Abstract
Guanylin peptides (GPs) and their receptor, guanylate cyclase C (GC-C), have recently become a topic of great interest in metabolic research. Guanylin and uroguanylin are the most investigated GPs and they belong to a larger family of natriuretic peptides. GPs play a physiological role in regulation of electrolyte balance via the intestine and the kidney by regulating the energy balance via their action in the brain. In addition to well-known cGMP signaling pathway, GPs activate a GC-C independent signaling pathway in the intestine, kidneys, as well as the brain. Even though the existence of two separate signaling pathways for other natriuretic peptides is well investigated, the GC-C independent signaling pathway is still a mystery. In this review, we summarize the recent discoveries related to the actions of GPs with special attention to the GC-C independent signaling pathway. We also discuss the main controversies in the field. Sex differences in GPs action via GC-C dependent and independent signaling pathway could address some of the discrepancies in literature. Here, we overview the role of GPs and their signaling pathways in the most common diseases of the modern world.
Keywords
Ca2+ signaling pathway, cGMP, Natriuretic peptides, Sex differences, Energy balance, Tripartite synapse, Hypothalamus, Midbrain
Natriuretic Peptides and Their Receptors
Guanylin peptides (GPs), guanylin (GN) and uroguanylin (UGN) belong to the family of natriuretic peptides (NPs), which includes atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP). NPs activate membrane guanylate cyclases (GC), also called particulate GCs, and lead to the production of their second messenger cyclic guanosine monophosphate (cGMP), which acts on cGMP dependent protein kinases (PKG), cGMPregulated phosphodiesterases (PDE) and cGMP–gated channels.
To this day, there are seven known GCs, spanning from GC-A to GC-G, all in the form of homodimers with each subunit composed of a chain of ~1500 amino acids. The intracellular domain has a juxtamembranous protein kinase-homology domain, an amphipathic α–helical separation domain and a cGMP forming GC domain on the C-terminal. The extracellular domain, which is connected to the intracellular part by short transmembrane domain, is a binding site for NPs [for a review, see 1]. GC-A is a receptor for ANP and BNP, which are mainly released from the atria due to atrial stretch. GC-A has a main role in maintaining blood pressure and intravascular volume by its effects on kidneys, adrenals, and central nervous system [2]. Other important effects on metabolism are the induction of lipolysis in human adipocytes [3], an increase in the energy expenditure in brown adipose tissue (BAT) and the “browning” of white adipose tissue (WAT) [4], secretion of the adipokine adiponectin [5] and the improvement of insulin sensitivity in muscle and liver [6,7]. GC-B is the receptor for CNP. Besides their expression in many tissues, their main role is in the physiological regulation of skeletal growth [8]. Other effects include embryonal axonal branching, oocyte maturation, inhibition of hypertrophic cardiomyocyte growth and the proliferation of fibroblasts and vascular smooth muscle cells [for a review, see 1].
ANP, BNP, and CNP, but not GPs, also bind to another receptor which does not have GC activity, called natriuretic peptide receptor C (NPR-C) [9]. All three hormones bind to NPR-C with a similar affinity which is alike the affinity for GC-A or GC-B [10-12]. This GC independent receptor is also called the clearance receptor since it is suggested that its main role is to remove NPs from the circulation and modulate their physiological effects [13]. Further research showed that removing NPs is not NPR-C’s only function as it exerts other functions as well. NPR-C is coupled to the pertussis toxin-sensitive adenylate cyclase inhibitory G protein (Gi) and contains specific Gi- activator domain, therefore NPs, via activation of the NPR-C, can decrease cyclic adenosine monophosphate (cAMP) levels [14,15]. Furthermore, NPR-C can activate the β-isoform of phospholipase C (PLCβ) by β subunit of the same Gi protein and lead to an increase in intracellular Ca2+ concentration [16]. NPR-C is widely expressed in many tissues and cell lines and in most tissues its expression is higher than that of the GC receptors for NPs [17].
