pilot study further evaluated the BV-AVD regimen with and without consolidative radiotherapy for limited-stage, unfavorable HL [47]. Patients received 4 cycles of BV-AVD, and divided in one of four cohorts, if PET/CT was negative after four cycles or PET/CT positive, but subsequent biopsy was negative, to receive either 30Gy ISRT, 20 Gy ISRT, 30Gy consolidation volume radiation therapy (CVRT) or no consolidation radiotherapy. Patient with a positive biopsy for HL went off study. 117 patients were enrolled with 30 patients in cohort 1 and 29 patients each in cohorts 2-4. There was a greater proportion of patients with bulky disease in cohorts 3 and 4. Complete response rates in cohorts 1-4 were 93%, 100%, 93%, and 97%, respectively. With a median follow-up of 3.8 years, 2-PFS was 93% (95% CI 83.9-1.0), 97% (95% CI 89.9-1.0), 90% (95% CI 78.5-1.0), and 97% (95% CI 89.9-1.0), respectively. These findings supported the safe reduction and even elimination of consolidative radiation among patients that are PET/CT negative after four cycles of BV-AVD. The larger BREACH trial was a multi-center, randomized, phase II trial evaluating the efficacy of BV-AVD also for patients with limited-stage, unfavorable HL [48]. Patients were eligible if they had limited-stage HL with ≥ 1 unfavorable EORTC/LYSA criterion. 170 patients were enrolled and randomized in a 2:1 ratio to either four cycles BV-AVD with IFRT or four cycles ABVD with IFRT. Primary endpoint was PET/CT response rate after two cycles based on 2007 Chesson criteria. After two cycles, PET/CT negativity (Deaville score 1-3) was observed in 82.3% of patients who received BV-AVD (90% CI 75.3-88) compared to 75.4% (90% CI 64.3-84.5) of patients who received ABVD. 2-PFS was 97.3% (95% CI 91.9-99.1) and 92.6% (95% CI 81.4-97.2), respectively. Strikingly, in patients that were PET/CT positive (Deaville score 4-5) after two cycles, 2-PFS was 90.9% (95% CI 74.4-97) for patients who received BV-AVD versus 70.7% (95% CI 39.4-87.9) for patients that received ABVD. Similarly, a non-randomized phase II trial was performed in 34 patients with non-bulky, limited stage HL [49]. All patients received a lead-in of two doses of BV followed by 4-6 cycles of BV-AVD based on PET/CT imaging obtained after the second cycle. No consolidative radiation was used. 38% of patients had unfavorable risk factors. An ORR was observed in 100% of patients after the initial two doses of BV, with a CR rate of 52.9% (95% CI 35.1-70.2). After two additional cycles of BV-AVD, CR rate was 97.1% (95% CI 84.7-99.9) with one fatality from sepsis. Although BV-AVD was well tolerated, peripheral neuropathy was observed in 79% of patients with 24% having grade 3-4 adverse events. The same group subsequently evaluated 4-6 cycles of BV-AD for the treatment of limited-stage HL in the hope that the omission of vinblastine would reduce the incidence of peripheral neuropathy as a result of overlapping toxicity between BV and vinblastine [50]. Again, no consolidative radiation was used. 34 non-bulky patients with stage I-II HL were enrolled. 47% of patients had unfavorable disease factors. ORR and CR rates were 100% (95% CI 76.3-98.1) and 97% (95% CI 87-100), respectively, with a 5-PFS of 91% (95% CI 75-99). Peripheral neuropathy was observed in 59% of patients, with most cases being grade 1 and no patients were observed of grade 3 or higher.
Only limited data is currently available about the use of ICI in limited-stage HL. A phase II non-randomized trial evaluated single-agent pembrolizumab for three cycles followed by 4-6 cycles of AVD in patients with both limited and advanced-stage HL [51]. 30 patients were enrolled, including 12 patients with limited-stage, unfavorable disease. CMR rate after single-agent pembrolizumab was 42% in patients with limited-stage, unfavorable disease, and increased to 100% after two additional cycles of AVD. At a median follow-up of 33.1 months (range, 26.0-43.0), PFS and OS remained at 100%. An international phase II trial to confirm the findings is currently ongoing. The NIVAHL study also evaluated efficacy of ICI for the treatment of limited-stage HL [52]. In this larger phase II trial, 109 patients with limited-stage, unfavorable HL were enrolled based on GHSG criteria and randomized to either four cycles of nivolumab-AVD (N-AVD) or sequential treatment of four cycles nivolumab, followed by two cycles of N-AVD, followed by two cycles of AVD. Both arms received ISRT for consolidation. At final analysis, 3-PFS and 3-OS estimates for the total cohort were 99% (95% CI 97-100) and 100%, respectively. One case of histologically-proven primary progression was observed in a patient while on nivolumab monotherapy. Several studies are currently underway evaluating novel agents for the treatment of limited-stage HL. Clinical trials are currently underway evaluating the efficacy of BV with nivolumab for the frontline treatment of patients with limited-stage HL, including for elderly patients (NCT03712202, NCT02758717). Another phase II trial (INDIE) is evaluating the efficacy of a different PD-1 inhibitor, Tislelizumab, for patients with limited stage, unfavorable HL (NCT04837859).
In summary, these smaller studies have evaluated both BV and ICI in limited-stage HL, and the data that we have is encouraging. Large randomized trials are needed to evaluate BV-AVD or ICI with AVD versus the standard of care of ABVD with or without consolidative radiation therapy. Further, these trials should omit mandatory consolidative radiation therapy in the experimental arms. BV-AVD should be considered for patients with limited-stage disease with unfavorable disease characteristics, especially in patients with bulky disease or MMR > 1/3, and this is our standard practice at our institution. In our opinion, combined modality treatment for limited-stage HL is likely a thing of the past rapidly approaching extinction as current approaches utilizing chemo-immunotherapy are showing high cure rates without the need for consolidative radiation therapy.
