Abstract
The development of direct-acting antiviral agents for the treatment of Hepatitis C (HCV) has changed the practice of treating patients with HCV. In particular, organ transplant recipients who have not previously been exposed to HCV are now able to consider receiving an organ from a donor who is infected with HCV, and anticipate effective antiviral therapy after transplantation. As a result of the opioid epidemic, the proportion of young, relatively healthy individuals dying from overdose has risen. The proportion of these individuals who are infected with HCV is significant. Many institutions have begun consenting HCV naïve patients with organ failure for receipt of organs from donors who have HCV with positive nucleic acid testing (NAT). One of the most robust fields of solid organ transplantation with HCV NAT+ donors is kidney transplantation. There have been many different strategies and barriers addressed through years of literature. Post-transplant outcomes have not demonstrated significantly increased risk in regards to death or organ rejection compared to a control group of kidney transplant patients. In addition, patients who undergo antiviral therapy after organ transplantation have demonstrated high rates of sustained virologic response (SVR). This review paper examines the background of HCV NAT+ transplantation, examines several landmark research manuscripts, and addresses those areas of ongoing controversy regarding the considerations of transplantation with HCV NAT+ organs into HCV naïve recipients.
Keywords
Kidney transplant, Hepatitis C, Direct-acting antivirals, Solid organ transplant
Abbreviations
CMV: Cytomegalovirus; DAA: Direct-acting Antiviral Agents; D-/R-: Donor Negative/Recipient Negative; D+/R-: Donor Positive/Recipient Negative; eGFR: Estimated Glomerular Filtration Rate; FCH: Fibrosing Cholestatic Hepatitis; HCV: Hepatitis C; NAT: Nucleic Acid Testing; OPTN: Organ Procurement and Transplantation Network; POD: Post-Operative Day; QALY: Quality-Adjusted Life-Years; SOT: Solid Organ Transplant; SVR: Sustained Virologic Response
Introduction
The treatment of hepatitis C virus (HCV) has undergone significant changes within the past decade. One of these changes is the ability to treat hepatitis C with direct-acting antiviral agents (DAA) that do not involve the use of interferon [1]. This advancement in therapy has allowed for consideration of antiviral therapy in non-hepatic transplant recipients [2]. Previously, high rates of allograft rejection from immune stimulation by interferon precluded antiviral therapy in these patients [3-5]. In addition to tolerability, new treatment regimens for hepatitis C are significantly more effective than previous iterations, allowing for confidence in treatment efficacy [2]. These new DAA combinations have dramatically affected the practice of solid organ transplantation (SOT). The United States is simultaneously in the midst of an epidemic of opioid use that disproportionally affects younger and healthier individuals [6-8]. There is a high rate of HCV infection in these individuals. The opioid epidemic escalated dramatically in the 2010s, with 13.4% of organ donors dying of overdose in 2017, compared to 1.1% of organ donors dying of overdose in 2010 [7]. Overdose deaths rose again in 2019-2020, largely driven by illicitly manufactured fentanyl and concurrent stimulant use [9]. Of organ donors who died of drug overdose, the prevalence of HCV infection increased from 7.8% in 2000 to 30% in 2017 [7]. In the past, those people who were HCV positive were not considered to be organ donors to recipients that were not already infected with hepatitis C. The efficacy and tolerability of HCV therapy with new DAA regimens allows for these donor organs to be transplanted to patients with organ failure regardless of whether they are already infected with hepatitis C. They are then cured of hepatitis C after transplantation with antiviral therapy.
Early Thoughts Regarding Transplantation of HCV NAT+ Organs into HCV NAT- Recipients
Organs from HCV positive donors have been able to be transplanted into recipients with known HCV since the 1990s with acceptable outcomes [10-12]. However, when these organs were used as donors in HCV-negative recipients prior to DAAs, there were overall poor outcomes including graft loss and death [13]. Deaths were attributed to higher rates of infection, development of post-transplant diabetes, glomerulonephritis, and liver failure [13,14]. From 1995 through 2008, HCV antibody positive kidneys were 2.9 times more likely to be discarded compared to HCV negative kidneys [15]. In the setting of new DAA antiviral regimens for HCV, clinical practices have evolved with more institutions utilizing HCV NAT+ donor organs with thorough informed consent of the recipients. In the context of highly effective DAA therapy, one survey revealed that 82% of transplant candidates would at least potentially accept a HCV+ donor [16].