Guanylate Peptides and Guanylate Cyclase C
GN and UGN are small peptides that bind to GC-C and, like other NPs, increase production of cGMP. Human GN is a 15- and UGN a 16-amino acid peptide, respectively, with two disulphide bonds between cysteine positions 4–12 and 7-15 [18,19]. The main site of expression of GPs is the intestine, where they play their major role in sodium and water reabsorption. They are also expressed in many other tissues like the kidney, lungs, pancreas, adrenal glands, and reproductive system, where their role and signaling is still not fully discovered [20]. The only known receptor for GPs, that is known today, is GC-C. It can be found in the form of dimer and trimer [21,22]. Activity of the GC-C can be regulated by adenosine triphosphate (ATP) and protein kinase C (PKC). ATP leads to an increase in GC activity by stabilizing the active form of the receptor, while phosphorylation by PKC, which is different to the regulation of GC-A and GC-B, increases GC-C activity by 70% [21,23-26]. GCC, like GPs, is also widely expressed with the major expression site being the intestines but also the kidney, brain, embryonic and regenerating liver, adrenal glands, pancreas, lungs, male and female reproductive system, and lymph nodes [27-29]. The role of GC-C in many of those organs, similar to the function of GPs, is still unknown, since most studies were done on the gastrointestinal tract and kidneys, and, in recent years, in the brain.
GPs and GC-C in intestine
Since GPs are mainly produced after a salty meal in the intestines and released into the gut lumen and circulation, most of GPs/GC-C signaling has been investigated in the intestine [30]. cGMP, produced by activation of the GC-C on the apical membrane of enterocytes, leads to the activation of PKG II, the inhibition of the Na+/H+ exchanger, and an increase in intracellular cAMP and the activation of protein kinase A (PKA) directly, or by inhibition of its degradation via PDE3. PKG II and PKA activate cystic fibrosis transmembrane regulator (CFTR) that activates the Cl−/HCO3− exchanger [for a review, see 31]. In general, GPs released in the gut lumen lead to decreased Na+ reabsorption and increased Cl−, HCO3− and water secretion (Figure 1). Since GPs are also released into the circulation, and GC-C is widely distributed, they also have effects on other organs.

GPs and GC-C in the kidney
The function of GC-C in the kidneys is less known. The major effects of GPs in the kidney are contributed to their activation of the GC-C independent signaling pathway (see later). Even though the natriuresis, kaliuresis, and diuresis are still present in GC-C knock out (GC-C KO) male mice [32], the volume of urine is decreased in female GC-C KO mice compared to their wild-type (WT) littermates suggesting sex differences in GC-C function in the kidney [33]. To determine the importance of these sex differences and GC-C action in the kidney, further study is necessary.
GPs and GC-C in the brain
Recently, research of effects of UGN/GC-C signaling pathway in the brain and their role in metabolism became a hot topic. GC-C was shown in the rodent brain in neurons of the cerebral cortex, amygdala, midbrain, hypothalamus and Purkinje cells and neurons of deep nuclei of the cerebellum, but also confirmed in the human hypothalamus and prefrontal cortex [33-39].
In the amygdala, we showed a GC-C expression in basolateral amygdaloid nucleus and cortical amygdaloid area as well as sex-dependent effects on anxiety-like behavior with an increase in the GC-C mRNA expression 2 hours after feeding. This was shown only in female mice who exert higher anxiety level after a meal [35]. Since the sex differences in the function of GC-C are well known in different parts of the brain as well as in the kidney, further studies are necessary to resolve the important question of effects of female sex hormones and the importance of the phase of estrous cycle on GC-C function in female animals and women.
In the midbrain, GC-C is located on dopaminergic neurons of substantia nigra compacta and ventral tegmental area where UGN neuromodulates neurons by increasing the activity of group 1 metabotropic glutamate receptors (mGLUR) and muscarinic acetylcholine receptors (mAChR) which leads to an increase in the firing frequency, which is possibly mediated by cation channels of transient receptor potential canonical (TRPC) channel family. Thus, GC-C KO mice have symptoms similar to attention deficit and hyperactivity disorder (ADHD) with an increase in locomotor activity, higher level of novelty seeking, and deficits in sustaining attention [36].
