Abstract
For over a century, the therapeutic potential of electrical stimulation (ES) has intrigued scientists and clinicians alike. The ophthalmology field has largely centered its applications in posterior segment diseases of the eye, yet recent developments have brought corneal pathologies into sharper focus. This commentary aims to contextualize and critique the current landscape of ES in eye care, particularly the growing interest in the non-invasive techniques: transpalpebral electrical stimulation (TpES). Compared to transcorneal approaches, TpES not only preserves ocular surface integrity, but also enhances patient comfort and compliance—factors often overlooked in early clinical studies. Moreover, ES demonstrates promising neuroprotective, regenerative, and anti-inflammatory properties, extending its therapeutic relevance beyond neurons to glial and epithelial cell populations. Despite these advances, significant gaps remain. The diversity of stimulation parameters, variability in clinical protocols, and limited mechanistic understanding continue to hinder broader clinical translation. We argue that future progress depends on the development of standardized treatment regimens and the execution of large-scale, controlled trials. As such, ES stands at the intersection of innovation and implementation, offering an exciting, albeit complex, path forward in ocular therapeutics.
Keywords
Electrical stimulation, Transpalpebral electrical stimulation (TpES), Neuroprotection, Dry eye, Anti-inflammation
Commentary
For over a century, researchers have explored the therapeutic potential of electrical current. In the modern era, microcurrent electrical stimulation (ES) has been used as a promising modality for treating various disorders of the eye and nervous system [1,2]. Current investigations of ES in ocular diseases mainly target ocular diseases affecting the posterior segment, such as retinitis pigmentosa (RP), age-related macular degeneration (AMD), glaucoma, and optic nerve diseases [2-4]. Several studies have reported encouraging outcomes: ES increases visual acuity and visual field from retina and optic nerve diseases [5,6]; upregulates neurotrophin expression which promotes nerve growth and neuron survival [5]; enhances retinal blood flow in patients with RP [7]; and reduces the apoptosis of retinal ganglion cell while promoting neural regeneration [8]. Despite the overall encouraging outcomes, several critical aspects of electrical stimulation therapy remain the subject of ongoing debate, most notably the mode of delivery and the optimal stimulation parameters. In the present commentary, we seek to examine these salient issues in greater detail.
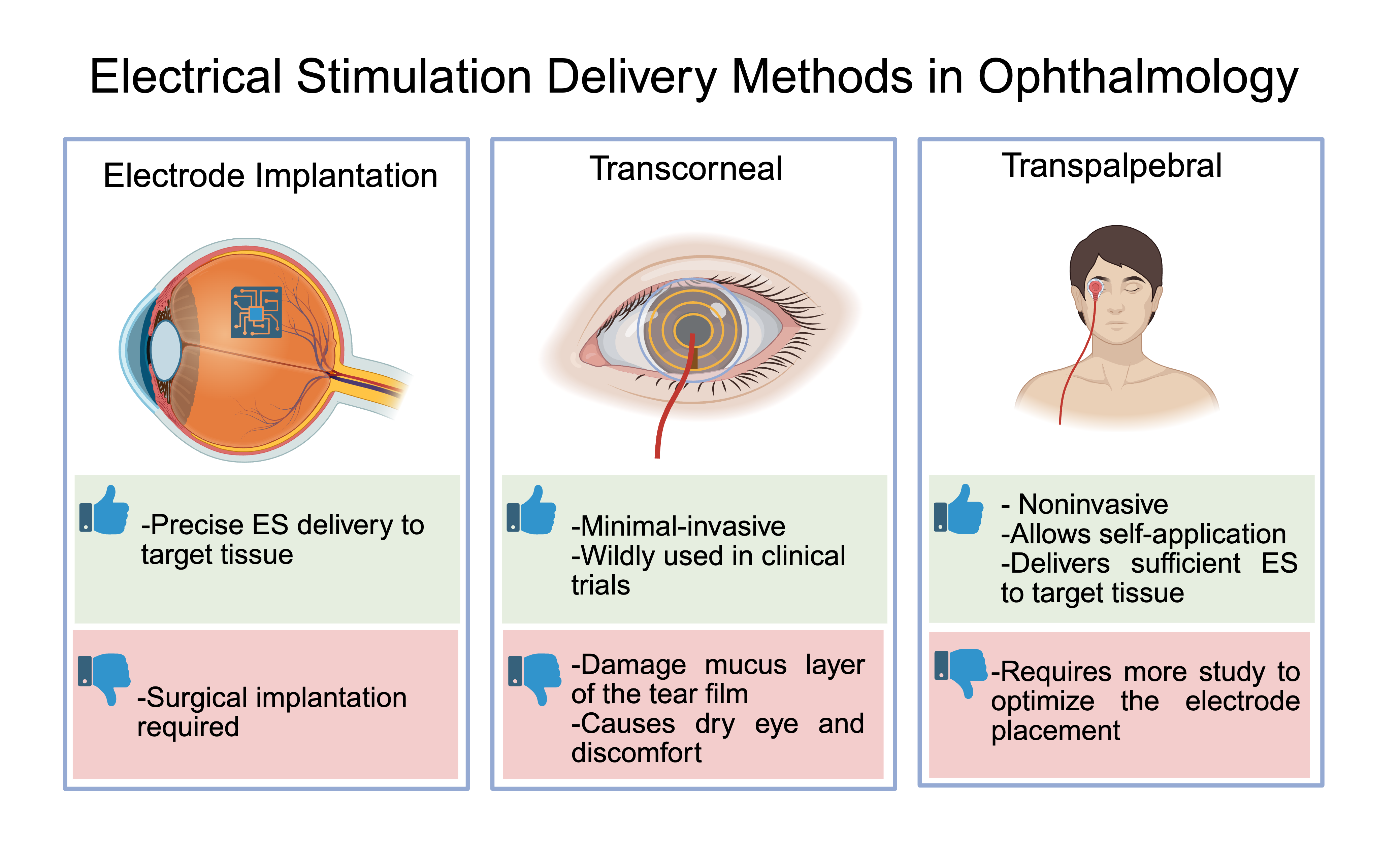
Two major methods of ES delivery have been reported in ophthalmic clinics: invasive approaches involving electrode implantation, and minimal- or non-invasive techniques administered either through the corneal surface or via the periocular skin (Figure 1). Since 2010, the vast majority of published studies have employed minimal- or non-invasive delivery methods and have been summarized by Liu et al. [3].
