Abstract
Bone mineral content and density (BMC, BMD) losses appear in various disuse conditions. To abate BMC and BMD losses, exercise may provide greater mechanical loads and impact forces to the human skeleton from muscle contractions that attach to various bones of interest. Specifically, stimuli imparted to bones refers to magnitudes, rates and frequencies of strains engendered by exerciseinduced mechanical loads and impact forces. Yet exercise’s impact on BMC and BMD has yielded mixed results due to limited delivery of one or more strain component. To perhaps best prevent BMC and BMD losses, exercise must routinely elicit high strain magnitudes, rates and frequencies to specific bones of interest. A paradigm that may best yield high strain magnitudes, rates and frequencies must entail dynamic high-speed activity; whereby successive repetitions see a light load abruptly and rapidly change its direction of movement and, when occurs, imparts large impact forces. A recent 30-workout intervention on an inertial exercise trainer (IET; Impulse Technologies, Newnan GA) produced large calcaneal BMC (+29%) and BMD (+33%) gains in ambulatory subjects. Throughout the intervention, Δ force/Δ time values achieved on the IET exceeded 2250 N.s-1 at a rate of 2.0-2.5 repetitions per second. Despite the promising results from healthy ambulatory subjects, similar research is needed with disuse conditions if the IET gain acceptance as an exercise modality used to treat bone loss.
Keywords
Magnitude, Rate, Frequency, Impact force, Osteogenic, Physical activity, Mechanotransduction
Bone Losses
Bone mineral content and density (BMC, BMD) losses, which may subsequently lead to osteopenia and osteoporosis, appear in various disuse (aging, space flight and its analogs, spinal cord injury, etc.) conditions. While diet and hormonal status impact BMC and BMD values, a major factor responsible for these losses are the reduced mechanical loads and impact forces imparted to bones. The human skeleton receives such stimuli from muscles that insert onto bony attachment points [1]. Individual motor patterns, sent by the central nervous system and executed by muscles, each impart unique strains upon the skeleton that exceed those experienced from ground reaction forces [1]. In turn, the strains evoke osteocyte remodeling and convert mechanotransduction stimuli into the appropriate bone adaptation [2-4]. In addition, impact forces imparted by physical activity have positive effects on BMC and BMD [5-8]. Yet while muscle plasticity allows adaptations to occur relatively quickly, the skeleton responds slowly, if at all, to mechanical load and impact force stimuli that hasten bone loss [1,9,10].
At the molecular level, heightened osteoclast activity from greater bone resorption accelerates BMC and BMD losses. This rise is most rapid with space flight; an abrupt loss of gravity evokes a dramatic and precipitous rise in bone resorption markers within an astronaut’s urine and blood, with little change to bone formation indices [11,12]. This led to claims space flight may aid the study of bone loss on Earth. An immediate cessation of mechanical loads and impact forces elicits BMC and BMD losses at faster rates that may in turn offer insights into the etiology of osteopenia and osteoporosis in ambulatory models [12]. Yet with BMD loss rates of 1-3% per month of microgravity exposure, and even higher losses to weight-bearing bones, some question if long-term manned flights, like those to Mars, are safe and feasible [1,12-16].
Such bone losses undermine human health and make this issue a priority for several entities. For instance, despite the NASA Human Research Program’s Pathway to Mars claim that the risk of fractures is acceptable for such missions, they currently cite seven bone-related gaps that need to be addressed [11], and others express concern of the magnitude and rate of BMC and BMD losses from space flight [12,16]. The United States military noted female recruits with low BMC and BMD values in basic training were predisposed to higher risks of skeletal fractures as compared to their male counterparts, and were therefore not well suited to withstand the rigors of basic training [17-20]. Among elderly Americans, 2010 saw in excess of a quarter million people 65 years and older incur hip fractures, with a projected 11.9% rise in their occurrence by 2030 [21]. Morbidity risk is high three to six months after hip fracture, with higher mortality rates seen in older persons [21-23]. Among the elderly, there is a four-fold higher mortality rate in those who had hip fractures versus age-matched controls [21-23]. Clearly BMC and BMD losses are a serious concern and priority for many entities to address.