GPs and GC-C in the hypothalamus
UGN role in metabolism, including regulation of glucose homeostasis, induction of satiety and changes in activity of brown adipose tissue, is mediated by GC-C located in the proopiomelanocortin expressing (POMC) neurons of the arcuate nucleus in the hypothalamus, both in rodent and humans, but also in POMC-negative neurons in female mice and women [33,37,38]. This suggests that a sex difference in UGN action on the metabolism should be expected. This hypothesis is supported by the GC-C expression upregulation after feeding, like in the intestine, but only in the hypothalamus of male mice [33,35,40]. After a meal, GC-C expression is differently regulated in different brain regions. It increases only in the female amygdala [35] and only in the male hypothalamus [33], suggesting a completely different physiological function of GC-C in male and female animals. Besides the difference in localization, GC-C is more expressed during diestrus compared with estrus, which probably affects the changes in appetite during the menstrual cycle (the less GC-C, the weaker the satiety effect of UGN) [33]. Even though metabolic studies in female subjects are still rare, the common mistake is to neglect the estrous cycle phase and feeding schedule when comparing food intake in male and female animals [38].
Intracerebroventricular (icv) administration of UGN leads to a decrease in 24-hour food intake and weight gain and it is not than surprising that GC-C KO mice have signs of metabolic syndrome with increased body mass, hyperinsulinemia, hyperleptinemia, and disturbed glucose metabolism [38,41]. Except satiety, UGN has also effects on BAT physiology, but its activity is different compared to other natriuretic peptides, like ANP.
BAT is specialized adipose tissue that expresses special proteins of thermogenesis, like uncoupling proteins (UCPs). BAT transforms energy given by glucose or fatty acids not to ATP, but rather to heat. It is activated by exposure to cold with a goal to warm up the body [42,43]. Since GC-C is not expressed in the BAT, UGN acts via hypothalamus-sympathetic nervous system axis. Chronic icv administration of UGN increases BAT thermogenic markers, like UCP1, UCP3, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), therefore GC-C KO mice have decreased levels of those markers [33,41]. This effect of UGN on BAT is probably mediated by the sympathetic nervous system since in the triple KO (β1, β2 and β3 adrenergic receptor knock out) mice this increase of UCP1 in BAT is abolished (Figure 2) [41].
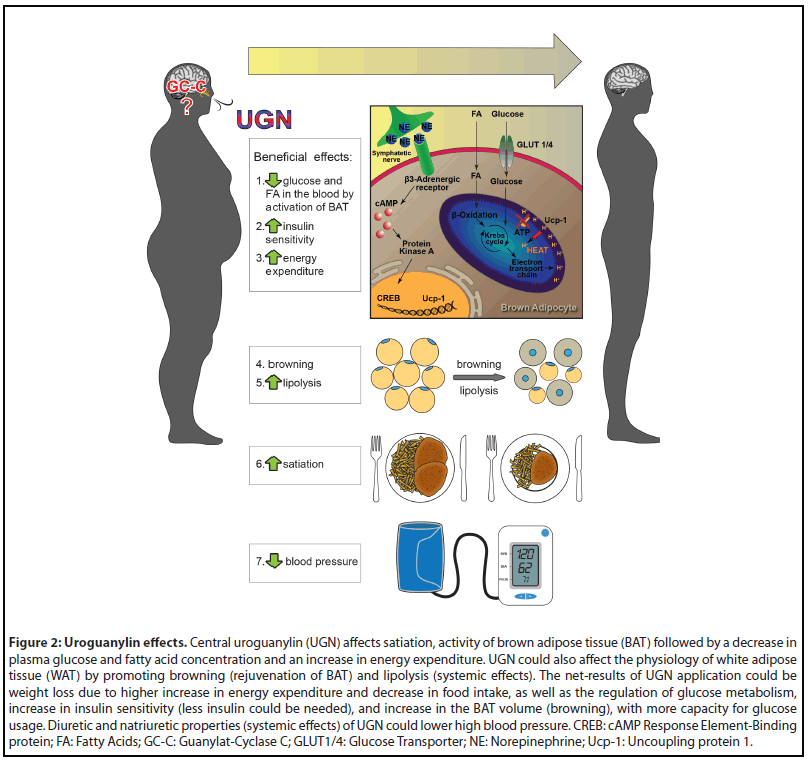
BAT activity is also increased after a meal and participates in diet induced thermogenesis (DIT). DIT is increased energy expenditure after a meal above basal rate. DIT occurs due to movement of the intestine, digestive and absorption processes, and activation of BAT [44]. GC-C KO mice almost completely lack activation of BAT and DIT after a meal while intranasal UGN administration increases BAT activity 2 and 4 hours after application in WT male mice, which was not seen in GC-C KO male mice [33]. The DIT and BAT activation after a meal as well as UGN effects of BAT activity are, again, in laboratory animals, as well as in humans, sex dependent but also phase of estrous cycle and age dependent [our unpublished results, 33]. The summarized hypothalamic effects of UGN are presented in Figure 2. The more detailed discussion of the matter is beyond the scope of this review.