Figure 1. Electrical stimulation delivery methods in ophthalmology.
Theoretically, electrode implantation enables optimal stimulation efficiency at the targeted disease site. However, the surgical procedure - whether subretinal or directed at the optic nerve stump - inevitably imposes mechanical and physiological stress on the retinal tissue. Furthermore, the distribution of electrical current within the human eye adheres to Ohm’s law, indicating that, with appropriate electrode placement and calibrated intensity, non-invasive electrical stimulation can effectively target and deliver current to the diseased area with precision. Lee et al. employed realistic finite element human head models to optimize the electrodes placement for electrical current to reach the outer retina [9]. This study offered valuable insights into the advantages of multichannel ES and research methods for seeking precise delivery of electrical stimulation.
Compared to the invasive neuromodulatory techniques, non-invasive ES is one of the least damaging neuromodulation treatments available, with minimal adverse effects such as headache and irritation reported that were resolved without medical intervention [3,8]. The most popular non-invasive ES methods include transcorneal ES (TcES) and transpalpebral ES (TpES), or sometimes termed after transcutaneous ES. Both transpalpebral and transcutaneous ES place electrodes on the skin around the orbit, however, the placement of electrodes varies slightly, as transpalpebral is connected to the eyelid and the transcutaneous ES is above or below the eyelid [12]. TcES involves direct contact of electrodes to the corneal surface [8], and has been the most commonly employed approach in published human studies. Although TcES treatment met the expected study outcome, 53% of study subjects experienced dry eye-like symptoms after TcES [10], with foreign body sensation reported as common side effects in patients [11]. Results from animal studies revealed that direct corneal contact with electrodes led to corneal epithelial damage, disturbing both membrane spanning and soluble mucin [12]. Mucin stabilizes tear film on the ocular surface [13], and damage to this layer can cause dry-eye irritation. Very few studies using trans-scleral approach in animal models resulted in the same side effect due to the direct contact with conjunctiva [14]. In comparison, TpES does not interfere with mucin production during treatment and has not been associated with ocular surface damage; rather, it has been shown to enhance tear production and alleviate dry eye [12]. Moreover, TpES has demonstrated neuroprotective and anti-inflammatory effects on the retina comparable to TcES, without causing ocular discomfort [6,15,16]. Thus, with respect to preserving ocular surface integrity, TpES represents a more favorable alternative to TcES. Studies with repeated, regular ES usage show the most improvement compared to patients who received a treatment once [3], therefore, the employment of TpES can improve a patient's experiences and enhance compliance during a treatment course by avoiding the unpleasant side effects.
