Abstract
In this review, we explore the potential applications of functionalized folic acid-polymer nanoparticles in nucleic acids delivery. Folic acid-chitosan (carbohydrates) and folic acid-PAMAM (dendrimers) of different polymer sizes were used to conjugate with DNA and tRNA in vitro. Thermodynamic analysis showed that conjugation of DNA and tRNA to nanoparticles occurred via hydrophilic, hydrophobic, H-bonding and van der Waals contacts. Major alterations of DNA and RNA morphology occurred by nanocarrier interaction. These results indicate that functionalized folic acid-nanoparticles can deliver DNA and RNA to target sites.
Keywords
DNA, tRNA, Gene delivery, Folic acid-polymer, Thermodynamics, Transmission electron microscopy
Introduction
The conjugation of polymers with multiple targeting ligands has become a popular approach for targeted gene and drug delivery [1,2]. Functionalized polymer–drug conjugates are increasingly used to obtain biodegradable, targeted tools to further enhance localized gene and drug delivery systems [1-3]. Folic acid (FA)-conjugated biodegradable polymers were tested as effective gene and drug delivery tools [4-7]. Folate receptors are cellular markers highly expressed in various cancer cells and on the surface of activated macrophages [8-12]. Chitosan (Ch), (1–4)-2-amino-2-deoxy-β-D-glucan, is a polysaccharide obtained from alkaline hydrolysis of chitin, one of the most abundant natural amino polysaccharides extracted from the exoskeleton of crustaceans and insect, from fungal cell walls, etc. [13,14]. There are amine groups (-NH2) and hydroxyl groups (-OH) along the chitosan chain, which can be used as cross-linkable functional groups to react with cross-linking agents for in-situ chemical cross-linking [13]. In addition, these amino groups can be protonated below pH 6.3 and hence chitosan can interact with DNA/ RNA phosphate groups in an electrostatic manner. Dendrimers are highly branched three-dimensional molecules, with defined molecular weight and a large number of controllable peripheral functionalities [15,16]. Polyamidoamines (PAMAMs) were historically the first class of dendrimers to be synthesized [17]. The peripheral –NH2 groups of PAMAM can also interact with DNA/ RNA phosphate groups by electrostatic forces [18]. The interaction of charged amino and imino groups with DNA/RNA phosphate groups is known to provoke DNA/ RNA condensation to nanoparticles [19,20]. Since DNA/ RNA transport through the cell membrane is an inefficient process, their condensation to nanoparticles is important to facilitate DNA delivery.
The application of dendrimers in gene delivery has been recently reviewed [21]. The bundling and aggregation of DNA by PAMAM is known [22-26]. Conjugated folic acid with synthetic and natural polymers has been extensively used in gene and drug delivery systems [27]. A gene delivery
system based on folic acid-polyethylene glycol (PEG)- chitosan-PAMAM was used for cancer cell targeting [28]. The conjugation of DNA with chitosan and folic acid was recently reported [29,30]. Chitosan-based formulation for the delivery of DNA and RNA is known [31]. Chitosan is widely used as a gene delivery vehicle due to its ability to condense DNA, facilitate transport and subsequently release plasmid DNA, allowing gene expression [32,33]. The fabrication and structural characterization of the functionalized folic acid-PAMAM and folic acid-chitosan complexes have been recently reported [34,35].
Due to the major applications of folic acid-polymer conjugates in gene and drug delivery systems, we are reviewing recent studies on the encapsulation of DNA and tRNA by functionalized folic-acid-polymer conjugates here. In this review, the loading efficacies of DNA and tRNA by folic acid-PAMAM (G3 and G4) and folic acid-chitosan (15 and 100 kDa) nanoparticles are reported, using spectroscopic, thermodynamic and transmission electron microscopy (TEM) image analysis. Thermodynamic analysis of the complexation process can provide the necessary physical chemical data on the stability of nanoparticles with potential biotechnological applications.