|
Setting |
Trial |
Treatment |
Dose |
Patients (age) |
Disease |
Outcome |
|
Limited Stage – Favorable |
Abramson [49] Phase 2 |
BV + AVD |
1.2 mg/kg days 1 & 15 followed by 1.2 mg/kg + AVD x 4-6 cycles |
34 (20-75yrs) |
21 Stage I/II favorable HL 13 Stage I/II unfavorable HL |
CR 52.9% BV only (95% CI 35.1-70.2); 97.1% BV-AVD x2 (95% CI 84.7-99.9); 3-PFS 94% (95% CI 78-98); 3-OS 97% (95% CI 81-100) |
|
Abramson [50] Phase 2 |
BV + AD |
1.2 mg/kg days 1 & 15 + AVD x 4-6 cycles |
34 (18-63yrs) |
18 Stage I/II favorable HL 16 Stage I/II unfavorable HL |
ORR 100% (95% CI 76.3-100); CR 97% (95% CI 87-100); 5-PFS 91% (95% CI 75-99) |
|
|
Limited Stage – Unfavorable |
Kumar [46] Phase 2
|
BV + AVD |
1.2 mg/kg q3w + AVD x4 cycles + RT |
30 (18-59yrs) |
Stage I/II unfavorable HL (GHSG criteria)
|
PET2 negativity 90% PET4 negativity 93% 1-PFS 93.5% (95% CI 84-102) |
|
Kumar [47] Phase 2 |
BV-AVD |
1.2 mg/kg q3w + AVD x4 cycles + RT (30 Gy ISRT, 20 Gy ISRT, 30 Gy CVRT, no RT) |
117 (18-59yrs) |
Stage I/II unfavorable HL (GHSG criteria) |
2-PFS 30Gy 93.1% (95%CI 83.9-1.0), 20Gy 96.6% (95%CI 89.9-1.0), 30Gy CVRT 89.7% (95%CI 78.5-1.0), 96.6% (95%CI 89.9-1.0). |
|
|
For Necker [48] Phase 2 BREACH |
BV+AVD vs ABVD |
1.2mg/kg q3w + AVD x4 cycles + RT vs ABVD x4 cycles + RT |
170 (18-60yrs) |
Stage I/II unfavorable HL (EORTC/LYSA) |
PET2 negativity 82.3% BV-AVD (90% CI 75.3-88) vs 75.4% ABVD (90% CI 64.3-84.5); 2-PFS 97.3% BV-AVD (95% CI 91.9-99.1) vs 92.6% ABVD (95% CI 81.4-97.2). |
|
|
Brockelman [52] Phase 2 NIVAHL |
A: Nivolumab + AVD B: Nivolumab, N-AVD, AVD |
A: 240 mg q3w + AVD + RT B: 240 mg q3w, 240 mg q3w + AVD x2, AVD x2 + RT |
109 (18-60yrs) |
Stage I/II unfavorable HL (GHSG criteria) |
3-PFS 99% N-AVD (95 CI 97-100) and 100% in sequential cohort. 3-OS 100% and 100%. |
|
|
Allen [51] Phase 2 KeyNote-667 |
Pembrolizumab + AVD |
2 mg/kg q3w x3 cycles followed by AVD x 4-6 cycles |
30 (21-77yrs) |
12 Stage I/II unfavorable HL 18 Stage III/IV HL |
CMR 37% (monotherapy pembro); 100% (pembro + 2x AVD); 3-PFS and 3-OS 100%
|
|
|
Advanced Stage |
Connors [59] Phase 3 ECHELON-1 |
BV + AVD vs ABVD |
1.2 mg/kg q3w + AVD x6 cycles vs ABVD x6 cycles |
1334 (26-53yrs) |
Stage III/IV HL |
6-PFS 82.3% BV-AVD vs 74.5% ABVD (HR 0.68, 95% CI 0.53-0.68); 6-OS 93.9% BV-AVD (95% CI 91.6-95.5) vs 89.4% ABVD (95% CI 86.6-91.7) |
|
Herrera [62] Phase 3 SWOG-S1826 |
Nivolumab + AVD vs BV + AVD |
240 mg q3w + AVD x 6 cycles vs 1.2 mg/kg q3w + AVD x 6 cycles |
970 (12-83yrs) |
Stage III/IV HL |
2-PFS 92% N-AVD vs 83% BV-AVD (HR 0.45; 95%CI 0.3-0.65)
|
|
|
Borchmann Phase 3 GHSG-HD21 |
BV+ECADD vs escBEACOPP |
1.2mg/kg + ECADD q3w x 4-6 cycles vs escBEACOPP x 4-6 cycles |
1500 (18-61yrs) |
Stage III/IV HL |
3-PFS 94.9% BV-ECADD vs 92.3% (HR 0.63, 95% CI 0.42-0.94) |
|
|
BV: Brentuximab Vedotin; yrs: years; HL: Hodgkin Lymphoma; CR: Complete Remission; CI: Confidence Interval; 3-PFS: 3-year Progression-Free-Survival; 3-OS: 3-year Overall Survival; PET2: PET/CT after 2 cycles; Nivo: Nivolumab; q3w: each 3 weeks; CMR: Complete Metabolic Remission. |
||||||
Advanced-Stage Hodgkin Lymphoma
Frontline therapy for advanced-stage HL (stage III-IV) remains curative intent due to its high susceptibility to chemotherapy. As a result, over 80% of patients with advanced-stage HL can be cured with multi-drug combination chemotherapy approaches. After the initial introduction of MOPP, and subsequent development of ABVD, both regimens were compared, not only against each other, but also as an alternating hybrid regimen (MOPP vs ABVD vs MOPP/ABVD) in newly diagnosed patients with advanced-stage HL [53]. This study demonstrated superiority of ABVD and MOPP/ABVD over MOPP alone, but also significant less toxicity of ABVD when compared to MOPP-containing regimens. This study established ABVD as a standard of care for patients with advanced-stage HL. Subsequent trials comparing ABVD with more complex multi-drug regimens failed to show similar outcomes, except for maybe therapy with escalated BEACOPP [54]. However, while treatment with escalated BEACOPP has been shown to result in higher PFS rates when compared to ABVD (5-PFS 93% vs 75%; HR 0.3, p=0.007), no significant difference have been observed in OS (5-OS 99% vs 92%; HR 0.18; p-0.06). Studies then tried to determine whether PET/CT could be used for risk adapted management. As part of the RATHL trial, 1214 patients with a new diagnosis of advanced-stage HL received two cycles of ABVD and subsequently underwent interim PET/CT after two cycles [55,56]. Patients with a negative PET/CT (Deaville 1-3) were randomly assigned to either continue ABVD or omit bleomycin (AVD) for the next 3-6 cycles. Patients with a positive PET/CT (Deaville 4-5) had treatment intensified with BEACOPP. For all patients, 7-year PFS and 7-year OS were 78.2% (95% CI 75.6-80.5) and 91.6% (95% CI 89.7-93.2), respectively. 3-PFS was 85.5% (95% CI 81.9-88.4) for the ABVD arm and 84.3% (95% CI 80.6-87.3) for the AVD arm, which fell within the predefined margin of non-inferiority. Patients who failed to obtain a negative PET/CT after two cycles, and proceeded with BEACOPP, had significantly inferior outcomes with observed 7-year PFS of 65.9% (95% CI 58.1-72.6) and 7-year OS of 83.2% (95% CI 76.2-88.3). Similar inferior outcomes were observed for patients with positive interim PET/CTs in the SWOG S0816 and GITIL/FIL HD 0607 trials, highlighting the need to escalate treatment in these patients [57,58].
After showing efficacy in R/R HL, BV was further evaluated in advanced-stage HL by incorporating it into established backbone regimens to not only improve efficacy but also with the hope to reduce toxicity. ECHELON-1 was a multi-center, randomized phase III clinical trial that involved patients with previously untreated stage III and IV HL (Table 2) [59,60]. A total of 1334 patients were enrolled and randomly assigned to receive either six cycles of BV-AVD (n=664) or ABVD (n=670). Interim PET/CT was performed after the second cycle. Long-term follow-up revealed a 6-OS benefit favoring BV-AVD over ABVD in both interim PET/CT negative patients (94.9% vs 90.6%; HR 0.53, 95% CI 0.34-0.86) as well as in interim PET/CT positive patients (95% vs 77%; HR 0.16, 95% CI 0.04-0.72). 6-PFS also favored BV-AVD over ABVD (82.3% vs 74.5%; HR 0.68, 95% CI 0.53-0.86) in all patient subgroups independent of stage, age, and IPS. A significantly higher incidence of peripheral neuropathy was observed in the BV-AVD arm, as well as incidence of neutropenic sepsis mandating addition of growth factor support. Based on ECHELON-1, BV in combination with chemotherapy was approved by the FDA in 2018 for previously untreated patients with stage III or IV HL. A follow-up phase II trial looked at sequential treatment of BV and AVD to minimize toxicity and improve curability for older patients with HL [61]. 48 patients with a median age of 69 years (range, 60-88) were enrolled and received two lead-in doses of single-agent BV, followed by six cycles of AVD, followed by four consolidative doses of BV. 81% of patients had advanced-stage HL, and 19% limited-stage, unfavorable disease. 52% of patients completed all 12 cycles of therapy. 2-EFS, 2-PFS and 2-OS were 80%, 84%, and 93%, respectively. Geriatric-based measures were strongly associated with survival. The currently ongoing GHSH HD21 trial attempts to further improve outcomes in this setting, by combining BV with ECADD (etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone). This phase III trial included 1500 patients with advanced HL who are randomized in a 1:1 ratio to either receive two cycles of BV-ECADD or two cycles of escalated BEACOPP before undergoing interim PET/CT. Patients that are PET/CT negative, will receive two additional cycles of the prescribed regimen, whereas patients that are PET/CT positive, will receive an additional four cycles. Data presented at the 2024 annual meeting of the American Society of Clinical Oncology revealed that at a median follow-up of 48 months, estimated 4-year PFS for patients receiving BV-ECADD was superior compared to patients who received escalated BEACOPP (94.3% vs 90.9%; HR 0.66, 95% CI 0.45-0.97), with similar 4-year OS.