By virtue of its protective effects on the ocular surface, TpES has opened new avenues for the treatment of corneal diseases. In cornea, TpES successfully promoted corneal nerve regeneration after injury [17], and prevent the onset of dry eye following photorefractive keratectomy (PRK) surgery [18]. In the neural retina, which is the extension of the central nervous system, ES demonstrated excellent neural protection and regeneration effects in retinal ganglion cells (RGC) and optic nerve by promoting both intrinsic and extrinsic growth-supportive mechanisms. In preclinical models of optic nerve crush injury, short-term sessions of low-frequency ES have been shown to stimulate RGC survival and axonal regeneration [19,20]. Mechanistically, ES activates upregulation of key regenerative markers such as BDNF [21], and GAP-43 [19], both of which contribute to a pro-growth transcriptional environment. Recent advances underscore the potent synergy between ES and epigenetic reprogramming in promoting optic nerve regeneration. Ashok et al. demonstrated that low-intensity biphasic ES robustly stimulates RGC survival and axonal outgrowth via elevating levels of DNA demethylase TET1 enzyme and GAP43, while reducing the repressive H3K9me3 histone mark—thereby inducing DNA demethylation and chromatin relaxation that favors regenerative gene expression [19]. Adding another mechanistic perspective, Chang et al. demonstrated glutamate-mediated ES neuroprotection and regeneration in primary RGCs from mice, which directed long-distance RGC axon length and number, reiterating the neuro-regenerative potential of ES [22]. A recent case report demonstrated that daily TpES over 60 days led to marked improvements in visual acuity, contrast sensitivity, and visual field performance in a patient with long-standing non-arteritic anterior ischemic optic neuropathy (NAION), without any reported adverse effects [23]. This study highlights the potential of TpES as a safe, noninvasive modality for functional visual recovery in chronic optic neuropathies, warranting further investigation in larger clinical trials. TpES offers a promising foundation for developing therapies aimed at restoring vision after optic neuropathy.
Beyond its role in directly promoting neural regeneration, ES also protects neurons by modulating the immune reaction. The anti-inflammatory potential of ES was first recognized decades ago in the context of muscle and joint pain management [24]. At that time, its mechanisms were attributed to increased blood flow, oscillatory or torsional effects, and pH modulation. More recently, Lennikov et al. demonstrated that ES directly suppresses microglial cell activity, a key mediator of immune responses in the retina [25]. Notably, the anti-inflammatory effects of ES in non-neural cells appear to be mediated through the inhibition of Ca2+ signaling pathways, which differs from its mechanisms in neurons [25]. The molecular action by which ES modulates Ca2+ signaling is through negatively regulating energy metabolism which depletes intracellular Ca2+ stores (Lennikov A, Personal Communication, 2025). Furthermore, ES has been shown to enhance progenitor cell potential in Müller cells [26], offering promising avenues for tissue regeneration and repair.
The stimulation parameters include ES amplitude, frequency of the alternating current, duration of each treatment session, frequency of the treatment, and duration of each treatment course. As summarized by Li et al., up to 2024, there are various parameters used, ranging from 4 μA for 5 seconds to 50 mV for several hours [27]. Due to the multitude of parameters involved, identifying the most effective ES settings remains a significant challenge. Moreover, translating ES protocols optimized in cell or various animal models to human applications is inherently difficult. Despite these challenges, researchers continue to make progress in optimizing ES protocols. Merimoto et al. demonstrated that repeated ES yields greater therapeutic benefits compared to a single application, and symmetric pulses without an inter-pulse interval were the most effective waveform [28]. Although treatment frequencies vary among research groups, the most adopted regimen in mice ranges from daily to every other day. One study reported administering ES every three days, but they extended the treatment period to eight weeks to achieve RGC protection [29]. In our own studies using the Rho-/- mouse model, we observed that administering ES every other day achieved photoreceptor protection comparable to that of daily treatment [6]. In larger animal models such as rats and Mongolian gerbils, typical stimulation frequencies reduce to 1-3 times per week, while in rabbits, once-weekly treatment is more common (Table 1). Collectively, these findings suggest a trend toward reduced stimulation frequency in larger species, likely attributable to differences in metabolic rate and cellular turnover, which are generally higher in smaller animals.