Stability of DNA and RNA Conjugates with Folic Acid-polymer Nanoparticles
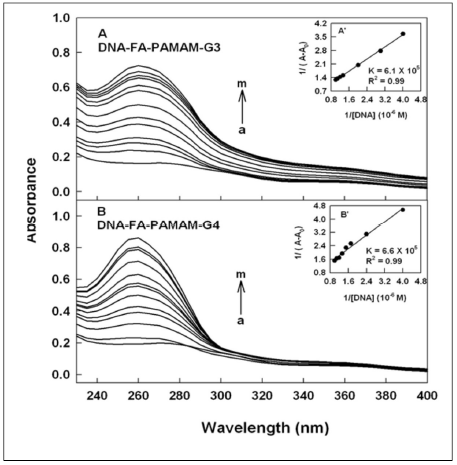
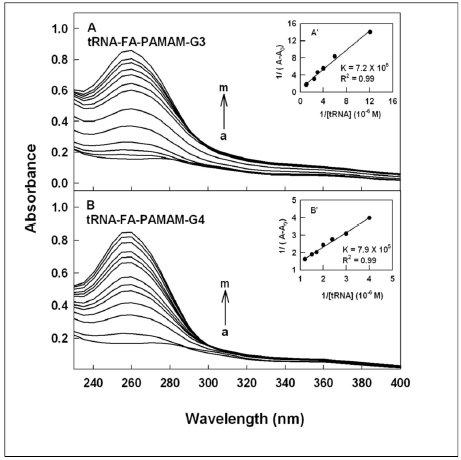
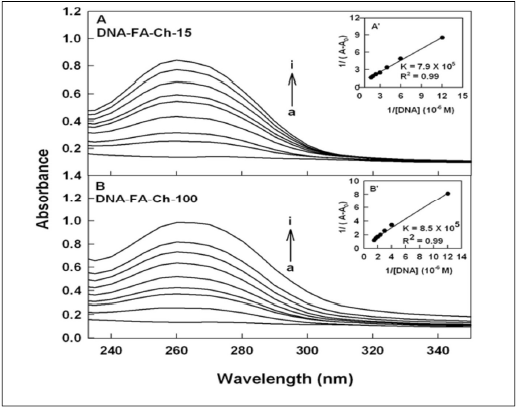
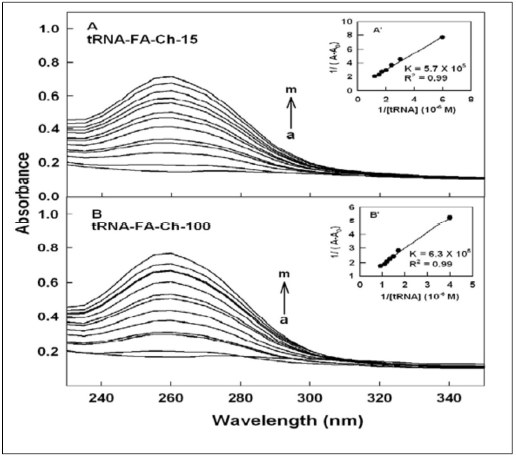
DNA and tRNA interactions with folic acid-PAMAM and folic acid-chitosan complexes induced major alterations of the folic acid-polymer absorption spectra. The observed changes were used to calculate the binding constants of DNA and tRNA complexes with folic acid-polymer nanoparticles. The ultraviolet (UV) spectra of DNA and tRNA with polymer nanoparticles are shown in Figures 1-4. DNA and tRNA complexation occurred with an increase in the folic acid-polymer absorption band at 260 nm. The DNA and tRNA binding constants were calculated as previously reported [36] and the results are shown in Figures 1-4 and Table 1. These results showed that more stable DNA/tRNA-FA-polymer conjugation occurred as FA-PAMAM and FA-Ch sizes increased (from G3 to G4 and chitosan-15 to chitosan-100 kDa), with an order of stability of FA-PAMAM-G4 > FA-PAMAM-G3 as well as FA-Ch-100 > FA-Ch-15 (Table 1). The increased stability of FA-PAMAM-G4 is related to the presence of additional terminal charged -NH2 groups on PAMAM-G4 (64 groups) as compared to PAMAM-G3 (32 groups) and that of FACh- 100 compared with FA-Ch-15, as these charged amino groups are involved in biomolecular interactions. Evidence regarding hydrophobic, hydrophilic or H-bonding interactions comes from the thermodynamic analysis of DNA/tRNA-FA-polymer conjugates, as discussed in the next section.
Figure 1. UV-visible spectra of folic acid-PAMAM and their DNA conjugates (pH 7.2) with FA-PAMAM-G3 (A) and FA-PAMAM-G4 (B), with polymer 60 μM (a) and its DNA complexes (b-m) with DNA at 1, 3, 5, 10, 20, 30, 40, 50, 60, 70 and 80 μM. Inset: plot of 1/(A-A0) vs (1/ DNA concentration) and binding constants (K) for DNA-acid-PAMAM conjugates.
Figure 2. UV-visible spectra of folic acid-PAMAM and their tRNA conjugates (pH 7.2) with FA-PAMAM-G3 (A) and FAPAMAM- G4 (B) with polymer 60 μM (a) and its tRNA complexes (b-m) with tRNA at 1, 3, 5, 10, 20, 30, 40, 50, 60, 70 and 80 μM.Inset: plot of 1/(A-A0) vs (1/ tRNA concentration) and binding constants (K) for tRNA-acid-PAMAM conjugates.