Studies have also incorporated ICI with chemotherapy regimens for the frontline setting of patients with advanced HL. A pilot phase II trial evaluated concurrent administration of pembrolizumab with AVD in 30 patients with HL [51]. Patients of all stages were enrolled in the study, with the majority having advanced disease (60%). 2-PFS and 2-OS were 97% (95% CI 90-100) and 100%, respectively. None of the four patients that were found to have end-of-treatment positive PET/CT, yet negative ctDNA, have relapsed to date. More recently, the long-awaited results of the SWOG-S1826 trial were published after the second interim analysis had already shown promising results with achievement of the efficacy threshold [62]. This multicenter phase III study was a collaboration between the North American adult and pediatric cooperative groups. 994 patients with newly diagnosed, advanced-stage HL were enrolled and randomized between six cycles of either Nivolumab+AVD (n=496) or BV+AVD (n=498). 970 patients were eligible for evaluation and stratified based on age, IPS and intended use of RT for residual disease. The median age was 27 years (range, 12-84 years). With a median follow-up of 2.1 years (range, 0-4.2 years), 2-PFS was 92% (95% CI 89-94) after N+AVD compared to 83% (95% CI 79-86) after BV+AVD (HR 0.45; 95%CI 0.3-0.65). 2-OS was 99% in the N+AVD group and 98% in the BV+AVD group (HR 0.39; 95%CI 0.15-1.0). Only seven patients received end of treatment RT across arms. Overall, N+AVD was also better tolerated than BV+AVD. Fewer patients stopped treatment early on the N+AVD arm (7.6% versus 12.0% of patients treated with BV+AVD) and fewer deaths occurred during treatment with N+AVD (0.6% versus 1.7% for patients treated with BV+AVD). Grade ≥ 3 side effects occurred more frequently with BV+AVD, especially the occurrence of peripheral neuropathy (32% versus 2.9% for patients treated with N+AVD). Grade ≥ 3 neutropenia was more frequently seen with N+AVD (48% versus 26% for patients treated with BV+AVD). Immune-related adverse events were infrequent with nivolumab, and rates of pneumonitis, dermatitis and colitis were similar between arms. Hypothyroidism (7%) and hyperthyroidism occurred more frequently with N+AVD (7% and 3%, respectively) than with BV+AVD (0.01% and 0%, respectively). The differences in toxicity were particularly pronounced in patients older than 60 years of age. Rutherford et al. performed a subset analysis of the SWOG-S1826 trial data of patients aged 60 years and over and presented the data at the 2023 annual meeting of the American Society of Hematology. At the time of analysis, 97 patients ≥ 60 years had been enrolled with 48 patients being randomized to N+AVD and 49 patients to BV+AVD. The median age was 66 years (range, 60-83 years). 61% of patients had stage IV disease and 47% had IPS 4-7. After a median follow-up of 12.1 months, a superior PFS was observed in the N+AVD arm (HR 0.35; 95% CI 0.12-1.02). 1-PFS was 93% (95% CI 79-98) for N+AVD and 64% (95% CI 45-77) for BV+AVD. 1-OS was 95% (95% CI 83-99) for N+AVD and 83% (95% CI 67-92) for BV+AVD. In the N+AVD group there were 2 deaths observed (sepsis) and 3 patients with progression/relapse, whereas in the BV+AVD group 7 deaths were observed (sepsis, pneumonitis, unknown) and 8 patients with progression/relapse. Treatment was discontinued early in 5 patients (10%) on N+AVD and 16 patients (33%) on BV+AVD. This analysis highlighted that not only does N+AVD have an improved PFS when compared with BV+AVD in patients ≥60 years with advanced stage HL, it also is better tolerated in this patient population. Currently several trials are ongoing that are incorporating both BV and ICI with chemotherapy for the treatment of advanced stage HL (NCT06045195, NCT03907488). The phase II Pembo-FLASH trial is evaluating the efficacy of a combination of Pembrolizumab + EACOPP using a response-adapted approach in advanced stage HL (NCT06045195).
Based on the recent results of the SWOG-S1826 trial, N+AVD will become a new standard of care for the frontline treatment of patients with advanced HL. Although we are still awaiting long-term follow-up data, current results support that N+AVD is the preferred treatment over BV+AVD not only for patients ≥ 60 years of age, but also for younger patients with advanced stage HL. These recommendations also reflect the practice at our institution.
Relapsed and Refractory Hodgkin Lymphoma
Although the majority of patients with HL will obtain cure after frontline treatment, 10-15% of patients with limited-stage disease and 15-30% of patients with advanced-stage disease will eventually relapse. Moreover, in approximately 10-15% of patients, the disease will be refractory to initial treatment. The management of these patients has long been salvage treatment followed by, if eligible, high-dose-therapy with autologous stem cell rescue (HDT/ASCR). This recommendation is based off results from two phase III trials that revealed significant improvements in EFS, PFS and time-from-treatment-failure (TTF) for patients with R/R HL treated with HDT/ASCR when compared to patients treated with conventional salvage chemotherapy alone [63,64]. Although a significant proportion of patients can be cured with HDT/ASCR, approximately 40-50% of patients still relapse [65]. Prognostic models have been developed to predict the outcome of patients with R/R HL undergoing HDT/ASCR. Although over the years, several prognostic factors for TTF and OS have been identified, all studies highlight that response to salvage treatment is one of the most important predictors of outcome, and that the presence of metabolically active residual disease before undergoing HDT/ASCR confers an inferior prognosis [66-69].
Although optimal cytoreduction is crucial before HDT/ASCR, no randomized phase III trials exist in this setting to give evidence-based recommendations. Commonly used regimens include ICE (ifosfamide, carboplatin, etoposide), DHAP (dexamethasone, high-dose cytarabine, cisplatin), ESHAP (etoposide, methylprednisolone, high-dose cytarabine, cisplatin), GCD (gemcitabine, cisplatin, dexamethasone), and GVD (gemcitabine, vinorelbine, liposomal doxorubicin) [68,70-74]. ORR and CR rates are about similar between regimens and vary between 59-89% and 19-50%, respectively. Bendamustine in combination with gemcitabine and vinorelbine (BeGEV) was evaluated as well in a phase II trial as salvage regimen before HDT/ASCR in patients with R/R HL [75]. ORR was found to be 83%, with 73% of patients obtaining a CR.