Challenges of Antiviral Therapy
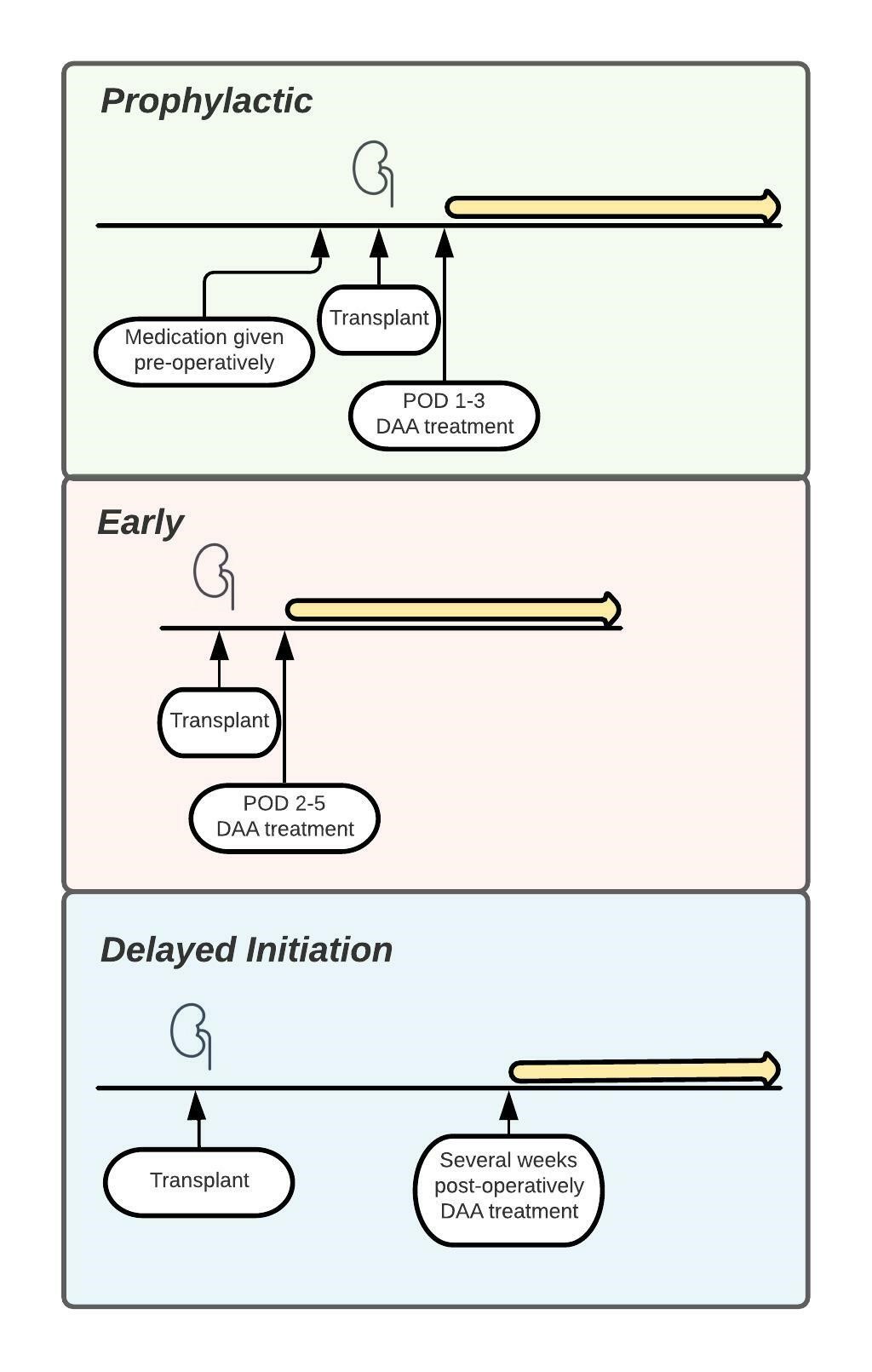
Since the advent of effective DAA therapy for HCV, several transplant centers have reported their experience with transplantation of HCV NAT+ kidneys into HCV naïve recipients (Table 1). Transplant centers have instituted varying strategies for antiviral therapy of HCV after liver transplantation. Specifics regarding the timing of antiviral treatment range from initiating treatment pre-operatively with one dose of DAAs to administering DAAs immediately post-operatively, to starting treatment several weeks post-operatively. Strategies are therefore categorized as prophylactic/pre-emptive/early approach versus delayed initiation (Figure 1). There are several factors that might influence timing of antiviral therapy including availability of antiviral therapy in the inpatient setting, testing turn-around time, cost of DAAs, route of drug delivery, and drug-drug interactions.