In human studies, the typical intensity of ES ranges from 100 to 1000 μA, depending on the individual’s electrical phosphene threshold, with a standard stimulation frequency of 20 Hz. Treatment sessions generally last for 30 minutes, and treatment intervals vary, including single sessions, as well as daily, weekly, or monthly regimens. However, most of these reports are limited by small sample sizes and lack appropriate controls. In contrast, studies employing larger sample sizes and randomized controlled designs have demonstrated functional improvements; all employed repeated stimulation [30,31]. These results indicate that repeated stimulation may be more effective than single-dose stimulation.
In conclusion, ES holds significant promise as a non-pharmacological modality for promoting neuroprotection, enhancing regeneration, and restoring function in various ocular diseases. Advances in non-invasive delivery methods, particularly transcutaneous ES, have broadened the potential for clinical application while minimizing harm to the ocular surface. Nonetheless, variability in stimulation parameters, limited understanding of underlying mechanisms, and the scarcity of large-scale, well-controlled clinical trials remain critical challenges. Moving forward, standardized protocols, mechanistic studies, and robust clinical validation will be essential to fully realize the therapeutic potential of ES in ophthalmology. With continued interdisciplinary collaboration and technological innovation, ES may become an integral component of future treatment strategies for both retinal and corneal pathologies.
|
Reference |
Species |
ES setting |
Treatment groups |
Outcome |
|
Yang et al. [12] |
C57BL6J Rho-/- mice N=8 |
Biphasic ramp waveform, 300 µAmp, 20 Hz 4 mins daily for 14 days
|
2 groups:
|
TcES induces corneal epithelial damage (disrupts mucin homeostasis) where TpES does not |
|
Jassim et al. [29] |
DBA2/J mouse model of glaucoma N=31 |
Biphasic ramp waveform 100 μA, 1 ms pulse duration, at a frequency of 20 Hz Every 3 days for 8 weeks, 10 mins per treatment |
3 groups:
|
TES retina experienced lower inflammation, and restoration of homeostasis in retina and optic nerve was observed. |
|
Yoo et al. [17] |
New Zealand White Rabbits N=9 |
2 Hz vs 20 Hz treatment Pulse: 7mA Phase duration: 250 µs Interphase interval: 5 µs 30 mins daily for 28 days
|
3 groups:
|
2 Hz had better neuro-regeneration. There are no statistically significant differences in epithelial wound healing between groups. |
|
Fu et al. [32] |
Mongolian gerbils N=30 |
Bipolar rectangular current with pulse duration of 1 ms/phase, intensity of 100 μA, 20 Hz for 1 hour One treatment immediately, then three days later, then twice a week for the next month (on days 1 and 4 of that week)
|
2 groups:
|
Higher retinal ganglion cell survival for TcES treated groups. Suppression of microglia cell activation was also observed. |
|
Naycheva et al. [30] |
RAO: CRAO N=12 BRAO N=1 |
Rectangular bi-phasic current (5 ms positive then 5 ms negative) 30 min sessions, 1 initial session, then six consecutive weekly sessions after |
3 groups:
|
Some treated patients show an increase in visual field area. The 150% group showed a statistically significant increase in a-wave slope, correlating to photoreceptor activity increase after TES. |
|
Schatz et al. [31] |
Advanced RP patients N=24 |
Rectangular bi-phasic current (5 ms positive then 5 ms negative) 30 min sessions, 1 initial session, then six consecutive weekly sessions after |
3 groups:
|
150% group found statistically significant increase in visual field compared to other groups |
|
Gunes et al. [6] |
C57BL6J mice (control) Rho -/- mice |
Biphasic rectangular (100 μA, 20 Hz) or biphasic ramp (100 μA, 20 Hz) One eye for 7 days after 6 weeks of age |
1 min of stimulation in 4 transorbital sites around one eye (4 minutes of stimulation total). 2 sites were on the upper eyelid and the other two sites on the lower eyelid
|
Significant improvement in both visual acuity and contrast sensitivity by ramp. Rectangular waveform saw increase in visual acuity only. Daily or every other day treatment required to see benefits of treatment. |
|
Bittner et al. [7] |
RP patients N=21 |
rectangular biphasic current, 5-ms positive, directly followed by 5-ms negative with amplitudes up to 750 μA at a frequency of 20 Hz, for 30 min during six weekly sessions |
3 groups
|
57% of TcES patients saw significant visual improvement compared to 29% of electro-acupuncture patients and 0% of control patients. |
|
Ashok et al. [19] |
B6.Cg-Tg (Thy1-YFP) 16Jrs/J (Thy-1 YFP) mice N=6 |
TpES at a biphasic ramp waveform (150 μAmp with 50 ms pulse duration) was applied for 20 min in each mouse. TpES was conducted every other day starting 24 h after optic nerve crush for a period of 4 weeks. |
2 groups:
|
Significant improvement in RGC morphology and pSTR (RGC function) |
|
RAO: Retinal Artery Occlusion; RP: Retinitis Pigmentosa; TpES: Transpalpebral Electrical Stimulation; TcES: Transcorneal Electrical Stimulation; TES: Transcutaneous Electrical Stimulation |
||||
Disclosure
None
Acknowledgement
The graphical abstract is designed using BioRender.