High level CD30 expression is an almost universal finding on HRS cells in HL. However, initial efforts in phase I and II studies to target CD30 in patients with R/R HL using unconjugated monoclonal antibodies did not show significant anti-tumor activity [76-78]. These findings prompted further development of CD30 antibody-drug conjugates, which would have to rely less on the host immune response for clinical efficacy in patients that likely already have an impaired antibody-dependent cell-mediated immunity as a result of their tumor microenvironment. Initial phase I trials evaluating BV for R/R HL in heavily pre-treated patients revealed ORR of 50-59% and CR rates in the 25-35% [79,80] (Table 3). This prompted further evaluation of the efficacy of BV for the treatment of R/R HL in a pivotal phase II trial that looked at the efficacy and safety of single-agent BV in 102 patients with R/R HL who had progressed after HDT/ASCR [80]. 75% of patients had an ORR, and 34% of patients obtained a CR. Median PFS was 5.6 months (95% CI 5.0-9.0) and median duration of response for those in CR was 20.5 months (95% CI 10.8-NE). 5-year end-of-study results revealed a PFS rate of 22% (95% CI, 13-31%) and OS rate of 41% (95% CI, 31-51%) [81]. 13 patients remained in CR at 5 years. Based on these results, the FDA approved single-agent BV in 2011 for treatment of patients with HL who had progressed after HDT/ASCR, or who had at least two prior lines of therapy and were deemed not a candidate for HDT/ASCR.
After this initial approval, BV was further evaluated for treatment in the second-line, before HDT/ASCR, in combination with chemotherapy. Using a PET-adapted strategy, a trial conducted at Memorial Sloan Kettering Cancer Center evaluated the combination of BV with ICE as part of a non-randomized, single-Centre phase II clinical trial for patients with R/R HL [82]. 46 patients were enrolled, but only 45 patients received treatment with two cycles of BV. After completion of two cycles of BV, patients received a PET/CT. Patient who achieved a PET/CT negative status (Deauville score of 1 or 2) underwent directly HDT/ASCR. Patients who had persistent abnormalities on PET/CT (Deaville 3-5) received two cycles of augmented ICE before consideration of HDT/ASCR. After BV treatment, 12 patients (27%, 95% CI 13-40%) were found to be PET/CT negative and 33 patients (73%, 95% CI 60-86) were PET/CT positive. One patient withdrew consent, 32 patients received augmented ICE. 22 patients (69%, 95% CI 53-85) were found to be PET/CT negative after two cycles augmented ICE. Overall, 34 patients (76%, 95% CI 60-86) achieved PET/CT negativity. The combination of BV with ICE was overall well tolerated with few grade 3-4 adverse events. Similarly, BV has been evaluated in combination with other regimens to further improve CR rates before HDT/ASCR, including platinum-containing regimens such as ESHAP and DHAP, as well as, bendamustine [83-85]. In addition to improving CR rates before HDT/ASCR, BV has been evaluated as consolidation therapy after HDT/ASCR in the phase III AETHERA trial [86,87]. 329 BV-naïve patients with high-risk of relapse after HDT/ASCR, defined as either having relapsed or progressive HL within 12 months after frontline therapy, a history of refractory disease, or extranidal involvement before HDT/ASCR, where randomly assigned to either BV or placebo for up to 16 cycles starting 30-45 days after HDT/ASCR. At 5-year follow-up, a sustained PFS benefit was observed for patients treated with BV versus placebo (59% vs 41%; HR 0.521, 95% CI 0.379-0.717). Although neuropathy was observed with BV consolidation, it resolved in 90% of patients. Based on this trial, BV was approved by the FDA for consolidation post HDT/ASCR in 2015.
Overtime, immune checkpoint inhibitors (ICI) were found to have efficacy in HL. Initial studies, evaluated both PD-1 inhibitors, nivolumab (CheckMate-205) and pembrolizumab (KEYNOTE-087), as single-agents for the treatment of patients with R/R HL who progressed after HDT/ASCR [88,89]. Studies revealed strong efficacy, durable responses with a known toxicity profile. Pembrolizumab was subsequently compared head-to-head to BV in a multicenter, randomized, phase III trial (KEYNOTE-204) [90]. 304 patients with R/R HL who were ineligible or who had relapsed after HDT/ASCR were enrolled and randomly assigned to either pembrolizumab (151 patients) or BV (153 patients). After a median time to cutoff of 25.7 months, median PFS was 13.2 months in the pembrolizumab arm versus 8.3 months in the BV arm (HR 0.65; 95% CI 0.48-0.88). Based on the above results, the FDA approved single-agent nivolumab for patients with R/R HL whose disease had progressed after HDT/ASCR in 2016, and single-agent pembrolizumab for the treatment of patients with R/R HL whose disease has relapsed after three lines of therapy in 2017.
ICI were further evaluated as first salvage treatment in combination with either BV or chemotherapy. The combination of nivolumab with BV was evaluated as a chemotherapy-sparing option as part of a phase 1/2 trial in 91 patients with R/R HL [91]. After four cycles, ORR was found to be 87% (95% CI 75.5-91.3) with 67% (95% CI 56.4-76.5) of patients achieving a CR. Estimated 3-PFS was 77% (95% CI 65-86) with median PFS not reached. The estimated 3-OS was 93% (95% CI 85-97). No new toxicities were observed, and the adverse event profile of the combination was similar to each agent administered individually. Using a response-adapted approach, nivolumab alone or nivolumab with ICE was further evaluated in a phase II trial in 43 patients with R/R HL [92]. Patient would receive up to six cycles of nivolumab and upon achieving CR proceed to HDT/ASCR. Patients with progressive disease at any point, or patients not in CR after six cycles, would receive two cycles of nivolumab plus ICE (N-ICE). ORR and CR rates in patients with monotherapy nivolumab was 81% and 71%, respectively. Patients who received N-ICE had an ORR and CR of 93% and 91%, respectively. 2-PFS was found to be 72% (95% CI 56-83%) and 2-OS 95% (95% CI 82-99). Impressive results were seen in a phase II trial combining the other ICI, pembrolizumab, with GVD for second-line treatment of R/R HL [93]. Patients received 2-4 cycles of pembrolizumab with GVD. 39 patients were enrolled of which 41% of patients had primary refractory disease and 38% had relapsed within one year after frontline treatment. 31 patients received two cycles, and the other eight patients received four cycles. ORR and CR rates were 100% (95% CI 91-100) and 95% (95% CI 82-99), respectively. Thirty-six patients (95%) proceeded with HDR/ASCR. With a median follow-up of 13.5 months (range 2.66-27.06) after HDT/ASCR, all transplanted patients remained in remission.