The Impact of Strains and Exercise on Bone
As a treatment to ameliorate BMC and BMD loss, exercise increases mechanical loads and impact forces to the human skeleton from muscle contractions that attach to various bones of interest [1,24,25]. Perhaps a specific type of exercise stimulus and modality must be consistently applied over time to best abate bone loss [15,26-28]. Stimuli refers to the magnitudes, rates and frequencies of strains engendered by exercise-induced mechanical loads and impact forces imparted by normal and shearing activities [3,29-31]. While strain is a general term to denote bone deformation relative to its initial length, magnitude is the absolute degree of distortion imparted by mechanical stimuli [3,24,29]. In contrast frequency refers to how often strains are imparted, while rate is the change in magnitude per unit time [1,3,29].
The application of strains, and their subsequent skeletal adaptations, were initially examined in vitro with animal bones [3,29,31-35]. They generally showed once strain magnitudes exceed osteogenic thresholds, the rate and frequency determine the extent of BMC and BMD accretion [3,29,31-35]. Results from animal data led to in vivo studies with human subjects who first submitted to surgical strain gauge implantation [1,46]. In humans, it is believed magnitudes in excess of 450N exceed the osteogenic threshold that elicit positive bone changes, while strain rates greater than 10,000 microstrain per second are required to induce osteogenesis [37,38]. Peak tibial forces in humans ranged from ~1-4 times subject’s body mass [36]. Human walking and running elicited magnitudes of 430 and 850 microstrain respectively [1]. Higher magnitudes (~2000 microstrain) were noted in humans from uphill and downhill running [1]. It was concluded running produces strain magnitudes in humans comparable to those for high-impact exercises, yet with higher strain rates [1]. However limited amounts of such data exist; as of 2011 only 40 humans underwent tibial strain gauge implantation due to the considerable risks (pain, swelling, tenderness, risk of infection) associated with this surgery [1].
To circumvent this problem, recent research on strains and their subsequent adaptations used rats, some of whom received strain magnitudes comparable to those produced by human ambulation [39]. With no crossover, rats were assigned to one of five groups. Two of which were assigned to either a control or sham condition, while the other three received peak tensile strains comparable to either low- (450 microstrain; akin to human walking), medium- (850 microstrain; akin to human uphill running) or high-intensity (1250 microstrain; akin to human vertical jumping) compressive loads five days a week for eight weeks [39]. Results produced significantly better bone outcomes in the high-intensity versus a sham group; the latter was treated identically to the three treatment groups sans the tensile strains [39]. No significant differences were found between the sham and control groups, or among the three strain groups, at any time points [39]. Sadly, little data was collected on all three strain features concurrently in either animals or humans [3,32].
Given that the results of strain application and subsequent skeletal adaptations from early animal bone studies [3,29,31-35] were promising, investigators attempted to create similar human BMC and BMD changes from chronic exercise interventions. However, BMC and BMD results produced by chronic human exercise studies only elicited minor changes [8,25,28,40-43]. They include chronic interventions in which subjects received whole body vibration [42,43] or wore an accelerometer as they performed high-speed low-impact aerobic activity [28,40]. Chronic walking did not abate BMD losses in post-menopausal women [41]. Even among athletes this problem exists. For instance, little benefit occurs from race walking, despite long participatory histories of the sport’s elites [8]. Limited benefits were attributed to weak ground reaction (impact) forces and the resultant low strain magnitudes [8]. Other athletes who routinely engage in exercise with low impact forces also see lower BMD values [6]. Thus, physical activities with high bone strain rates and frequencies but low strain magnitudes yield little [8,28,40,42,43] or no [41] BMC and BMD improvements.
Since strain magnitudes for many forms of exercise for active adults are relatively low, this led to the assessment of resistive exercise’s impact on BMC and BMD. Resistive exercise typically offers higher absolute mechanical loads to exert muscular force against, and are assumed to impart greater strain magnitudes [25,44]. Yet results from longterm (3-48 months) resistance exercise studies with heavy (70-90 % 1RM) loads 2-5 days per week only yielded small (+1-3%) BMC and BMD gains [44-46]. While such paradigms routinely produced small changes, when they merely attenuated bone loss in disuse models they were deemed beneficial treatments [44,47]. A combined 26- week aerobic and resistive exercise program led to small (+1.7-2.5%) BMD gains in female cancer survivors [48]. However, when similar combined exercise programs were administered over a six-month period on the International Space Station significant lower body BMD losses (~-1.6%) persisted, regardless if astronauts engaged in a more traditional in-flight countermeasure prescription or one performed at a higher absolute exercise intensity [49].