Figure 3. UV-visible spectra of folic acid-chitosan and their DNA conjugates (pH 7.2) with FA-chotsan-15 (A) and chitosan-100 kDa (B) with folic acid-chitosan at 60 μM (free acid) (a) and its DNA complexes (b-i) for chitosan-15 at 1, 5, 10, 20, 30, 40, 50 and 60 μM and (b-i) for chitosan-100 kDa at 1, 5, 10, 20, 30, 40, 50 and 60 μM. Inset: plot of 1/(A-A0) vs (1/ DNA concentration) and binding constants (K) for DNA-acid-chitosan conjugates.
Figure 4. UV-visible spectra of folic acid-chitosans and their tRNA conjugates (pH 7.2) with acid-chotsan-15 (A) and acidchitosan- 100 kDa (B) with folic acid-chitosan at 60 μM (free conjugate) (a) and its tRNA complexes (b-m) for acid-chitosan-15 at 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μM and (b-m) for acid-chitosan-100 kDa at 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μM. Inset: plot of 1/(A-A0) vs (1/ tRNA concentration) and binding constants (K) for tRNA-acid-chitosan conjugates.
| Complexes | K x 105 M-1 | n | LE% |
|---|---|---|---|
| DNA-folic-acid-PAMAM-G3 | 6.1 | 1.1 | 40 |
| DNA-folic-acid-PAMAM-G4 | 6.6 | 1.2 | 50 |
| tRNA-folic-acid-PAMAM-G3 | 7.2 | 1 | 35 |
| tRNA-folic-acid-PAMAM-G4 | 7.9 | 1.1 | 45 |
| DNA-folic-acid-chitosan-15 | 7.9 | 1 | 45 |
| DNA-folic-acid-chitosan-100 | 8.5 | 1.2 | 50 |
| tRNA-folic-acid-chitosan-15 | 5.7 | 1 | 40 |
| tRNA-folic-acid-chitosan100 | 6.3 | 1.3 | 50 |
Thermodynamics of DNA/tRNA Bindingto Folic Acid-polymer Nanoparticles
Based on thermodynamic analysis, the ΔG, ΔH and ΔS of the nature of DNA/tRNA-FA-polymer interactions can be determined [37,38]. The thermodynamic parameters for the interaction of DNA and tRNA with folic acidpolymer conjugates at 298.15 K are presented in Table 2. The negative sign of ΔG shows that the binding process between DNA/tRNA with FA-polymer conjugate is spontaneous. Furthermore, all the DNA/tRNA-FApolymer nanoparticles have negative ΔH, which means that the complex formation between DNA and tRNA and FA-polymer complex is an exothermic reaction. The negative ΔH and negative ΔS for DNA/tRNA-FA-polymer nanocarrier show that H-bonding and van der Waals interactions are prevailing in the complex formation (Table 2). However, hyrophobic and H-bonding contacts are also observed in the case of negative ΔH and positive ΔS (Table 2). The thermodynamic analysis of DNA/tRNA-FApolymer interactions shows the importance of the binding constant (K), ΔH, ΔS and ΔG in determining what type of interaction is predominant in these conjugates [37,38]. The enthalpy value provides more contribution to ΔG than entropy for DNA/tRNA-FA-polymer conjugates, indicating that the binding process is enthalpy driven (Table 2). It should be noted that the enthalpy contribution to the free energy of binding results from the formation of H-bonding and van der Waals interactions and, therefore, the negative value indicates that enthalpy changes are favoring the DNA and tRNA-folic acid-polymer conjugation.
| Complexes | ΔH0 (kJ.mol-1±2) |
ΔS0 (J.mol-1±2) |
ΔG0 (kJ.Mol-1±2) |
|---|---|---|---|
| DNA-folic-acid-PAMAM-G3 | -14.56 | -0.70 | 14.35 |
| DNA-folic-acid-PAMAM-G4 | -19.66 | -17.59 | -14.41 |
| tRNA-folic-acid-PAMAM-G3 | -13.19 | 4.37 | -14.50 |
| tRNA-folic-acid-PAMAM-G4 | -14.36 | 0.92 | -14.64 |
| DNA-folic-acid-chitosan-15 | -12.00 | 8.83 | -14.63 |
| DNA-folic-acid-chitosan-100 | -17.54 | -9.43 | -14.73 |
| tRNA-folic-acid-chitosan-15 | -10.07 | 13.97 | -14.24 |
| tRNA-folic-acid-chitosan100 | -14.72 | -1.16 | -14.37 |
The loading efficacy for DNA and tRNA to FA-polymer conjugates was determined, as previously reported [39]. The loading efficacy was estimated to be 35-50% (FAPAMAM- G3 and FA-Ch-15), which increased to 50-55% (FA-PAMAM-G4 and FA-Ch-100), in DNA/tRNA-FApolymer nanoconjugates (Table 1). This result shows the important role of polymer size in DNA and RNAnancocarrier interactions.