Although allogenic stem cell transplant (allosct) has been used for decades as salvage strategy for R/R HL and is able to provide durable disease control for some patients, the reality is that as a result of a lack of potential donors and the significant treatment-associated toxicity in patients that have already been heavily pre-treated, this is not an option for most patients that have progressed after HDT/ASCR [94]. In addition to the novel agents discussed, chimeric antigen receptor T (CAR-T) cell therapy has been explored in this setting. The currently ongoing CHARIOT trial is a multi-center, phase II trial enrolling patients between 12-75 years of age with R/R HL and progression after at least three lines of therapy, including BV and ICI. Patients were treated with anti-CD30 CAR-T cells after lymphodepletion using bendamustine and fludarabine. The study allowed for second CAR-T injection in patients who progressed after initial response. Interim results were published at the 2022 annual conference of the American Society of Hematology. At that time, 15 patients had been enrolled. The median number of prior therapies was six (range: 3-18). Seven patients received a second infusion. ORR after the first infusion was 73.3%, with a CR rate of 60%. Of the five patients that received a second infusion and had data available, ORR was 100% with a CR rate of 60%. Overall, treatment was well tolerated. One case of grade 1 cytokine release syndrome was observed and no neurotoxicity. Several trials are underway to evaluate novel immunotherapy agents and combinations for the treatment of patient with R/R HL. One trial is currently evaluating combination treatment with BV and the BTK inhibitor ibrutinib for patients with R/R HL after at least one prior line of therapy (NCT02744612). However, preliminary results have shown significant toxicity likely limiting future development. Several trials are combining nivolumab with other agents, including ipilimumab (anti-CTLA4), axatilimab (anti-CSF-1R), and azacytidine for treatment of R/R HL upon progression after HDT/ASCR (NCT05723055, NCT01896999, and NCT0516297). Another trial is evaluating the combination of nivolumab with gemcitabine and bendamustine for patients with R/R HL also upon progression after HDR/ASCR (NCT03739619). A novel PD-1 inhibitor, prolgolimab, is currently being evaluated as single-agent and in combination with bendamustine for patients with R/R HL with progression after first line treatment (NCT05757466). Finally, the combination of BV with nivolumab is currently being evaluated for patients with R/R HL as post HDT/ASCR consolidation treatment (NCT03057795).
Based on the currently available data in the R/R setting, we favor an individualized approach that takes into consideration prior front-line treatment, presence of primary refractory or early relapsed (< one year) disease and the presence of comorbidities such as auto-immune disorders. Especially in patients with primary refractory or early relapsed disease, we would favor a regimen consisting of an ICI with either chemotherapy, or BV, if not received before as part of salvage treatment. All patients who obtain a CMR should undergo HDT/ASCR, if eligible. Consolidative radiation therapy should be used as appropriate in patients at high risk of treatment failure.
|
Setting |
Trial |
Treatment |
Dose |
Patients (age) |
Disease |
Outcome |
|
Salvage Treatment Post-HDT/ASCR |
Forero-Torres [77] Phase 2
|
SGN-30 (CD30 mAB) |
6 mg/kg or 12 mg/kg weekly |
79 (20-82 yrs) |
38 R/R HL 41 ALCL
|
ORR R/R HL 0/38 (0%) Stable disease R/R HL 11/38 (28.9%) |
|
Ansell [78] Phase 1/2 |
MDX-060 (CD30 mAB) |
0.1, 1, 5, 10 mg/kg weekly |
48 (19-78 yrs) |
40 R/R HL 6 ALCL 2 CD30+ TCL |
CR R/R HL 1/40 (2.5%) PR R/R HL 2/40 (5%) |
|
|
Younes [80] Phase 1 |
BV (SGN-35) |
0.1-3.6 mg/kg q3w |
45 (20-87 yrs) |
42 R/R HL 2 ALCL 1 AITL |
CR R/R HL 9/26 (35%) PR R/R HL 4/26 (15%) |
|
|
Fanale [79] Phase 1 |
BV |
0.4-1.4 mg/kg weekly |
44 (12-82 yrs) |
38 R/R HL 5 ALCL 1 PTCL-NOS |
ORR R/R HL 54% CR R/R HL 25% PR R/R HL 22% |
|
|
Younes [80] Phase 2 |
BV |
1.8 mg/kg q3w up to 16 cycles
|
102 (15-77 yrs) |
R/R HL
|
ORR 75%; CR 34% mPFS 5.6mo (95% CI 5.0-9.0); mOS 22.4mo (95% CI 21.7-NE) |
|
|
Salvage Treatment Pre-HDT/ASCR |
Moskowitz [82] Phase 2 |
BV ± augmICE |
1.2 mg/kg q3w x2 ± augmICE x2 cycles |
45 (13-65 yrs) |
R/R HL
|
CMR after BV x2 27% (95% CI 13-40); CMR after BV x2 + ICE x2 76% (95% CI 62-89) |
|
Lacasce [85] Phase 1/2 |
BV + bendamustine |
1.8 mg/kg q3w + bendamustine 90 mg/m2 x2-6 cycles |
55 (19-79 yrs) |
R/R HL
|
ORR 92.5% (95% CI 81.8-97.9%); CR 73.6% (95% CI 59.7-84.7); 2-PFS 69.8% |
|
|
Garcia [84] Phase 1/2 |
BV + ESHAP |
1.2 mg/kg q3w + ESHAP x 3x cycles |
66 (18-66 yrs) |
R/R HL |
ORR 91% (95% CI 84-98); CR 70% (95% CI 59-81); mPFS 71% (95% CI 65-77) mOS 91% (95% CI 84-98)
|
|
|
Maintenance Post-HDT/ASCR |
Moskowitz [86] Phase 3 AETHERA |
BV vs placebo |
1.8 mg/kg q3w up to 16 cycles vs placebo |
329 (18-71 yrs) |
R/R HL |
5-PFS 59% vs 41% (HR 0.52; 95% CI 0.38-0.72)
|
|
Salvage Treatment Post-HDT/ASCR |
Armand [88] Phase 2 CheckMate-205 |
Nivolumab |
3 mg/kg q3w until progression or toxicity |
243 (26-46 yrs) |