Guanylate Cyclase C Independent Signaling Pathway for Guanylin Peptides
As we previously mentioned, ANP, BNP and CNP, except activating their GC receptors, also activate NPR-C which lacks GC activity. NPR-C, besides its role in removing the NPs from circulation, has many different physiological functions [9]. To this day, GC-C is the only known receptor for GPs but for many years there was published evidence of the existence of other GC-C independent signaling pathways for GPs.
GC-C independent signaling pathway in the intestine
In the intestine, the localization of binding sites for Escherichia coli heat-stable enterotoxin (STa) and GC-C mRNA are not identical [45]. STa is the strongest exogenous GC-C agonist that could activate all GPs receptors. Two binding sites are identified. One has a higher affinity for STa than GC with 5% of STa binding sites. Lower affinity GC receptor has 95% of STa binding sites [46]. Even though GC-C is dominantly located on the apical membrane, GC independent binding sites are located on the basolateral site of the colonocytes [47]. Another evidence of the existence of a novel signaling pathway was shown on a cell culture of rat enterocytes where STa administration lead to an increase in intracellular Ca2+ concentration (Figure 1) [48].
GC-C independent signaling pathway in the kidney
When studying GPs’ effects on kidneys, more evidence of GC-C independent signaling pathway for GPs exists. Renal effects of GPs are still present in GC-C KO mice and GPs and STa still change membrane potential of principal cells of cortical collecting ducts isolated from GC-C KO mice [32,49]. Further evidence of the existence and importance of the GC-C independent signaling pathway in human physiology is that GPs depolarize human principal cells of cortical collecting ducts where the GC-C is not expressed, while the same cells are hyperpolarized by cGMP [50]. Therefore, it is not surprising that GC-C KO male mice have normal blood pressure while UGN KO male mice develop hypertension showing an important role of UGN via GC-C independent signaling pathway in the kidney (diuresis and natriuresis) in blood pressure regulation (Figure 2) [51,52]. Future studies will determine the importance of the GC-C independent signaling pathway in female subjects.
GC-C independent signaling pathway in the brain
The importance of the UGN and GC-C dependent and/ or independent signaling pathways on metabolism is controversial. As Valentino et al. showed that GC-C KO mice have signs of metabolic syndrome as mentioned above [38], Begg et al. showed that GC-C KO mice have normal body weight, glucose tolerance, and that the central administration of GC-C agonists did not reduce food intake according to their WT littermates, showing that UGN/GC-C role on satiety and metabolism is UGN dependent, but GC-C independent [37].