In the real-world experience of treating HCV, the hepatitis C genotype is determined and patients are initiated on a DAA regimen that is preferred and covered by their health insurance provider [17,18]. Those patients who lack medical insurance and the personal resources to pay for antiviral therapy may qualify for medication coverage by the pharmaceutical company itself. Typically, renal transplant recipients have medical insurance that will provide coverage for antiviral medications. The retail cost of an 8 to 12-week treatment course of DAAs is considerable, making insurance authorization processes very stringent. Early clinical trials in which antiviral therapy was administered in the inpatient setting involved the cost of antiviral therapy being paid by the transplant center, or by pharmaceutical companies that were involved in the specific clinical trial [19]. Edmonds found that in a real-world setting following solid organ transplants from HCV+ donors, it took patients about 45 days from transplant to receive their first dose of antiviral medication. Only 65% received insurance approval on initial submission, but with eventual insurance coverage or copay assistance programs provided by the pharmaceutical companies, the out-of-pocket costs for patients ended up being negligible [20]. In a cohort of 52 transplant patients in a North American medical center, significantly more patients had DAA initiation delays with commercial prescription coverage plans compared to governmental ones [21].
Treatment considerations may also include issues of drug delivery. Antiviral treatment regimens that are initiated early after transplantation may be started while patients are still intubated and unable to swallow pills. There is limited pharmacokinetic data on delivery of DAAs in crushed form via enteral access tube [22-24]. In addition, there are several drug-drug interactions to be aware of as well including immunosuppressants, amiodarone, proton pump inhibitors, azole family drugs [25-27]. However, despite all of these considerations, patients in available reports ultimately received treatment with various DAA regimens including glecaprevir/pibrentasvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir, and sofosbuvir/ledipasvir [19,28-33].
Available Trials and Reports of HCV D+/R- Kidney Transplantation
As noted in Table 1, treatment success is extremely high. Those rare patients who have been noted to have persistent viremia despite DAA therapy have had concerns raised by the author about lack of treatment adherence and patients subsequently declining further antiviral treatment although eligible [19]. Every other study, with over 300 patients analyzed in total, reported a 100% sustained virological response not counting the patients whose virologic response was still pending.