A: 63 R/R HL, BV naïve; B: 80 R/R HL, BV after HDT/ASCR C: 100 R/R HL, BV before HDT/ASCR |
A: ORR 65% (95% CI 52-77); CR 29%, PR 37% B: ORR 68% (95% CI 56-78); CR 13%, PR 55% C: ORR 73% (95% CI 63-81); CR 12%. PR 61% |
|
Kuruvilla [90] Phase 3 KeyNote-204 |
Pembrolizumab vs BV |
Pembrolizumab 200 mg q3w vs BV 1.8 mg/kg q3w |
304 (28-53 yrs) |
R/R HL |
mPFS pembro 13.2mo vs 8.3mo BV (HR 0.65; 95% CI 0.48-0.88) |
|
|
Salvage Treatment Pre-HDT/ASCR |
Mei [92] Phase 1/2
|
Nivolumab ± Nivolumab-ICE |
240 mg q3w x3-6 cycles ± Nivolumab-ICE x2 cycles |
43 (19-79yrs) |
R/R HL |
ORR 93% (95% CI 81.8-97.9) CR 91%; 2-PFS 72% (95% CI 56-83); 2-OS 95% (95% CI 82-99%) |
|
Moskowitz [93] Phase 2 |
Pembrolizumab + GVD |
200 mg + GVD q3w x2-4 |
39 (21-72 yrs) |
R/R HL |
ORR 100% (95% CI 91-100) CR 95% (95% CI 82-99); PR 5% (95%CI 1-18) |
|
|
Advani [91] Phase 1/2 |
Nivolumab + BV |
Nivolumab 3 mg/kg + BV 1.8 mg/kg q3w x4 cycles |
91 (18-69 yrs) |
R/R HL |
ORR 85% (95% CI 75.5-91.3); 3-PFS 77% (95% CI 65-86); 3-OS 93% (95% CI 85-97) |
|
|
HDT/ASCR: High-Dose Therapy with Autologous Stem Cell Rescue; MAB: Monoclonal Antibody; R/R: Relapsed / Refractory; HL: Hodgkin Lymphoma; yrs: years; ALCS: Anaplastic Large-Cell Lymphoma; CR: Complete Remission; ORR: Overall Response Rate; PR: Partial Remission; q3w: each 3 weeks; TCL: T-cell Lymphoma; BV: Brentuximab Vedotin; PTCL: Peripheral T-Cell Lymphoma; NOS: Not Otherwise Specified; CI: Confidence Interval; CMR: Complete Metabolic Remission; MPFS: Median Progression-Free-Survival; MOS: Median Overall Survival; AICE: Augmented ICE; 2-PFS: 2-year Progression-Free-Survival. |
||||||
Future Directions
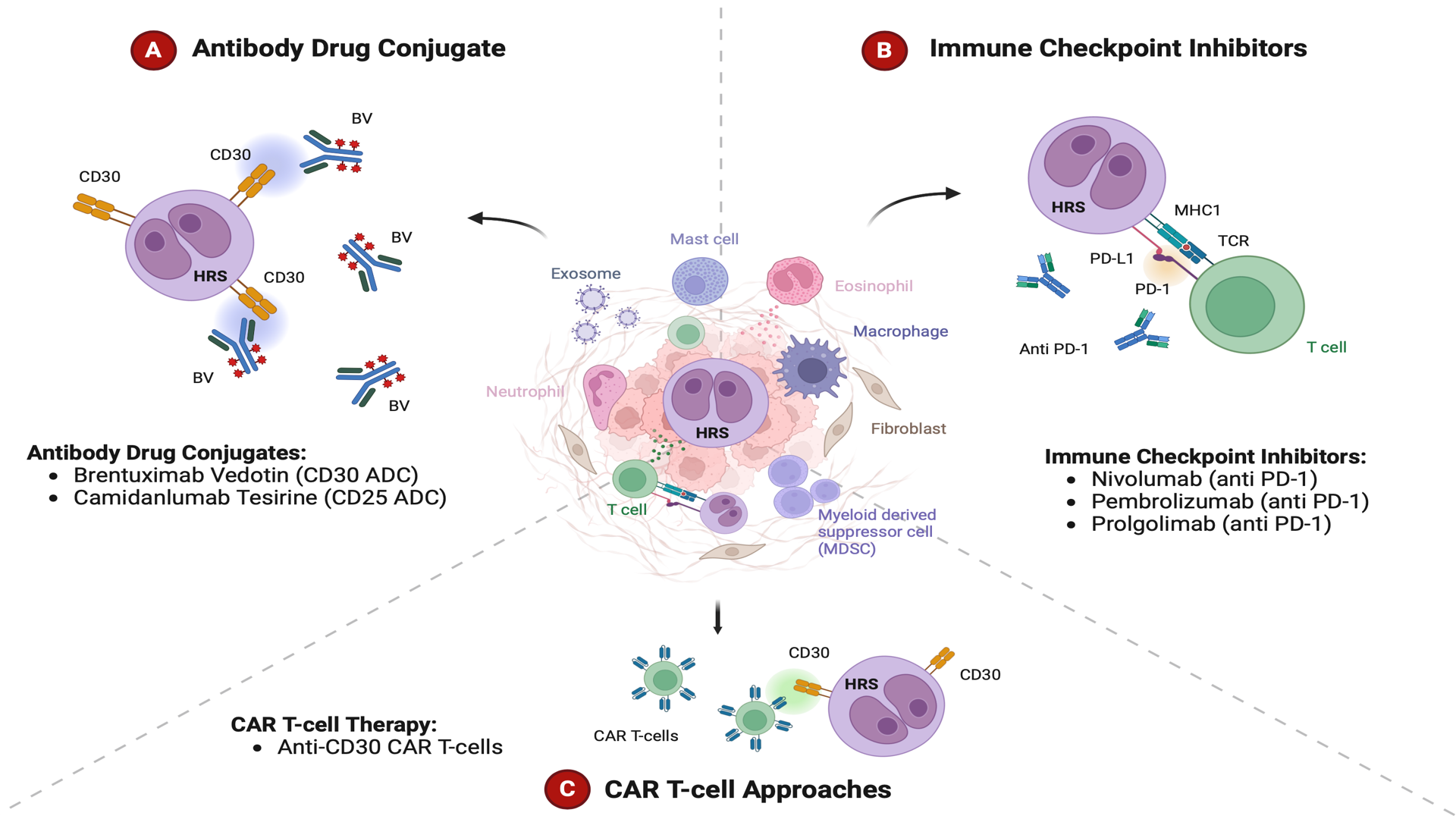
The treatment landscape of HL is evolving. BV and ICI have become integrated in salvage therapies and the combination of ICI with chemotherapy is challenging the paradigm of chemotherapy as a bridge to HDT/ASCR (Figure 1). However, the optimal sequencing of these new agents remains unclear as they are increasingly used in the upfront setting as well. In limited-stage HL, large frontline trials are needed to evaluate the combination of BV with AVD or ICI with AVD versus the standard of care of ABVD with or without consolidative radiotherapy. After four decades, these new approaches will hopefully further improve initial cure rates without the need for consolidative radiation. Trials are currently underway exploring the combination of BV with Nivolumab for the frontline treatment of limited-stage HL, and if found effective, this could be a chemo- and radiotherapy-free option (NCT03712202, NCT02758717). For advanced stage HL, the results of the phase III SWOG S1826 trial support that the combination of nivolumab with AVD establishes a new standard of care for the frontline treatment of both younger and older patients in this setting. Despite these improvements, new treatment strategies are needed, because there is still a group of patients that will progress even after treatment with HDT/ASCR, BV and ICI. Trials evaluating new approaches are currently underway and include newer generation of immune checkpoint inhibitors (tislelizumab, prolgolimab, and retifanlimab-dlwr), JAK-STAT inhibitors, anti-CD25 ADC (camidanlumab tesirine), anti-LAG3 antibodies (favezelimab), and CAR-T cell approaches [95]. Future strategies will likely incorporate the use of ctDNA to identify patients at high risk for treatment failure [96]. Immunotherapy has changed the treatment landscape of HL over the last decade, and primes the future for immunotherapy for all, reducing our dependence on chemotherapy and radiation for this disease.
Figure 1. Novel immunotherapy approaches for the treatment of Hodgkin Lymphoma. Among the novel therapeutic approaches for Hodgkin Lymphoma are the CD30-directed antibody-drug-conjugate brentuximab vedotin (A), the immune checkpoint inhibitors nivolumab and pembrolizumab (B), and chimeric antigen receptor (CAR) T-cell approaches that target CD30 (C).
References
2. Tousseyn TA, King RL, Fend F, Feldman AL, Brousset P, Jaffe ES. Evolution in the definition and diagnosis of the Hodgkin lymphomas and related entities. Virchows Arch Int J Pathol. 2023;482(1):207-26.
3. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36(7):1720-48.
4. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
5. Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence Patterns and Outcomes for Hodgkin Lymphoma Patients in the United States. Adv Hematol. 2011;2011:1-11.
6. Desai S, Guddati AK. Bimodal Age Distribution in Cancer Incidence. World J Oncol. 2022;13(6):329-36.
7. Shanbhag S, Ambinder RF. Hodgkin lymphoma: A review and update on recent progress. CA Cancer J Clin. 2018;68(2):116-32.
8. Roswarski JL, Longo DL. Hodgkin lymphoma: Focus on evolving treatment paradigms. Best Pract Res Clin Haematol. 2023;36(4):101510.
9. Kumar A, Burger IA, Zhang Z, Drill EN, Migliacci JC, Ng A, et al. Definition of bulky disease in early stage Hodgkin lymphoma in computed tomography era: prognostic significance of measurements in the coronal and transverse planes. Haematologica. 2016;101(10):1237-43.
10. Mauch P, Goodman R, Hellman S. The significance of mediastinal involvement in early stage Hodgkin's disease. Cancer. 1978;42(3):1039-45.