Power training inclusion into chronic (5-50 month) resistive exercise programs evoked larger (+0-13.5%) BMC and BMD gains, which led some to imply, with its high movement rates and impact forces, may reduce bone loss in disuse models [25,46,50]. Impact forces from an average of 14.3 years of tennis play evoked greater dominant arm muscle volume (+9.7%) and BMC (+13.5%) gains versus non-dominant arm values [51]. Yet resistive exercise on flywheel-based hardware, and concurrent to bisphosphonate therapy, only partially abated leg muscle and bone losses from a 90-day bed rest [52]. Long-term studies [51,53] demonstrate the relationship mechanical loads and impact forces have on bone, yet like many forms of physical activity that saw little BMC and BMD gains from small strain magnitudes, typical resistive exercise paradigms also offer little benefit [9,44,45].
Evidence implies resistance exercise imparts strain magnitudes that routinely exceed osteogenic thresholds but, as its repetitions occur slowly, low strain rates and frequencies limit its benefits [29,44,45,51]. Even when combined with Ca+2 and vitamin D supplementation, resistive exercise only limits the risk of bone fractures by merely maintaining BMD [25]. Since it is difficult to raise BMD values, it was concluded an emphasis on early lifestyle preventative measures may best limit age-related bone loss [25]. Efforts to limit BMC and BMD losses with exercise produced modest benefits in healthy ambulatory subjects [8,25,40], and only partial loss attenuation in disuse models [48,53]. Sadly, most forms of exercise lack one or more strain feature, as even among active adults, strain magnitudes imparted to bones for many forms of physical activity are relatively low.
High-speed, High-impact Exercise and its Effect on Bone
Like animal data, human exercise sees an inverse relationship between strain magnitudes and the other two features. Perhaps relationships among strain magnitudes, rates and frequencies must exhibit a happy medium between each feature, whereby none is neglected by mechanical stimuli imparted to bones. Thus, to evoke osteogenesis, exercise may have to routinely elicit high strain magnitudes, rates and frequencies to bones [26,54-56]. Given limited bone loss mitigation seen with traditional exercise, maybe a better paradigm must entail dynamic high-speed activity [26,54-56]. For exercise to enable dynamic high-speed activity, a light load must be moved rapidly; whereby successive repetitions see abrupt and rapid changes in movement direction to a component of exercise hardware that imparts large impact forces [7]. Such a paradigm would elicit high strain magnitudes, rates and frequencies that would substitute large impact forces for heavy exercise loads necessary to elicit high strain magnitudes. Power training results imply large impact forces ensure high strain magnitudes despite light loads [46,50].
Such a paradigm describes an intervention given to postmenopausal women for six weeks [50]. With a repeated measures design and no crossover, subjects received either a callisthenic-based high-speed highimpact exercise paradigm as they wore a weighted vest, or served as untreated controls, meaning they neither exercised or wore the vest [50]. The intervention group trained twice weekly against progressively heavier yet modest vest loads. Results showed pelvic BMD rose 1.6% from the intervention while controls incurred a 1% loss. It was concluded the intervention imparted higher impact forces when ground contact was made from the callisthenic exercises [50]. Another low-load high-repetition exercise paradigm produced significant BMD gains (4-8%) at several skeletal sites after 25 weeks [55]. Both studies had paradigms whereby all strain features were high and none were neglected; in turn, they each evoked osteogenesis more rapidly than was seen with standard exercise modes [50,55].