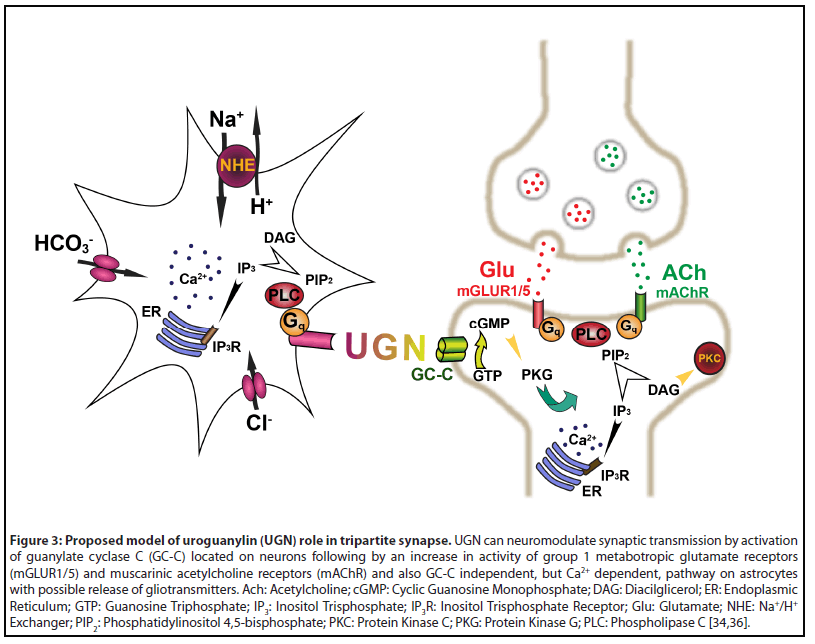
We also noticed the possible existence of a novel signaling pathway for GPs in the brain. In physiological conditions, GC-C is strictly located in neurons of the mouse and human brain, but not in the astrocytes [33-35]. Surprisingly, UGN still has effects on astrocyte function. UGN increases intracellular Ca2+ concentration in astrocytes of different brain regions (cerebral cortex, cerebellar cortex, and hypothalamus). This, Ca2+ signaling pathway, is present in GC-C KO animals. Furthermore, cultured astrocytes hyperpolarize in the presence of UGN which is opposite to the depolarization caused by cell permeable cGMP. Therefore, cGMP is not the second messenger for UGN in these cells. In astrocytes, this GC-C independent signaling pathway changes intracellular pH by change in H+ and HCO3- transport [34].
Ca2+ signaling is the most important mechanism for astrocytes in modulating neuronal function as shown in the model of the tripartite synapse. In this model, astrocytes are, along with presynaptic and postsynaptic neuron, part of the synapse and contribute to synaptic transmission as a third party by releasing several known neurotransmitters like glutamate, ATP, D-serine, and GABA. These neurotransmitters are also called gliotransmitters as they are released from glial cells [for a review, see 53]. As GC-C is located only in neurons where UGN acts as a neuromodulator [36] and astrocytes showed presence of GC-C independent signaling pathway [34], we propose a model of UGN action on the tripartite synapse (Figure 3). UGN can act as a neuromodulator both in neurons directly by GC-C or indirectly by activating the GC-C independent signaling pathway in astrocytes. An increase in intracellular Ca2+ concentration in astrocytes can lead to the acidification of interstitial fluid and possible release of gliotransmitters.
Future Aspects
As shown in this review, GPs and GC-C are present in many systems of the body but the GC-C independent signaling pathway is only investigated in some, like the brain, the intestine, and the kidney. The presence of GC-C independent signaling pathway for GPs should be determined in other systems wherever GC-C/cGMP signaling pathway exist (such as lungs, reproductive system, pancreas) since both signaling pathways can be present and act simultaneously. The special interest is the involvement of the GC-C independent signaling pathway in pathophysiological conditions such as asthma, cystic fibrosis, stroke, unexplained male, or female infertility etc. While using GPs centrally or systemic in treatment of diabetes, obesity, or hypertension, it is still not known whether its effect would be due to activation of the dependent or the independent GC-C signaling pathway (Figure 2).