Effect of Folic Acid-polymer Conjugation on DNA and tRNA Morphology
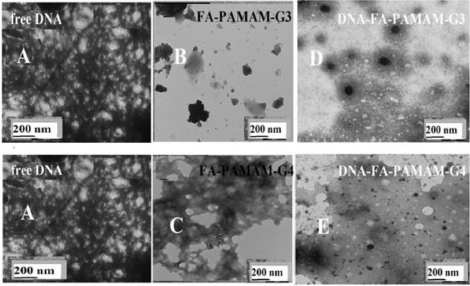
The morphological changes of DNA and tRNA by folic acid-polymer nanoparticles were monitored, using TEM. The TEM analysis of the free FA-PAMAM-G3 and FAPAMAM- G4 and their DNA and tRNA conjugates in aqueous solution at pH 7.2 are shown in Figures 5 and 6. The TEM images of the free DNA and tRNA show major spherical aggregates, with particle sizes ranging from 3 to 10 nm with a mean diameter of 5 to 6 nm (Figures 5A and 6A), which is in agreement with literature reports [40-45]. Marked differences were also observed in the morphology of the FA-PAMAM aggregates. TEM images showed the appearance of irregular shaped aggregates dispersed in solutions of FA-PAMAM-G3 and FA-PAMAM-G4 (Figure 5B and 5C) [35]. Upon addition of DNA and tRNA to FAPAMAM conjugates, DNA and tRNA aggregates became more evident in the TEM images (Figures 5 and 6D and E), revealing that the conjugation of DNA and tRNA by FAPAMAM caused an increase in DNA/tRNA aggregation. The aggregate size analysis showed a major increase in the diameter of DNA and tRNA aggregates (Figures 5 and 6D and E). The DNA and tRNA aggregate formation were more pronounced in FA-PAMAM-G4 than that in FAPAMAM- G3, indicating more perturbations of nucleic acid structures by higher generation PAMAM (Figures 5 and 6D and E). Similar structural changes were observed upon testosterone conjugation with DNA and tRNA [46,47].
Figure 5. TEM images showing the morphology of DNA (A) with folic acid-PAMAM-G3 (B) and folic acid-PAMAM-G4 (C) and their DNA conjugates (D and E) at pH 7.2 at 24°C. The concentrations of DNA and folic acid –PAMAM were 60 μM in all samples.
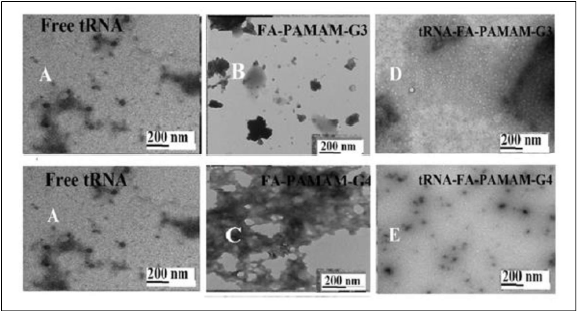
Figure 6. TEM images showing the morphology of tRNA (A) with folic acid-PAMAM-G3 (B) and folic acid-PAMAM-G4 (C) and their tRNA conjugates (D and E) at pH 7.2 at 24°C. The concentrations of tRNA and folic acid –PAMAM were 60 μM in all samples.
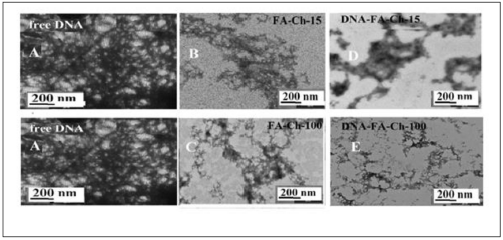
In the presence of folic acid-chitosan nanoparticles, major changes were also observed in the TEM images of DNA and tRNA aggregates. The TEM images of DNA and tRNA, in the presence and absence of folic acid-chitosan conjugates in aqueous solution at pH 7.2, are shown in Figures 6 and 7. The TEM photographs of the free DNA and tRNA exhibit major spherical aggregates, with the particle size ranging from 3 to 10 nm with a mean diameter of 5 to 6 nm (Figures 6A and 7A), which is in agreement with other reports [34,48,49]. Marked differences were also observed in the morphology of the acid–chitosan aggregates.