| Author/Trial | Study design | Included patients (kidney transplants) | Treatment strategy | Outcomes |
|---|---|---|---|---|
| Goldberg et al.(28) 2017 THINKER-1 |
Single-center, open-label, nonrandomized trial | 10 HCV D+/R- | Early (Tested POD 3) 12 week DAA, same for all |
- 100% SVR - Median eGFR at 6 months was 63 mL/min |
| Reese et al.(29) 2018 THINKER-2 |
Single-center, open-label, nonrandomized trial | Additional 10 HCV D+/R- from THINKER-1 | Early (Tested POD 3) 12 or 16 week DAA based on resistance testing, same DAA for all |
- 100% SVR - No significant difference in eGFR at 6 months in HCV+ vs. HCV- - No cases of rejection |
| Durand et al.(30) 2018 EXPANDER |
Single-center, open-label, nonrandomized trial | 10 HCV D+/R- | Early/Prophylactic (1 dose pre-operative then POD 1) 12 week DAA based on genotype |
- 100% SVR - No treatment related adverse events - Median eGFR at 12 weeks was 64 mL/min |
| Molnar et al.(31) 2019 |
Single-center, retrospective cohort study | 53 HCV D+/R- | Delayed (Median POD 76) 12-16 week DAA determined by hepatologist |
- 100% SVR - 1 patient developed FCH but resolved - 4 patients had acute rejection - Median eGFR at SVR12 was 67 mL/min - 34% developed BK viremia, 60% developed CMV viremia |
| La Hoz et al.(34) 2019 |
Retrospective analysis of OPTN registry | 548 HCV D+/R- and 36,934 HCV D-/R- | Not reported | - HCV D+/R- had significantly lower delayed graft function and better eGFR at 6 months - No difference in graft failure or survival |
| Gupta et al.(19) 2020 DAPPeR |
Single-center, open-label nonrandomized trial | 50 HCV D+/R- | Early/Prophylactic (1 dose pre-operative then POD 1 or POD 1-3) 12 week DAA based on genotype and resistance testing |
- 83% SVR - Only 6 required full DAA treatment - 1 death, 1 graft loss |
| Kapila et al.(35) 2020 |
Single-center, prospective cohort study | 64 HCV D+/R- | Early (Tested POD 3-5) DAA based on genotype and insurance approval |
- 71% SVR (with other 29% pending) - 2 patients developed FCH but resolved - 1 patient died of unknown cause |
| Sise et al.(32) 2020 MYTHIC |
Multicenter, prospective cohort study | 30 HCV D+/R- | Early (POD 2-5) 8 week DAA, same for all |
- 100% SVR - 3 patients developed rejection - 1 patient died of sepsis after SVR - 3 patients had BK viremia - Median eGFR at 6 months was 57 mL/min |
| Durand et al.(36) 2021 |
Single-center, open-label, nonrandomized trial | 10 HCV D+/R- | Early/Prophylactic (1 dose pre-operative then POD 1) 4 week DAA, same for all |
- 100% SVR - No episodes of rejection - 1 graft failure from venous thrombosis |
| Molnar et al.(37) 2021 |
Single-center, retrospective cohort study | 124 (65 HCV D+/R- and 59 HCV D-/R-) Additional work following 2019 study |
Delayed (Median POD 76) 12-16 week DAA determined by hepatologist |
- 100% SVR - Similar eGFR between two groups at 3, 6, 9, and 12 months - No difference in proportion of rejection, readmission, or death - HCV+ recipients had lower risk of graft loss |
| Concepcion et al.(33) 2021 |
Single-center, retrospective cohort study | 100 (50 HCV D+/R- and 50 HCV D-/R-) | Delayed (POD 29 +/- 11) 12-week DAA based on genotype and insurance approval |
- 100% SVR - At 6 months, no difference between SCr and eGFR - No difference in rates of rejection, readmission, complications |
Figure 1. Prophylactic, early approach strategies versus delayed initiation.
Outcomes
Those patients who were consented to receive a HCV+ donor kidney have been noted to have a significantly reduced time to transplant of between 23.5 to 58 days [28,32,35]. Although this waitlist time has increased over time as HCV+ transplants have become more widely utilized, this is still much shorter than the waitlist time for general deceased donor kidney transplant (DDKT). From the Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR) 2019 annual report, only approximately 25% of candidates for kidney transplantation receive a DDKT within 5 years. The median waitlist time has not been able to be calculated in over a decade due to half of the patients on the waitlist being listed since at least 2008 [38]. Considering the cost and reduced quality of life of patients with end-stage renal disease who are treated with maintenance dialysis, there is a substantial benefit that the patient could be afforded if willing to accept an HCV viremic transplant. Eckman described significantly increased effectiveness, measured in quality-adjusted life-years (QALYs) and U.S. dollars, from receiving an HCV+ kidney and subsequent antiviral treatment when compared to a HCV- kidney transplant. He estimated that unless the wait time for a HCV- kidney was less than 3.1 years, it was more effective to accept a HCV+ kidney. In this cost-effectiveness analysis, the median waitlist time was assumed to be 4.0 years for HCV- kidney transplants and 1.56 years for HCV+ kidney transplants to HCV- recipients. Costs of dialysis, DAA treatment, and overall mortality pre-transplant and post-transplant were major factors [39].