The researchers should also pay attention to the interpretation of the results on the GC-C KO animals and determine the effects on the GP KO animals, like the UGN KO mice, where all signaling pathways for GPs are diminished. Discovery and cloning of the GC-C independent receptor or diorphanization of one of the already known orphan receptors that bind GPs should be the most important task for the future. Knowing the new receptor will bring the field of GPs’ roles in physiology to a new era.
Conclusion
Many recent studies and controversial results considering roles of UGN in metabolism and brain physiology indicate that the GPs/GC-C axis is a very interesting field of research. This axis gives us novel insights into physiology of energy balance, glucose metabolism and pathophysiology of the most common diseases of the modern world. Signaling pathways for GPs in the intestine and kidneys are more investigated than in other tissues where GPs and GC-C are expressed. Like other NPs, except GC, they have a GC independent receptor, but there is growing evidence that GPs also have other GC-C independent signaling pathway. In addition to age and sex, estrous cycle has an effect on GPs’ function, which should be better investigated in the future. Discovery of the new receptor for GPs involved in the GC-C independent, but Ca2+ dependent, signaling pathway will surely help to distinguish GPs’ action whether they are GC-C dependent or independent and clear discrepancies and controversies in the field.
Conflict of Interest
The authors report no conflict of interest.
Acknowledgements
This manuscript is funded by the Scientific Centre of Excellence for Basic, Clinical and Translational Neuroscience (project "Experimental and clinical research of hypoxicischemic damage in perinatal and adult brain"; GA KK01.1.1.01.0007 funded by the European Union through the European Regional Development Fund) and by the Croatian Science Foundation research grant (IP-2018-01- 7416). We thank Josip Dugandžic for his expertise in graphic preparation.
Author Contributions Statement
Ivan Strinic and Nikola Habek wrote the manuscript. Aleksandra Dugandžic revised the manuscript.
References
2. Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70(3): 665-99.
3. Sengenes C, Berlan M, De GI, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14(10):1345-51.
4. Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022-36.
5. Birkenfeld AL, Boschmann M, Engeli S, Moro C, Arafat AM, Luft FC, et al. Atrial natriuretic peptide and adiponectin interactions in man. PLoS One 2012;7(8):e43238.
6. Jung DY, Ha H, Lee E, Hu X, Tran DA, Tsitsilianos N, et al. BNP is a novel cardiomyokine that regulates hepatic insulin action in diet induced obese mice. Diabetes. 2014;63:A10-A10.
7. Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122(12):4675-79.
8. Vasques GA, Arnhold IJ, Jorge AA. Role of the natriuretic peptide system in normal growth and growth disorders. Horm Res Paediatr. 2014;82(4): 222-9.
9. Rose RA, Giles WR. Natriuretic peptide C receptor signalling in the heart and vasculature. J Physiol. 2008;586(2):353-66.
10. Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev. 1993;45(4):455-97.
11. Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, et al. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238(4827):675-8.
12. Levin ER. Natriuretic peptide C-receptor: more than a clearance receptor. Am J Physiol Endocrinol Metab. 1993;264(4 Pt 1):E483-9.
13. Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M, et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci U S A. 1999;96(13):7403-8.
14. Anand-Srivastava MB, Sehl PD, Lowe DG. Cytoplasmic domain of natriuretic peptide receptor-C inhibits adenylyl cyclase. Involvement of a pertussis toxin-sensitive G protein. J Biol Chem. 1996;271(32):19324-9.
15. Pagano M, Anand-Srivastava MB. Cytoplasmic domain of natriuretic peptide receptor C constitutes Gi activator sequences that inhibit adenylyl cyclase activity. J Biol Chem. 2001;276(25):22064-70.
16. Murthy KS, Makhlouf GM. Identification of the G proteinactivating domain of the natriuretic peptide clearance receptor (NPR-C) J Biol Chem. 1999;274(25):17587-92.