Figure 7. TEM images showing the morphology of DNA (A) with folic acid-chitosan-15 (B) and folic acid-chitosan-100 kDa (C) and their DNA conjugates (D and E) at pH 7.2 at 24°C. The concentrations of DNA and folic acid -chitosan were 60 μM in all samples.
TEM photographs showed the appearance of irregular shaped aggregates dispersed in solutions of Ch-15 and Ch- 100 kD conjugated with folic acid (Figures 6B and 7B). The conjugated Ch-15 and Ch-100 with folic acid exhibit major changes in polymer morphology [34]. Upon addition of DNA and tRNA to folic acid-chitosan conjugates, DNA and tRNA aggregates became more evident in the TEM images (Figures 6D and 7E), revealing that the conjugation of DNA and tRNA by folic acid-chitosan nanoparticles caused an increase in the DNA and tRNA aggregation. The aggregate size analysis showed a major increase in diameter of DNA aggregates (Figures 6D and E). It is important to note that conjugation of folic acid-polymer nanocarrier also induced major morphological changes on DNA and tRNA structures (Figures 5-7).
Conclusions and Outlook
Based on thermodynamic analysis, the ΔG, ΔH and ΔS of the nature of DNA/tRNA-FA-polymer interactions can be determined [37,38]. The thermodynamic parameters for the interaction of DNA and tRNA with folic acidpolymer conjugates at 298.15 K are presented in Table 2. The negative sign of ΔG shows that the binding process between DNA/tRNA with FA-polymer conjugate is spontaneous. Furthermore, all the DNA/tRNA-FApolymer nanoparticles have negative ΔH, which means that the complex formation between DNA and tRNA and FA-polymer complex is an exothermic reaction. The negative ΔH and negative ΔS for DNA/tRNA-FA-polymer nanocarrier show that H-bonding and van der Waals interactions are prevailing in the complex formation (Table 2). However, hyrophobic and H-bonding contacts are also observed in the case of negative ΔH and positive ΔS (Table 2).
The conjugation of polymer with multiple targeting ligands has become a popular approach for targeted gene and drug delivery [1-3]. Folic acid-conjugated with biodegradable polymers were tested as effective gene and drug delivery tools [4-12]. These multivalent polymers have great utility in controlled release and targeting studies of different bioactive molecules. Chitosan and PAMAM dendrimers and their functionalized folic acid nanoparticles were often used for drug and gene delivery [50-63]. DNA, RNA and drug bindings to functionalized folic acid-polymer nanocarrier occurred via hydrophilic, hydrophobic, H-bonding and van der Waals contacts. As polymer size increased, the stability and loading efficacy of nucleic acids and drug-polymer conjugation also increased. Major alterations of DNA and RNA morphology were observed upon nanocarrier complexation, as the condensation of DNA by these and other ligands facilitate cellular transport [64-67]. These results show that functionalized folic acidpolymer conjugates can be used to deliver DNA and RNA to target sites. Future research should be focused on the conjugation of polymers with multiple targeting ligands to develop effective functionalized nanocarrier for targeted gene and drug delivery systems.
Funding
The financial support from Natural Sciences and Engineering Research Council of Canada (NSERC) for this review is highly appreciated.
References
2. Van Dongen MA, Dougherty CA, Banaszak Holl MM. Multivalent polymers for drug delivery and imaging: the challenges of conjugation. Biomacromolecules. 2014 Sep 8;15(9):3215-34.
3. Samal SK, Dash M, Van Vlierberghe S, Kaplan DL, Chiellini E, Van Blitterswijk C, et al. Cationic polymers and their therapeutic potential. Chemical Society Reviews. 2012;41(21):7147-94.
4. Wang Y, Cao X, Guo R, Shen M, Zhang M, Zhu M, Shi X. Targeted delivery of doxorubicin into cancer cells using a folic acid–dendrimer conjugate. Polymer Chemistry. 2011;2(8):1754-60.
5. Roger E, Kalscheuer S, Kirtane A, Guru BR, Grill AE, Whittum-Hudson J, Panyam J. Folic acid functionalized nanoparticles for enhanced oral drug delivery. Molecular Pharmaceutics. 2012 Jul 2;9(7):2103-10.
6. Rollett A, Reiter T, Nogueira P, Cardinale M, Loureiro A, Gomes A, et al. Folic acid-functionalized human serum albumin nanocapsules for targeted drug delivery to chronically activated macrophages. International Journal of Pharmaceutics. 2012 May 10;427(2):460-6.
7. Wong PT, Choi SK. Mechanisms and implications of dual-acting methotrexate in folate-targeted nanotherapeutic delivery. International Journal of Molecular Sciences. 2015 Jan;16(1):1772-90.