Although several studies described rapid post-transplant viremia occurring approximately 0-3 days post-operatively, there are differing conclusions as to whether or not this viremia is clinically significant [28,35]. Durand and Gupta both instituted prophylactic dosing with one dose immediately pre-operatively and then again starting POD1 to try to prevent any viremia from occurring [19,30,36]. Durand achieved more success with an extended 4 weeks of prophylactic treatment [36]. However, with delayed treatment, mimicking real-world experience, there were found to be no difference in rejection, readmission, and death [33,37].
Risks of HCV viremia have noted to potentially include fibrosing cholestatic hepatitis (FCH), BK viremia, and rejection. In this review, there were only 3 noted cases of FCH that resolved in a timely fashion with initiation of DAA therapy [31,35,40]. There were no studies in this review of kidney transplants that noted a significantly increased rate of organ rejection, however studies have previously noted increased rates of graft dysfunction and acute cellular rejection in heart transplants and liver transplants [41-44]. Molnar also noted high levels of BK viremia and CMV viremia in concurrence with HCV viremia, but overall in another more recent study found that a HCV+ renal transplant did not significantly increase a patient’s risk of developing BK viremia compared to HCV- transplants, although there was a higher risk of severe viremia in those patients affected [45].
Future Considerations
The use of HCV NAT+ donor kidneys has become very widespread with many transplant centers adopting this strategy. The utility of these organs in HCV naïve recipients has gained acceptance, although there remain concerns about whether subsequent HCV viremia leads to adverse events such as rejection, severe hepatic dysfunction, death, or other adverse outcomes. To date, there has not been evidence to suggest that a real-world delay in initiation of antiviral therapy after kidney transplantation results in higher risks of adverse outcomes. However, more research must be done to confirm the safety of this approach due to low sample size of current studies [33,37]. In addition, with increased use of HCV+ donor solid organ transplantation, there may be modified and expedited insurance authorization for DAA making the agents available while inpatient or very shortly after discharge. The specific pharmacologic agents used may still be guided by genotype, insurance coverage, and hepatologist recommendation. Further research will be needed to specify the pharmacokinetic interactions with other medications and the bioavailability of direct acting antiviral medications in crushed or alternative formulations. Avenues of research have included trialing kidney pump perfusion to reduce the viral inoculation load of HCV. Viral levels decreased but were not statistically or clinically significant. All patients still had detectable viremia and required DAA [46]. However, future efforts in biomedical engineering may be able to modify transmission of viruses between donor and recipients in SOT. Long-term outcomes are limited due to the paradigm shift in practice only evolving within the last 4 years. However, HCV has been present for several decades and current data suggests that once a patient has attained SVR, there is a very low recurrence risk. Simmons has cited the risk of viral recurrence to be 1.85 per 1000 person-years and many “recurrences” are in fact due to reinfection with a different HCV strain [47]. Long-term outcomes of those patients who have already undergone HCV+ kidney transplantation will be helpful to fully elucidate the benefits of the approach of utilizing HCV viremic donor organs.
Conclusions
While organ donation has increased over the past few decades, so have HCV infected donors as a result of the opioid epidemic [7]. Solid organ transplants from HCV+ donors have been done in liver transplantation for several decades but have recently expanded to other organs including kidney, heart, and lung. With the new generation of direct-acting antiviral agents, treatment of HCV after transplant has become accepted in several medical institutions and highly successful. From this review of 11 different trials, studies, and retrospective analyses, all available data has shown near 100% SVR in HCV- kidney transplant recipients who received organs from HCV+ donors. There are few reported adverse events but those of note include fibrosing cholestatic hepatitis, rejection, and BK and CMV viremia. Studies have not demonstrated any significant difference in readmission and death rates between D+/R- and D-/R- transplants [34]. There is also no proven consequence of a delayed approach with “real world” treatment of HCV [33,37]. HCV viremic organ donation has been proven to be a safe and effective.