17. Anand-Srivastava MB. Natriuretic peptide receptor-C signaling and regulation. Peptides. 2005;26(6):1044-59.
18. de Sauvage FJ, Keshav S, Kuang WJ, Gillett N, Henzel W, Goeddel DV. Precursor, structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992;89(19):9089-93.
19. Kita T, Smith CE, Fok KF, Duffin KL, Moore WM, Karabatsos PJ, et al. Characterization of human uroguanylin: A member of the guanylin peptide family. Am J Physiol Renal Physiol. 1994;266(2 Pt 2):F342-8.
20. Sindic A. Current Understanding of Guanylin Peptides Actions. ISRN Nephrol. 2013;2013:813648.
21. Vaandrager AB, Van der Wiel E, De Jonge HR. Heat-stable enterotoxin activation of immunopurified guanylyl cyclase C. Modulation by adenine nucleotides. J Biol Chem. 1993;268(26):19598-603.
22. Vaandrager AB, Van Der Wiel E, Hom ML, Luthjens LH, De Jonge HR. Heatstable enterotoxin receptor/guanylyl cyclase C is an oligomer consisting of functionally distinct subunits, which are non-covalently linked in the intestine. J Biol Chem. 1994;269(23):16409-15.
23. Bhandari R, Suguna K, Visweswariah SS. Guanylyl cyclase C receptor: regulation of catalytic activity by ATP. Biosci Rep. 1999;19(3):179-88.
24. Gazzano H, Wu HI, Waldman SA. Activation of particulate guanylate cyclase by Escherichia coli heat-stable enterotoxin is regulated by adenine nucleotides. Infect Immun. 1991;59(4):1552-7.
25. Crane JK, Shanks KL. Phosphorylation and activation of the intestinal guanylyl cyclase receptor for Escherichia coli heat-stable toxin by protein kinase C. Mol Cell Biochem. 1996;165(2):111-20.
26. Crane JK, Wehner MS, Bolen EJ, Sando JJ, Linden J, Guerrant RL, et al. Regulation of intestinal guanylate cyclase by the heat-stable enterotoxin of Escherichia coli (STa) and protein kinase C. Infect Immun. 1992;60(12):5004-12.
27. Fan X, Wang Y, London RM, Eber SL, Krause WJ, Freeman RH, et al. Signaling Pathways for Guanylin and Uroguanylin in the Digestive, Renal, Central Nervous, Reproductive, and Lymphoid Systems. Society. 1997;138(11):4636-48.
28. Range SP, Holland ED, Basten GP, Knox AJ. Regulation of guanosine 3',5'-cyclic monophosphate in ovine tracheal epithelial cells. Br J Pharmacol. 1997;120(7):1249-54.
29. Schulz S, Chrisman TD, Garbers DL. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem. 1992;267(23):16019-21.
30. Kita T, Kitamura K, Sakata J, Eto T. Marked increase of guanylin secretion in response to salt loading in the rat small intestine. Am J Physiol. 1999;277(5):G960-6.
31. Sindic A, Schlatter E. Cellular effects of guanylin and uroguanylin. J Am Soc Nephrol. 2006;17(3):607-16.
32. Carrithers SL, Ott CE, Hill MJ, Johnson BR, Cai W, Chang JJ, et al. Guanylin and uroguanylin induce natriuresis in mice lacking guanylyl cyclase-C receptor. Kidney Int. 2004;65(1):40-53.
33. Habek N, Dobrivojevic Radmilovic M, Kordic M, Ilic K, Grgic S. Activation of brown adipose tissue in diet-induced thermogenesis is GC-C dependent. Pflugers Arch. 2020;472(3):405-17.
34. Habek N, Ratko M, Dugandžic A. Uroguanylin increases Ca2+ concentration in astrocytes via guanylate cyclase C-independent signaling pathway. Croat Med J. 2021;62(3):250-63.