8. Carron PM, Crowley A, O’Shea D, McCann M, Howe O, Hunt M, Devereux M. Targeting the folate receptor: improving efficacy in inorganic medicinal chemistry. Current Medicinal Chemistry. 2018 Jul 1;25(23):2675- 708.
9. Marchetti C, Palaia I, Giorgini M, De Medici C, Iadarola R, Vertechy L, et al. Targeted drug delivery via folate receptors in recurrent ovarian cancer: a review. OncoTargets and Therapy. 2014;7:1223.
10. Tang Z, Li D, Sun H, Guo X, Chen Y, Zhou S. Quantitative control of active targeting of nanocarriers to tumor cells through optimization of folate ligand density. Biomaterials. 2014 Sep 1;35(27):8015-27.
11. Bwatanglang IB, Mohammad F, Yusof NA, Abdullah J, Alitheen NB, Hussein MZ, et al. In vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. Journal of Colloid and Interface Science. 2016 Oct 15;480:146-58.
12. Vlahov IR, Leamon CP. Engineering folate–drug conjugates to target cancer: from chemistry to clinic. Bioconjugate Chemistry. 2012 Jul 18;23(7):1357-69.
13. Pujana MA, Pérez-Álvarez L, Iturbe LC, Katime I. Biodegradable chitosan nanogels crosslinked with genipin. Carbohydrate Polymers. 2013 May 15;94(2):836-42.
14. Soares PA, Bourbon AI, Vicente AA, Andrade CA, Barros Jr W, Correia MT, et al. Development and characterization of hydrogels based on natural polysaccharides: Policaju and chitosan. Materials Science and Engineering: C. 2014 Sep 1;42:219-26.
15. Akbarzadeh A, Khalilov R, Mostafavi E, Annabi N, Abasi E, Kafshdooz T, et al. Role of dendrimers in advanced drug delivery and biomedical applications: a review. Experimental Oncology. 2018 Oct 1;40(3):178-83.
16. Frechet JM. Functional polymers and dendrimers: reactivity, molecular architecture, and interfacial energy. Science. 1994 Mar 25;263(5154):1710-5.
17. Esfand R, Tomalia DA. Poly (amidoamine)(PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discovery Today. 2001 Apr 15;6(8):427-36.
18. Santhakumaran LM, Thomas T, Thomas TJ. Enhanced cellular uptake of a triplex-forming oligonucleotide by nanoparticle formation in the presence of polypropylenimine dendrimers. Nucleic Acids Research. 2004 Jan 1;32(7):2102-12.
19. Thomas TJ, Thomas T. Collapse of DNA in packaging and cellular transport. International journal of Biological Macromolecules. 2018 Apr 1;109:36-48.
20. Thomas TJ, Tajmir-Riahi HA, Thomas T. Polyamine– DNA interactions and development of gene delivery vehicles. Amino Acids. 2016 Oct;48(10):2423-31.
21. Hu J, Hu K, Cheng Y. Tailoring the dendrimer core for efficient gene delivery. Acta Biomaterialia. 2016 Apr 15;35:1-1.
22. Perico A. Electrostatic theory of the assembly of PAMAM dendrimers and DNA. Biopolymers. 2016 May;105(5):276-86.
23. Froehlich E, Mandeville JS, Weinert CM, Kreplak L, Tajmir-Riahi HA. Bundling and aggregation of DNA by cationic dendrimers. Biomacromolecules. 2011 Feb 14;12(2):511-7.
24. Navarro G, de ILarduya CT. Activated and nonactivated PAMAM dendrimers for gene delivery in vitro and in vivo. Nanomedicine: Nanotechnology, Biology and Medicine. 2009 Sep 1;5(3):287-97.
25. Zhong H, He ZG, Li Z, Li GY, Shen SR, Li XL. Studies on polyamidoamine dendrimers as efficient gene delivery vector. Journal of Biomaterials Applications. 2008 May;22(6):527-44.
26. Meng HM, Zhang X, Lv Y, Zhao Z, Wang NN, Fu T, et al. DNA dendrimer: an efficient nanocarrier of functional nucleic acids for intracellular molecular sensing. ACS Nano. 2014 Jun 24;8(6):6171-81.
27. Liu Y, Li K, Pan J, Liu B, Feng SS. Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials. 2010 Jan 1;31(2):330-8.
28. Agudelo D, Kreplak L, Tajmir-Riahi HA. Microscopic and spectroscopic analysis of chitosan–DNA conjugates. Carbohydrate Polymers. 2016 Feb 10;137:207-13.
29. Agudelo D, Kreplak L, Tajmir-Riahi HA. tRNA conjugation with chitosan nanoparticles: An AFM imaging study. International Journal of Biological Macromolecules. 2016 Apr 1;85:150-6.