References
2. Weinfurtner K, Reddy KR. Hepatitis C viraemic organs in solid organ transplantation. J Hepatol. 2021;74(3):716-33.
3. Wei F, Liu J, Liu F, Hu H, Ren H, Hu P. Interferon-based anti-viral therapy for hepatitis C virus infection after renal transplantation: an updated meta-analysis. PLoS One. 2014;9(4):e90611.
4. Fabrizi F, Martin P, Ponticelli C. Hepatitis C virus infection and renal transplantation. Am J Kidney Dis. 2001;38(5):919-34.
5. Magnone M, Holley JL, Shapiro R, Scantlebury V, McCauley J, Jordan M, et al. Interferon-alpha-induced acute renal allograft rejection. Transplantation. 1995;59(7):1068-70.
6. Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(50-51):1445-52.
7. Durand CM, Bowring MG, Thomas AG, Kucirka LM, Massie AB, Cameron A, et al. The Drug Overdose Epidemic and Deceased-Donor Transplantation in the United States: A National Registry Study. Ann Intern Med. 2018;168(10):702-11.
8. Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419-27.
9. O’Donnell J TL, Gladden RM, Davis NL, Bitting J. Trends in and Characteristics of Drug Overdose Deaths Involving Illicitly Manufactured Fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep; 2021. p. 1740-6.
10. Saab S, Ghobrial RM, Ibrahim AB, Kunder G, Durazo F, Han S, et al. Hepatitis C positive grafts may be used in orthotopic liver transplantation: a matched analysis. Am J Transplant. 2003;3(9):1167-72.
11. Morales JM, Campistol JM, Domínguez-Gil B, Andrés A, Esforzado N, Oppenheimer F, et al. Long-term experience with kidney transplantation from hepatitis C-positive donors into hepatitis C-positive recipients. Am J Transplant. 2010;10(11):2453-62.
12. Pereira BJ, Milford EL, Kirkman RL, Levey AS. Transmission of hepatitis C virus by organ transplantation. N Engl J Med. 1991;325(7):454-60.
13. Gupta G, Kang L, Yu JW, Limkemann AJ, Garcia V, Bandyopadhyay D, et al. Long-term outcomes and transmission rates in hepatitis C virus-positive donor to hepatitis C virus-negative kidney transplant recipients: Analysis of United States national data. Clin Transplant. 2017;31(10).
14. Bucci JR, Lentine KL, Agodoa LY, Peters TG, Schnitzler MA, Abbott KC. Outcomes associated with recipient and donor hepatitis C serology status after kidney transplantation in the United States: analysis of the USRDS/UNOS database. Clin Transpl. 2004:51-61.
15. Kucirka LM, Singer AL, Ros RL, Montgomery RA, Dagher NN, Segev DL. Underutilization of hepatitis C-positive kidneys for hepatitis C-positive recipients. Am J Transplant. 2010;10(5):1238-46.
16. McCauley M, Mussell A, Goldberg D, Sawinski D, Molina RN, Tomlin R, et al. Race, Risk, and Willingness of End-Stage Renal Disease Patients Without Hepatitis C Virus to Accept an HCV-Infected Kidney Transplant. Transplantation. 2018;102(4):e163-e70.
17. González-Grande R, Jiménez-Pérez M, González Arjona C, Mostazo Torres J. New approaches in the treatment of hepatitis C. World J Gastroenterol. 2016;22(4):1421-32.
18. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69(2):461-511.
19. Gupta G, Yakubu I, Bhati CS, Zhang Y, Kang L, Patterson JA, et al. Ultra-short duration direct acting antiviral prophylaxis to prevent virus transmission from hepatitis C viremic donors to hepatitis C negative kidney transplant recipients. Am J Transplant. 2020;20(3):739-51.