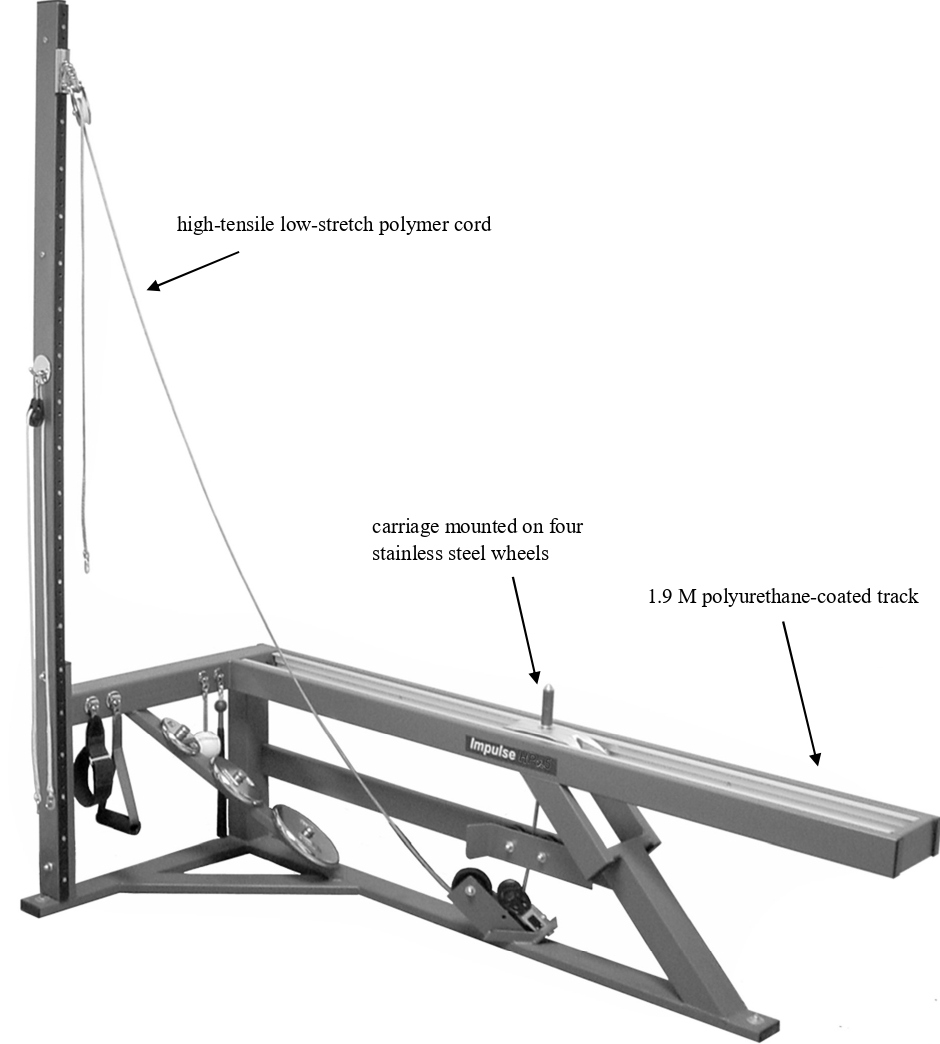
Recently, BMC and BMD changes were examined from chronic training done on an exercise modality specifically designed to impart high bone strain magnitudes, rates, frequencies and impact forces [26,56]. Called an inertial exercise trainer (IET; Impulse Training Systems, Newnan GA), it is equipped with a polyurethane-coated 1.9 m track onto which a 1 kg carriage rolls upon stainless steel wheels as forces are applied [26,56]. With as little as ~0.45N of force needed to initiate the carriage’s oscillatory movement on the track, repetitions occur at high velocities, impulses (force/time ratio) and rates of acceleration. A high-tensile low-stretch polymer cord is interwoven amongst pulleys that connect the carriage to an attachment handle that enables users to apply forces. Rapid changes in carriage direction at the start of each new repetition see the engaged muscles and bones receive an impact force. In essence the IET substitutes high impact forces for heavy loads to offer strain magnitudes that induce osteogenesis, just as was done in power training and callisthenic-based studies [7,46,50,55]. Yet without heavy loads, the IET still imparts high strain rates and frequencies. A side view picture of the IET appears in Figure 1.
Figure 1: Side view of the IET (Impulse Training Systems; Newnan, GA).
Numerous exercise movements may be done on the device and, since the carriage moves parallel to the Earth’s surface, the IET does not require gravity to operate [26,56]. Thus, it has the potential to serve as in-flight hardware to counteract weight-bearing musculoskeletal losses incurred with long-term space flights. Recent research saw, with 3.4 kg of mass added to the carriage, average Δ force/Δ time values approach 2250 N.s-1 yet still allowed performance of 2.0-2.5 repetitions per second [26]. Combined with large impact forces over successive repetitions, the IET imparts high bone strain magnitudes, rates, and frequencies. Given the numerous exercises that may be done on the IET, skeletal sites most prone to losses may be targeted by workouts.