References
2. Sanie-Jahromi F, Azizi A, Shariat S, Johari M. Effect of Electrical Stimulation on Ocular Cells: A Means for Improving Ocular Tissue Engineering and Treatments of Eye Diseases. Biomed Res Int. 2021 Nov 17;2021:6548554.
3. Liu J, Ma AKH, So KF, Lee VWH, Chiu K. Mechanisms of electrical stimulation in eye diseases: A narrative review. Adv Ophthalmol Pract Res. 2022 May 5;2(2):100060.
4. Colombo L, Baldesi J, Martella S, Quisisana C, Antico A, Mapelli L, et al. Managing Retinitis Pigmentosa: A Literature Review of Current Non-Surgical Approaches. Journal of Clinical Medicine. 2025 Jan 8;14(2):330.
5. Yu WS, Kwon SH, Agadagba SK, Chan LLH, Wong KH, Lim LW. Neuroprotective Effects and Therapeutic Potential of Transcorneal Electrical Stimulation for Depression. Cells. 2021 Sep 21;10(9):2492.
6. Gunes K, Chang K, Lennikov A, Tai WL, Chen J, ElZaridi F, et al. Preservation of vision by transpalpebral electrical stimulation in mice with inherited retinal degeneration. Front Cell Dev Biol. 2024 Aug 14;12:1412909.
7. Bittner AK, Seger K, Salveson R, Kayser S, Morrison N, Vargas P, et al. Randomized controlled trial of electro-stimulation therapies to modulate retinal blood flow and visual function in retinitis pigmentosa. Acta Ophthalmol. 2018 May;96(3):e366-76.
8. Agadagba SK, Lim LW, Chan LLH. Advances in transcorneal electrical stimulation: From the eye to the brain. Front Cell Neurosci. 2023 Mar 2;17:1134857.
9. Lee S, Park J, Kwon J, Kim DH, Im CH. Multi-channel transorbital electrical stimulation for effective stimulation of posterior retina. Sci Rep. 2021 May 7;11(1):9745.
10. Schatz A, Pach J, Gosheva M, Naycheva L, Willmann G, Wilhelm B, et al. Transcorneal Electrical Stimulation for Patients With Retinitis Pigmentosa: A Prospective, Randomized, Sham-Controlled Follow-up Study Over 1 Year. Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):257-69.
11. Hahm BJ, Shin YW, Shim EJ, Jeon HJ, Seo JM, Chung H, et al. Depression and the vision-related quality of life in patients with retinitis pigmentosa. Br J Ophthalmol. 2008 May;92(5):650-4.
12. Yang M, Lennikov A, Chang K, Ashok A, Lee C, Cho KS, et al. Transcorneal but not transpalpebral electrical stimulation disrupts mucin homeostasis of the ocular surface. BMC Ophthalmol. 2022 Dec 15;22(1):490.
13. Watanabe H. Significance of Mucin on the Ocular Surface. Cornea. 2002 Mar;21(2 Suppl 1):S17-22.
14. Liu F, Zhang M, Xiong G, Han X, Lee VWH, So KF, et al. Trans-Sclera Electrical Stimulation Improves Retinal Function in a Mouse Model of Retinitis Pigmentosa. Life (Basel) [Internet]. 2022 Nov 17;12(11):1917.
15. Yu H, Enayati S, Chang K, Cho K, Lee SW, Talib M, et al. Noninvasive Electrical Stimulation Improves Photoreceptor Survival and Retinal Function in Mice with Inherited Photoreceptor Degeneration. Invest Ophthalmol Vis Sci. 2020 Apr 9;61(4):5.