30. Wang M, Hu H, Sun Y, Qiu L, Zhang J, Guan G, et al. A pH-sensitive gene delivery system based on folic acid-PEGchitosan– PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials. 2013 Dec 1;34(38):10120-32.
31. Morris VB, Pillai CK, Sharma CP. Folic acid-conjugated depolymerized quaternized chitosan as potential targeted gene delivery vector. Polymer International. 2011 Jul;60(7):1097-106.
32. Liu X, Zhang Y, Ma D, Tang H, Tan L, Xie Q, Yao S. Biocompatible multi-walled carbon nanotube-chitosan– folic acid nanoparticle hybrids as GFP gene delivery materials. Colloids and Surfaces B: Biointerfaces. 2013 Nov 1;111:224-31.
33. Du YZ, Cai LL, Li J, Zhao MD, Chen FY, Yuan H, Hu FQ. Receptor-mediated gene delivery by folic acid-modified stearic acid-grafted chitosan micelles. International Journal of Nanomedicine. 2011;6:1559.
34. Chanphai P, Konka V, Tajmir-Riahi HA. Folic acid– chitosan conjugation: A new drug delivery tool. Journal of Molecular Liquids. 2017 Jul 1;238:155-9.
35. Chanphai P, Tajmir-Riahi HA. Characterization of folic acid-PAMAM conjugates: drug loading efficacy and dendrimer morphology. Journal of Biomolecular Structure and Dynamics. 2018 May 19;36(7):1918-24.
36. Connors K. Binding constants: The measurement of molecular complex stability. New York: Wiley. 1987.
37. Bekale L, Agudelo D, Tajmir-Riahi HA. Effect of polymer molecular weight on chitosan–protein interaction. Colloids and Surfaces B: Biointerfaces. 2015 Jan 1;125:309-17.
38. Bekale L, Chanphai P, Sanyakamdhorn S, Agudelo D, Tajmir-Riahi HA. Microscopic and thermodynamic analysis of PEG–ß-lactoglobulin interaction. RSC Advances. 2014;4(59):31084-93.
39. Chandra S, Dietrich S, Lang H, Bahadur D. Dendrimer– doxorubicin conjugate for enhanced therapeutic effects for cancer. Journal of Materials Chemistry. 2011;21(15):5729-37.
40. Ottaviani MF, Matteini P, Brustolon M, Turro NJ, Jockusch S, Tomalia DA. Characterization of starburst dendrimers and vesicle solutions and their interactions by CW-and pulsed-EPR, TEM, and dynamic light scattering. The Journal of Physical Chemistry B. 1998 Jul 30;102(31):6029-39.
41. Yang H, Yu D, Wang H, Xie Q, Wu J, Wang J. Aggregation behavior of amphiphilic PAMAM-based hyperbranched polymer in the presence of conventional small molecular surfactants. Advances in Chemical Engineering and Science. 2013;3(3): 34510.
42. Ottaviani MF, Favuzza P, Bigazzi M, Turro NJ, Jockusch S, Tomalia DA. A TEM and EPR investigation of the competitive binding of uranyl ions to starburst dendrimers and liposomes: Potential use of dendrimers as uranyl ion sponges. Langmuir. 2000 Sep 19;16(19):7368- 72.
43. Zhang Y, Chen J, Xiao C, Li M, Tian H, Chen X. Cationic dendron-bearing lipids: Investigating structure– activity relationships for small interfering RNA delivery. Biomacromolecules. 2013 Dec 9;14(12):4289-300.
44. Ramireddy RR, Raghupathi KR, Torres DA, Thayumanavan S. Stimuli sensitive amphiphilic dendrimers. New Journal of Chemistry. 2012;36(2):340-9.
45. Sanyakamdhorn S, Bekale L, Agudelo D, Tajmir- Riahi HA. Structural analysis of doxorubicin-polymer conjugates. Colloids and Surfaces B: Biointerfaces. 2015 Nov 1;135:175-82.
46. Sanyakamdhorn S, Agudelo D, Bekale L, Tajmir- Riahi HA. Targeted conjugation of breast anticancer drug tamoxifen and its metabolites with synthetic polymers. Colloids and Surfaces B: Biointerfaces. 2016 Sep 1;145:55- 63.
47. Ainalem ML, Nylander T. DNA condensation using cationic dendrimers—morphology and supramolecular structure of formed aggregates. Soft Matter. 2011;7(10):4577-94.
48. Manchanda R, Nimesh S. Controlled size chitosan nanoparticles as an efficient, biocompatible oligonucleotides delivery system. Journal of Applied Polymer Science. 2010 Nov 15;118(4):2071-7.