20. Edmonds C, Carver A, DeClercq J, Choi L, Peter M, Schlendorf K, et al. Access to hepatitis C direct-acting antiviral therapy in hepatitis C-positive donor to hepatitis C-negative recipient solid-organ transplantation in a real-world setting. Am J Surg. 2022 May;223(5):975-982.
21. Torabi J, Rocca JP, Ajaimy M, Melvin J, Campbell A, Akalin E, et al. Commercial insurance delays direct-acting antiviral treatment for hepatitis C kidney transplantation into uninfected recipients. Transpl Infect Dis. 2021;23(1):e13449.
22. Pijnenburg DWM, van Seyen M, Abbink EJ, Colbers A, Drenth JPH, Burger DM. Pharmacokinetic similarity demonstrated after crushing of the elbasvir/grazoprevir fixed-dose combination tablet for HCV infection. J Antimicrob Chemother. 2020;75(9):2661-5.
23. Oberoi RK, Zhao W, Sidhu DS, Viani RM, Trinh R, Liu W. A Phase 1 Study to Evaluate the Effect of Crushing, Cutting Into Half, or Grinding of Glecaprevir/Pibrentasvir Tablets on Exposures in Healthy Subjects. J Pharm Sci. 2018;107(6):1724-30.
24. Tanaka Y, Tateishi R, Koike K. Successful treatment of chronic hepatitis C virus infection with crushed glecaprevir/pibrentasvir administered via a percutaneous endoscopic gastrostomy tube: case report and review of the literature. Clin J Gastroenterol. 2019;12(6):588-91.
25. Garrison KL, German P, Mogalian E, Mathias A. The Drug-Drug Interaction Potential of Antiviral Agents for the Treatment of Chronic Hepatitis C Infection. Drug Metab Dispos. 2018;46(8):1212-25.
26. Gao LH, Nie QH, Zhao XT. Drug-Drug Interactions of Newly Approved Direct-Acting Antiviral Agents in Patients with Hepatitis C. Int J Gen Med. 2021;14:289-301.
27. Kahn JA. The use of organs from hepatitis C virus-viremic donors into uninfected recipients. Curr Opin Organ Transplant. 2020;25(6):620-5.
28. Goldberg DS, Abt PL, Blumberg EA, Van Deerlin VM, Levine M, Reddy KR, et al. Trial of Transplantation of HCV-Infected Kidneys into Uninfected Recipients. N Engl J Med. 2017;376(24):2394-5.
29. Reese PP, Abt PL, Blumberg EA, Van Deerlin VM, Bloom RD, Potluri VS, et al. Twelve-Month Outcomes After Transplant of Hepatitis C-Infected Kidneys Into Uninfected Recipients: A Single-Group Trial. Ann Intern Med. 2018;169(5):273-81.
30. Durand CM, Bowring MG, Brown DM, Chattergoon MA, Massaccesi G, Bair N, et al. Direct-Acting Antiviral Prophylaxis in Kidney Transplantation From Hepatitis C Virus-Infected Donors to Noninfected Recipients: An Open-Label Nonrandomized Trial. Ann Intern Med. 2018;168(8):533-40.
31. Molnar MZ, Nair S, Cseprekal O, Yazawa M, Talwar M, Balaraman V, et al. Transplantation of kidneys from hepatitis C-infected donors to hepatitis C-negative recipients: Single center experience. Am J Transplant. 2019;19(11):3046-57.
32. Sise ME, Goldberg DS, Kort JJ, Schaubel DE, Alloway RR, Durand CM, et al. Multicenter Study to Transplant Hepatitis C-Infected Kidneys (MYTHIC): An Open-Label Study of Combined Glecaprevir and Pibrentasvir to Treat Recipients of Transplanted Kidneys from Deceased Donors with Hepatitis C Virus Infection. J Am Soc Nephrol. 2020;31(11):2678-87.