16. Enayati S, Chang K, Lennikov A, Yang M, Lee C, Ashok A, et al. Optimal transcorneal electrical stimulation parameters for preserving photoreceptors in a mouse model of retinitis pigmentosa. Neural Regen Res. 2024 Nov 1;19(11):2543-52.
17. Yoo YS, Park S, Eun P, Park YM, Lim DH, Chung TY. Corneal Neuro-Regenerative Effect of Transcutaneous Electrical Stimulation in Rabbit Lamellar Keratectomy Model. Transl Vis Sci Technol. 2022 Oct 3;11(10):17.
18. Han G, Lim DH, Yoo YS, Shin EH, Park JY, Kim D, et al. Transcutaneous Electrical Stimulation for the Prevention of Dry Eye Disease after Photorefractive Keratectomy: Randomized Controlled Trial. Ophthalmol Sci. 2023 Jun;3(2):100242.
19. Ashok A, Tai WL, Lennikov A, Chang K, Chen J, Li B, et al. Electrical stimulation alters DNA methylation and promotes neurite outgrowth. J Cell Biochem. 2023 Oct;124(10):1530-45.
20. Kim T, Iseri E, Peng MG, Medvidovic S, Silliman T, Pahlavan P, et al. Electric field stimulation directs target-specific axon regeneration and partial restoration of vision after optic nerve crush injury. PLoS One. 2025 Jan 9;20(1):e0315562.
21. Ni YQ, Gan DK, Xu HD, Xu GZ, Da CD. Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration. Exp Neurol. 2009 Oct;219(2):439-52.
22. Chang K, Wu JG, Ma TL, Hsu SH, Cho KS, Yu Z, et al. Bioengineering strategy to promote CNS nerve growth and regeneration via chronic glutamate signaling. Acta Biomater. 2024 Dec;190:165-77.
23. Martella S, Ferri P, Colombo L, Torregrossa G, Mocciardini E, Baldesi J, et al. Restoring visual function in NAION by means of transpalpebral electrical stimulation: A case report. Eur J Ophthalmol. 2025 Jun 18;11206721251350826.
24. Odell RH Jr, Sorgnard RE. Anti-inflammatory effects of electronic signal treatment. Pain Physician. 2008 Nov-Dec; 11(6):891-907.
25. Lennikov A, Yang M, Chang K, Pan L, Saddala MS, Lee C, et al. Direct modulation of microglial function by electrical field. Front Cell Dev Biol. 2022 Sep 8;10:980775.
26. Enayati S, Chang K, Achour H, Cho KS, Xu F, Guo S, et al. Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential. Cells. 2020 Mar 23;9(3):781.
27. Li J, Zhou W, Liang L, Li Y, Xu K, Li X, et al. Noninvasive electrical stimulation as a neuroprotective strategy in retinal diseases: a systematic review of preclinical studies. J Transl Med. 2024 Jan 6;22(1):28.
28. Morimoto T, Miyoshi T, Sawai H, Fujikado T. Optimal parameters of transcorneal electrical stimulation (TES) to be neuroprotective of axotomized RGCs in adult rats. Exp Eye Res. 2010 Feb;90(2):285-91.
29. Jassim AH, Cavanaugh M, Shah JS, Willits R, Inman DM. Transcorneal Electrical Stimulation Reduces Neurodegenerative Process in a Mouse Model of Glaucoma. Ann Biomed Eng. 2021 Feb;49(2):858-70.
30. Naycheva L, Schatz A, Willmann G, Bartz-Schmidt KU, Zrenner E, Röck T, et al. Transcorneal electrical stimulation in patients with retinal artery occlusion: a prospective, randomized, sham-controlled pilot study. Ophthalmol Ther. 2013 Jun;2(1):25-39.
31. Schatz A, Röck T, Naycheva L, Willmann G, Wilhelm B, Peters T, et al. Transcorneal electrical stimulation for patients with retinitis pigmentosa: a prospective, randomized, sham-controlled exploratory study. Invest Ophthalmol Vis Sci. 2011 Jun 23;52(7):4485-96.
32. Fu L, Fung FK, Lo AC, Chan YK, So KF, Wong IY, et al. Transcorneal Electrical Stimulation Inhibits Retinal Microglial Activation and Enhances Retinal Ganglion Cell Survival After Acute Ocular Hypertensive Injury. Transl Vis Sci Technol. 2018 May 29;7(3):7.