11. El-Galaly TC, d'Amore F, Mylam KJ, de Nully Brown P, Bøgsted M, Bukh A, et al. Routine Bone Marrow Biopsy Has Little or No Therapeutic Consequence for Positron Emission Tomography/Computed Tomography–Staged Treatment-Naive Patients With Hodgkin Lymphoma. J Clin Oncol. 2012;30(36):4508-14.
12. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol. 2014;32(27):3059-67.
13. Klimm B, Goergen H, Fuchs M, von Tresckow B, Böll B, Meissner J, et al. Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: an analysis of international staging definitions. Ann Oncol Off J Eur Soc Med Oncol. 2013;24(12):3070-76.
14. Hasenclever D, Diehl V. A Prognostic Score for Advanced Hodgkin’s Disease. N Engl J Med. 1998;339(21):1506-14.
15. Weniger MA, Küppers R. Molecular biology of Hodgkin lymphoma. Leukemia. 2021;35(4):968-81.
16. Küppers R, Hansmann ML. The Hodgkin and Reed/Sternberg cell. Int J Biochem Cell Biol. 2005;37(3):511-7.
17. Rengstl B, Newrzela S, Heinrich T, Weiser C, Thalheimer FB, Schmid F, et al. Incomplete cytokinesis and re-fusion of small mononucleated Hodgkin cells lead to giant multinucleated Reed-Sternberg cells. Proc Natl Acad Sci U S A. 2013;110(51):20729-34.
18. Piccaluga PP, Agostinelli C, Gazzola A, Tripodo C, Bacci F, Sabattini E, et al. Pathobiology of hodgkin lymphoma. Adv Hematol. 2011;2011:920898.
19. Küppers R, Rajewsky K, Zhao M, Simons G, Laumann R, Fischer R, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci U S A. 1994;91(23):10962-6.
20. Kanzler H, Küppers R, Hansmann ML, Rajewsky K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J Exp Med. 1996;184(4):1495-105.
21. Marafioti T, Hummel M, Foss HD, Laumen H, Korbjuhn P, Anagnostopoulos I, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95(4):1443-50.
22. Deacon EM, Pallesen G, Niedobitek G, Crocker J, Brooks L, Rickinson AB, et al. Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med. 1993;177(2):339-49.
23. Vrzalikova K, Ibrahim M, Nagy E, Vockerodt M, Perry T, Wei W, et al. Co-Expression of the Epstein-Barr Virus-Encoded Latent Membrane Proteins and the Pathogenesis of Classic Hodgkin Lymphoma. Cancers. 2018;10(9):285.
24. Schwering I, Bräuninger A, Klein U, Jungnickel B, Tinguely M, Diehl V, et al. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101(4):1505-12.
25. Grewal RK, Chetty M, Abayomi EA, Tomuleasa C, Fromm JR. Use of flow cytometry in the phenotypic diagnosis of hodgkin's lymphoma. Cytometry B Clin Cytom. 2019;96(2):116-27.
26. Küppers R, Bräuninger A. Reprogramming of the tumour B-cell phenotype in Hodgkin lymphoma. Trends Immunol. 2006;27(5):203-5.
27. Jundt F, Acikgöz O, Kwon SH, Schwarzer R, Anagnostopoulos I, Wiesner B, et al. Aberrant expression of Notch1 interferes with the B-lymphoid phenotype of neoplastic B cells in classical Hodgkin lymphoma. Leukemia. 2008;22(8):1587-94.
28. Mathas S, Janz M, Hummel F, Hummel M, Wollert-Wulf B, Lusatis S, et al. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol. 2006;7(2):207-15.
29. Jardin F. NFkB Pathway and Hodgkin Lymphoma. Biomedicines. 2022;10(9):2153.
30. Weniger MA, Küppers R. NF-κB deregulation in Hodgkin lymphoma. Semin Cancer Biol. 2016;39:32-9.
31. Tiacci E, Ladewig E, Schiavoni G, Penson A, Fortini E, Pettirossi V, et al. Pervasive mutations of JAK-STAT pathway genes in classical Hodgkin lymphoma. Blood. 2018;131(22):2454-65.
32. Fernández S, Solórzano JL, Díaz E, Menéndez V, Maestre L, Palacios S, et al. JAK/STAT blockade reverses the malignant phenotype of Hodgkin and Reed-Sternberg cells. Blood Adv. 2023;7(15):4135-47.
33. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268-77.
34. Roemer MG, Advani RH, Ligon AH, Natkunam Y, Redd RA, Homer H, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(23):2690-7.
35. Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O'Donnell E, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18(6):1611-8.
36. Taylor JG, Truelove E, Clear A, Calaminici M, Gribben JG. PDL1 shapes the classical Hodgkin lymphoma microenvironment without inducing T-cell exhaustion. Haematologica. 2023;108(4):1068-82.
37. Brune MM, Juskevicius D, Haslbauer J, Dirnhofer S, Tzankov A. Genomic Landscape of Hodgkin Lymphoma. Cancers. 2021;13(4):682.
38. Liu WR, Shipp MA. Signaling pathways and immune evasion mechanisms in classical Hodgkin lymphoma. Hematol Am Soc Hematol Educ Program. 2017;2017(1):310-6.
39. Longo DL, Young RC, Wesley M, Hubbard SM, Duffey PL, Jaffe ES, et al. Twenty years of MOPP therapy for Hodgkin's disease. J Clin Oncol Off J Am Soc Clin Oncol. 1986;4(9):1295-306.
40. Bonadonna G, Valagussa P, Santoro A, Viviani S, Bonfante V, Banfi A. Hodgkin's disease: the Milan Cancer Institute experience with MOPP and ABVD. Recent Results Cancer Res Fortschritte Krebsforsch Progres Dans Rech Sur Cancer. 1989;117:169-74.
41. Engert A, Plütschow A, Eich HT, Lohri A, Dörken B, Borchmann P, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363(7):640-52.
42. Sasse S, Bröckelmann PJ, Goergen H, Plütschow A, Müller H, Kreissl S, Buerkle C, et al. Long-Term Follow-Up of Contemporary Treatment in Early-Stage Hodgkin Lymphoma: Updated Analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 Trials. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(18):1999-2007.
43. Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Wells WA, Winter JN, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N Engl J Med. 2012;366(5):399-408.
44. Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N Engl J Med. 2015;372(17):1598-607.
45. Eich HT, Diehl V, Görgen H, Pabst T, Markova J, Debus J, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(27):4199-206.
46. Kumar A, Casulo C, Yahalom J, Schöder H, Barr PM, Caron P, et al. Brentuximab vedotin and AVD followed by involved-site radiotherapy in early stage, unfavorable risk Hodgkin lymphoma. Blood. 2016;128(11):1458-64.
47. Kumar A, Casulo C, Advani RH, Budde E, Barr PM, Batlevi CL, et al. Brentuximab Vedotin Combined With Chemotherapy in Patients With Newly Diagnosed Early-Stage, Unfavorable-Risk Hodgkin Lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(20):2257-65.
48. Fornecker LM, Lazarovici J, Aurer I, Casasnovas RO, Gac AC, Bonnet C, et al. Brentuximab Vedotin Plus AVD for First-Line Treatment of Early-Stage Unfavorable Hodgkin Lymphoma (BREACH): A Multicenter, Open-Label, Randomized, Phase II Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2023;41(2):327-35.
49. Abramson JS, Arnason JE, LaCasce AS, Redd R, Barnes JA, Sokol L, et al. Brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine for nonbulky limited-stage classical Hodgkin lymphoma. Blood. 2019;134(7):606-13.