49. Chandra Hembram K, Prabha S, Chandra R, Ahmed B, Nimesh S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artificial cells, Nanomedicine, and Biotechnology. 2016 Jan 2;44(1):305- 14.
50. Yang PW, Lin TL, Lin TY, Yang CH, Hu Y, Jeng US. Packing DNA with disc-shaped bicelles. Soft Matter. 2013;9(48):11542-8.
51. Hainfeld JF, Sprinzl M, Mandiyan V, Tumminia SJ, Boublik M. Localization of a specific nucleotide in yeast tRNA by scanning transmission electron microscopy using an undecagold cluster. Journal of Structural Biology. 1991 Aug 1;107(1):1-5.
52. Eichman JD, Bielinska AU, Kukowska-Latallo JF, Baker Jr JR. The use of PAMAM dendrimers in the efficient transfer of genetic material into cells. Pharmaceutical Science & Technology Today. 2000 Jul 1;3(7):232-45.
53. Marini M, Falqui A, Moretti M, Limongi T, Allione M, Genovese A, et al. The structure of DNA by direct imaging. Science Advances. 2015 Aug 1;1(7):e1500734.
54. Chanphai P, Agudelo D, Vesper AR, Bérubé G, Tajmir-Riahi HA. Effect of testosterone and its aliphatic and aromatic dimers on DNA morphology. International Journal of Biological Macromolecules. 2017 Feb 1;95:850- 5.
55. Chanphai P, Agudelo D, Vesper AR, Bérubé G, Tajmir-Riahi HA. Testosterone and its dimers alter tRNA morphology. Journal of Pharmaceutical and Biomedical Analysis. 2017 Feb 5;134:269-74.
56. Sanyakamdhorn S, Agudelo D, Tajmir-Riahi HA. Review on the targeted conjugation of anticancer drugs doxorubicin and tamoxifen with synthetic polymers for drug delivery. Journal of Biomolecular Structure and Dynamics. 2017 Aug 18;35(11):2497-508.
57. Chanphai P, Bekale L, Sanyakamdhorn S, Agudelo D, Bérubé G, Thomas TJ, Tajmir-Riahi HA. PAMAM dendrimers in drug delivery: Loading efficacy and polymer morphology. Canadian Journal of Chemistry. 2017;95(9):891-6.
58. Sanyakamdhorn S, Agudelo D, Tajmir-Riahi HA. Encapsulation of antitumor drug doxorubicin and its analogue by chitosan nanoparticles. Biomacromolecules. 2013 Feb 11;14(2):557-63.
59. Agudelo D, Sanyakamdhorn S, Nafisi S, Tajmir-Riahi HA. Transporting antitumor drug tamoxifen and its metabolites, 4-hydroxytamoxifen and endoxifen by chitosan nanoparticles. PLoS One. 2013 Mar 20;8(3):e60250.
60. Chanphai P, Tajmir-Riahi HA. Binding efficacy of tRNA with folic acid-PAMAM nanoparticles. International Journal of Biological Macromolecules. 2018 Jul 15;114:851- 4.
61. Chanphai P, Tajmir-Riahi HA. tRNA conjugation with folic acid-chitosan conjugates. International Journal of Biological Macromolecules. 2017 Dec 1;105:810-5.
62. P. Chanphai, H. A. Tajmir-Riahi, DNA binding efficacy with functionalized folic acid-PAMAM nanoparticles. Chemico-Biological Interactions.290 (2018) 52–56.
63. Palmerston Mendes L, Pan J, Torchilin VP. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017 Sep;22(9):1401.
64. Agostinelli E, Vianello F, Magliulo G, Thomas T, Thomas TJ. Nanoparticle strategies for cancer therapeutics: Nucleic acids, polyamines, bovine serum amine oxidase and iron oxide nanoparticles. International Journal of Oncology. 2015 Jan 1;46(1):5-16.
65. Thomas RM, Thomas T, Wada M, Sigal LH, Shirahata A, Thomas TJ. Facilitation of the cellular uptake of a triplexforming oligonucleotide by novel polyamine analogues: Structure- activity relationships. Biochemistry. 1999 Oct 5;38(40):13328-37.
66. Vijayanathan V, Thomas T, Thomas TJ. DNA nanoparticles and development of DNA delivery vehicles for gene therapy. Biochemistry. 2002 Dec 3;41(48):14085- 94.
67. Vijayanathan V, Thomas T, Antony T, Shirahata A, Thomas TJ. Formation of DNA nanoparticles in the presence of novel polyamine analogues: a laser light scattering and atomic force microscopic study. Nucleic Acids Research. 2004 Jan 1;32(1):127-34.