35. Dugandzic A, Ratko M, Habek N. Anxiety-like behavior in female mice changes by feeding, possible effect of guanylate cyclase C. Eur J Neurosci. 2020;52(1):2781-90.
36. Gong R, Ding C, Hu J, Lu Y, Liu F, Mann E, et al. Role for the membrane receptor guanylyl cyclase-C in attention deficiency and hyperactive behavior. Science 2011;333(6049):1642-46.
37. Begg DP, Steinbrecher KA, Mul JD, Chambers AP, Kohli R, Haller A, et al. Effect of guanylate cyclase-C activity on energy and glucose homeostasis. Diabetes. 2014;63(11):3798-804.
38. Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, et al. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest. 2011;121(9):3578-88.
39. Colantuoni C, Lipska BK, Ye T, Hyde TM, Tao R, Leek JT et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519-23.
40. Scheving LA, Russell WE, Chong KM. Structure, glycosilation and localisation of rat intestinal guanylyl cyclase C: modulation by fasting. Am J Physiology. 1996;271(6 Pt 1):G959-68.
41. Folgueira C, Beiroa D, Callon A, Al-Massadi O, Barja-Fernandez S, Senra A, et al. Uroguanylin action in the brain reduces weight gain in obese mice via different efferent autonomic pathways. Diabetes. 2016;65(2):421-32.
42. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500-8.
43. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125(2): 478-86.
44. Hibi M, Oishi S, Matsushita M, Yoneshiro T, Yamaguchi T, Usui C, et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes. 2016;40(11):1655-61.
45. Qian X, Prabhakar S, Nandi A, Visweswariah S, Goy M. Expression of GC-C, a receptor-guanylate cyclase, and its endogenous ligands uroguanylin and guanylin along the rostrocaudal axis of the intestine. Endocrinology. 2000;141(9):3210-24.
46. Crane MR, Hugues M, O’Hanley PD, Waldman SA. Identification of two affinity states of low affinity receptors for Escherichia coli heatstable enterotoxin: correlation of occupation of lower affinity state with guanylate cyclase activation. Mol Pharmacol. 1992;41(6):1073- 80.
47. Albano F, Brasitus T, Mann EA, Guarino A, Giannella RA. Colonocyte basolateral membranes contain Escherichia coli heatstable enterotoxin receptors. Biochem Biophys Res Commun. 2001;284(2):331-4.
48. Ganguly U, Chaudhury AG, Basu A, Sen PC. STa-induced translocation of protein kinase C from cytosol to membrane in rat enterocytes. FEMS Microbiol Lett. 2001;204(1):65-9.
49. Sindic A, Velic A, Basoglu C, Hirsch JR, Edemir B, Kuhn M, et al. Uroguanylin and guanylin regulate transport of mouse cortical collecting duct independent of guanylate cyclase C. Kidney Int. 2005;68(3):1008-17.
50. Sindic A, Hirsch JR, Velic A, Piechota H, Schlatter E. Guanylin and uroguanylin regulate electrolyte transport in isolated human cortical collecting ducts. Kidney Int. 2005;67(4):1420-7.
51. Lorenz JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, et al. Uroguanylin knockout mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J Clin Invest. 2003;112(8):1244-54.
52. Kuhn M. Function and Dysfunction of Mammalian Membrane Guanylyl Cyclase Receptors: Lessons from Genetic Mouse Models and Implications for Human Diseases. In: Schmidt HHHW, Hofmann F, Stasch JP. (eds) cGMP: Generators, Effectors and Therapeutic Implications. Handbook of Experimental Pharmacology. Berlin, Heidelberg: Springer; 2009.
53. Bazargani, N., Attwell, D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19(2):182-9.
54. Sellers ZM, Mann E, Smith A, Ko KH, Giannella R, Cohen MB, et al. Heat-stable enterotoxin of Escherichia coli (STa) can stimulate duodenal HCO3(-) secretion via a novel GC-C- and CFTR-independent pathway. FASEB J. 2008;22(5):1306-16.