33. Concepcion BP, Binari LA, Schaefer H, Rega S, Feurer I, Shawar S, et al. Kidney Transplantation From Hepatitis C Viremic Deceased Donors to Aviremic Recipients in a Real-world Setting. Transplant Direct. 2021;7(10):e761.
34. La Hoz RM, Sandıkçı B, Ariyamuthu VK, Tanriover B. Short-term outcomes of deceased donor renal transplants of HCV uninfected recipients from HCV seropositive nonviremic donors and viremic donors in the era of direct-acting antivirals. Am J Transplant. 2019;19(11):3058-70.
35. Kapila N, Menon KVN, Al-Khalloufi K, Vanatta JM, Murgas C, Reino D, et al. Hepatitis C Virus NAT-Positive Solid Organ Allografts Transplanted Into Hepatitis C Virus-Negative Recipients: A Real-World Experience. Hepatology. 2020;72(1):32-41.
36. Durand CM, Barnaba B, Yu S, Brown DM, Chattergoon MA, Bair N, et al. Four-Week Direct-Acting Antiviral Prophylaxis for Kidney Transplantation From Hepatitis C-Viremic Donors to Hepatitis C-Negative Recipients: An Open-Label Nonrandomized Study. Ann Intern Med. 2021;174(1):137-8.
37. Molnar MZ, Azhar A, Tsujita M, Talwar M, Balaraman V, Bhalla A, et al. Transplantation of Kidneys From Hepatitis C Virus-Infected Donors to Hepatitis C Virus-Negative Recipients: One-Year Kidney Allograft Outcomes. Am J Kidney Dis. 2021;77(5):739-47.e1.
38. Hart A, Lentine KL, Smith JM, Miller JM, Skeans MA, Prentice M, et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am J Transplant. 2021;21 Suppl 2:21-137.
39. Eckman MH, Woodle ES, Thakar CV, Alloway RR, Sherman KE. Cost-effectiveness of Using Kidneys From HCV-Viremic Donors for Transplantation Into HCV-Uninfected Recipients. Am J Kidney Dis. 2020;75(6):857-67.
40. Kapila N, Al-Khalloufi K, Bejarano PA, Vanatta JM, Zervos XB. Fibrosing cholestatic hepatitis after kidney transplantation from HCV-viremic donors to HCV-negative recipients: A unique complication in the DAA era. Am J Transplant. 2020;20(2):600-5.
41. Woolley AE, Singh SK, Goldberg HJ, Mallidi HR, Givertz MM, Mehra MR, et al. Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients. N Engl J Med. 2019;380(17):1606-17.
42. Woolley AE, Singh SK. The curious phenomenon of early cardiac allograft rejection with hepatitis C?infected donor heart transplants. J Heart Lung Transplant. 2020 Nov;39(11):1208-1209.
43. Schlendorf KH, Zalawadiya S, Shah AS, Perri R, Wigger M, Brinkley DM, et al. Expanding Heart Transplant in the Era of Direct-Acting Antiviral Therapy for Hepatitis C. JAMA Cardiol. 2020;5(2):167-74.
44. Ting PS, Hamilton JP, Gurakar A, Urrunaga NH, Ma M, Glorioso J, et al. Hepatitis C-positive donor liver transplantation for hepatitis C seronegative recipients. Transpl Infect Dis. 2019;21(6):e13194.
45. Molnar MZ, Potluri VS, Schaubel DE, Sise ME, Concepcion BP, Forbes RC, et al. Association of donor hepatitis C virus infection status and risk of BK polyomavirus viremia after kidney transplantation. Am J Transplant. 2022 Feb;22(2):599-609.
46. Forbes RC, Concepcion BP, Clapper D, DuBray BJ, Shawar S, Schaefer HM, et al. The effect of pulsatile pump perfusion on hepatitis C transmission in kidney transplantation: A prospective pilot study. Clin Transplant. 2020;34(8):e13987.
47. Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of Late Relapse or Reinfection With Hepatitis C Virus After Achieving a Sustained Virological Response: A Systematic Review and Meta-analysis. Clin Infect Dis. 2016;62(6):683-94.