50. Abramson JS, Bengston E, Redd R, Barnes JA, Takvorian T, Sokol L, et al. Brentuximab vedotin plus doxorubicin and dacarbazine in nonbulky limited-stage classical Hodgkin lymphoma. Blood Adv. 2023;7(7):1130-6.
51. Allen PB, Lu X, Chen Q, O'Shea K, Chmiel JS, Slonim LB, et al. Sequential pembrolizumab and AVD are highly effective at any PD-L1 expression level in untreated Hodgkin lymphoma. Blood Adv. 2023;7(12):2670-76.
52. Bröckelmann PJ, Bühnen I, Meissner J, Trautmann-Grill K, Herhaus P, Halbsguth TV, et al. Nivolumab and Doxorubicin, Vinblastine, and Dacarbazine in Early-Stage Unfavorable Hodgkin Lymphoma: Final Analysis of the Randomized German Hodgkin Study Group Phase II NIVAHL Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2023;41(6):1193-9.
53. Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327(21):1478-84.
54. Mondello P, Musolino C, Dogliotti I, Bohn JP, Cavallo F, Ferrero S, et al. ABVD vs BEACOPP escalated in advanced-stage Hodgkin's lymphoma: Results from a multicenter European study. Am J Hematol. 2020;95(9):1030-7.
55. Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A, et al. Adapted Treatment Guided by Interim PET-CT Scan in Advanced Hodgkin’s Lymphoma. N Engl J Med. 2016;374(25):2419-29.
56. Luminari S, Fossa A, Trotman J, Molin D, d'Amore F, Enblad G, et al. Long-Term Follow-Up of the Response-Adjusted Therapy for Advanced Hodgkin Lymphoma Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2024;42(1):13-8.
57. Gallamini A, Rossi A, Patti C, Picardi M, Romano A, Cantonetti M, et al. Consolidation Radiotherapy Could Be Safely Omitted in Advanced Hodgkin Lymphoma With Large Nodal Mass in Complete Metabolic Response After ABVD: Final Analysis of the Randomized GITIL/FIL HD0607 Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(33):3905-13.
58. Stephens DM, Li H, Schöder H, Straus DJ, Moskowitz CH, LeBlanc M, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood. 2019;134(15):1238-46.
59. Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin's Lymphoma. N Engl J Med. 2018;378(4):331-44.
60. Ansell SM, Radford J, Connors JM, Długosz-Danecka M, Kim WS, Gallamini A, et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N Engl J Med. 2022;387(4):310-20.
61. Evens AM, Advani RH, Helenowski IB, Fanale M, Smith SM, Jovanovic BD, et al. Multicenter Phase II Study of Sequential Brentuximab Vedotin and Doxorubicin, Vinblastine, and Dacarbazine Chemotherapy for Older Patients With Untreated Classical Hodgkin Lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(30):3015-22.
62. Herrera AF, LeBlanc M, Castellino SM, Li H, Rutherford SC, Evens AM, et al. Nivolumab+AVD in Advanced-Stage Classic Hodgkin's Lymphoma. N Engl J Med. 2024;391(15):1379-89.
63. Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet Lond Engl. 2002;359(9323):2065-71.
64. Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: results of a BNLI randomised trial. Lancet Lond Engl. 1993;341(8852):1051-4.
65. von Tresckow B, Müller H, Eichenauer DA, Glossmann JP, Josting A, Böll B, et al. Outcome and risk factors of patients with Hodgkin Lymphoma who relapse or progress after autologous stem cell transplant. Leuk Lymphoma. 2014;55(8):1922-4.
66. Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's lymphoma study group. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(1):221-30.
67. Jabbour E, Hosing C, Ayers G, Nunez R, Anderlini P, Pro B, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109(12):2481-9.
68. Moskowitz CH, Yahalom J, Zelenetz AD, Zhang Z, Filippa D, Teruya-Feldstein J, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148(6):890-7.
69. Brice P, Bouabdallah R, Moreau P, Divine M, André M, Aoudjane M, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin’s disease: analysis of 280 patients from the French registry. Société Française de Greffe de Moëlle. Bone Marrow Transplant. 1997;20(1):21-6.
70. Josting A, Rudolph C, Reiser M, Mapara M, Sieber M, Kirchner HH, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol Off J Eur Soc Med Oncol. 2002;13(10):1628-35.
71. Labrador J, Cabrero-Calvo M, Pérez-López E, Mateos MV, Vázquez L, Caballero MD, et al. ESHAP as salvage therapy for relapsed or refractory Hodgkin’s lymphoma. Ann Hematol. 2014;93(10):1745-53.
72. Abali H, Urün Y, Oksüzoğlu B, Budakoğlu B, Yildirim N, Güler T, et al. Comparison of ICE (ifosfamide-carboplatin-etoposide) versus DHAP (cytosine arabinoside-cisplatin-dexamethasone) as salvage chemotherapy in patients with relapsed or refractory lymphoma. Cancer Invest. 2008;26(4):401-6.
73. Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol Off J Eur Soc Med Oncol. 2007;18(6):1071-9.
74. Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(31):3490-6.
75. Santoro A, Mazza R, Pulsoni A, Re A, Bonfichi M, Zilioli VR, et al. Bendamustine in Combination With Gemcitabine and Vinorelbine Is an Effective Regimen As Induction Chemotherapy Before Autologous Stem-Cell Transplantation for Relapsed or Refractory Hodgkin Lymphoma: Final Results of a Multicenter Phase II Study. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(27):3293-9.
76. Bartlett NL, Younes A, Carabasi MH, Forero A, Rosenblatt JD, Leonard JP, et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood. 2008;111(4):1848-54.
77. Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146(2):171-9.
78. Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin's lymphoma and anaplastic large-cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(19):2764-9.
79. Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18(1):248-55.
80. Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(18):2183-9.
81. Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562-6.
82. Moskowitz AJ, Schöder H, Yahalom J, McCall SJ, Fox SY, Gerecitano J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16(3):284-92.
83. Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, et al. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study. Haematologica. 2021;106(4):1129-37.
84. Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez AP, Pinana JL, et al. Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group). Ann Oncol Off J Eur Soc Med Oncol. 2019;30(4):612-20.
85. LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132(1):40-8.
86. Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Lond Engl. 2015;385(9980):1853-62.
87. Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639-42.
88. Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(14):1428-39.
89. Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(19):2125-32.
90. Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021;22(4):512-4.
91. Advani RH, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138(6):427-38.
92. Mei MG, Lee HJ, Palmer JM, Chen R, Tsai NC, Chen L, et al. Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood. 2022;139(25):3605-16.
93. Moskowitz AJ, Shah G, Schöder H, Ganesan N, Drill E, Hancock H, et al. Phase II Trial of Pembrolizumab Plus Gemcitabine, Vinorelbine, and Liposomal Doxorubicin as Second-Line Therapy for Relapsed or Refractory Classical Hodgkin Lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(28):3109-17.
94. Perales MA, Ceberio I, Armand P, Burns LJ, Chen R, Cole PD, et al. Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2015;21(6):971-83.
95. Hamadani M, Collins GP, Caimi PF, Samaniego F, Spira A, Davies A, et al. Camidanlumab tesirine in patients with relapsed or refractory lymphoma: a phase 1, open-label, multicentre, dose-escalation, dose-expansion study. Lancet Haematol. 2021;8(6):e433-5.
96. Maco M, Kupcova K, Herman V, Ondeckova I, Kozak T, Mocikova H, et al. Circulating tumor DNA in Hodgkin lymphoma. Ann Hematol. 2022;101(11):2393-03.