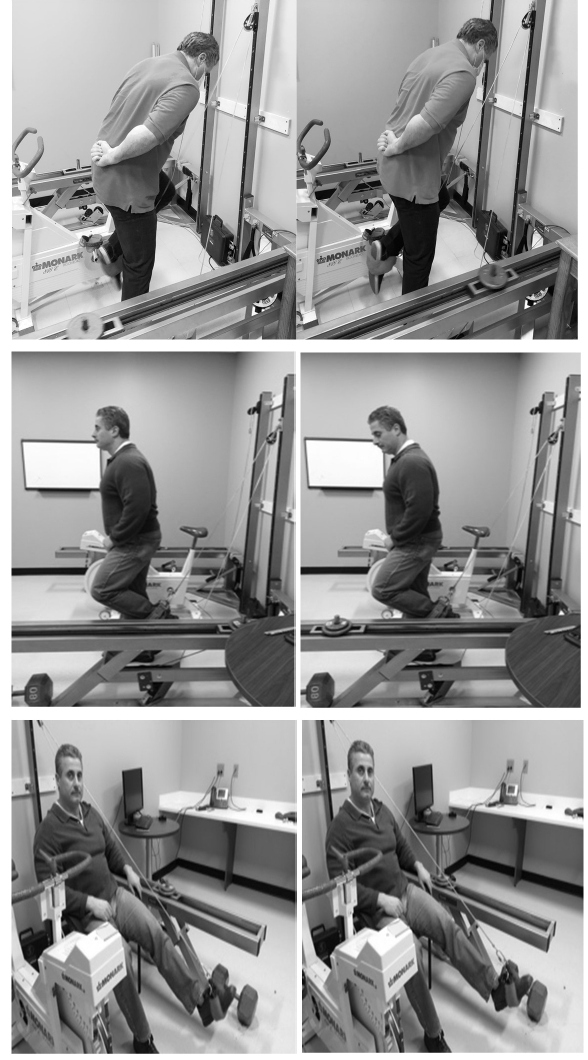
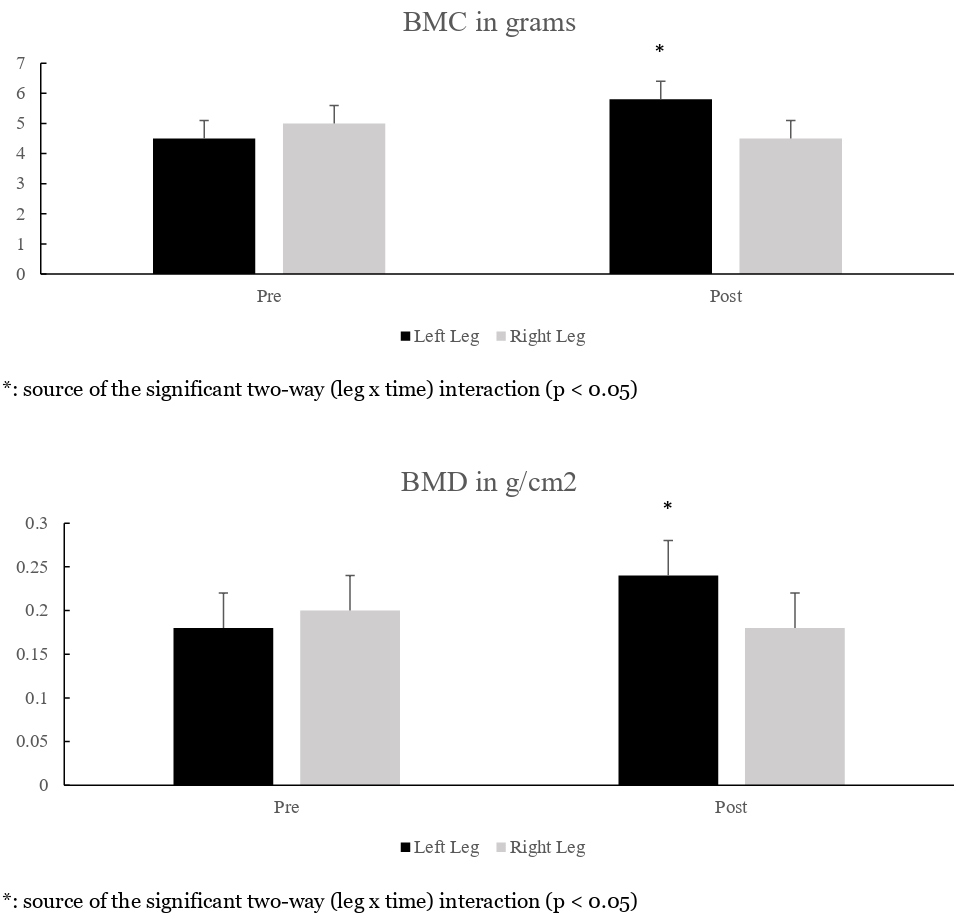
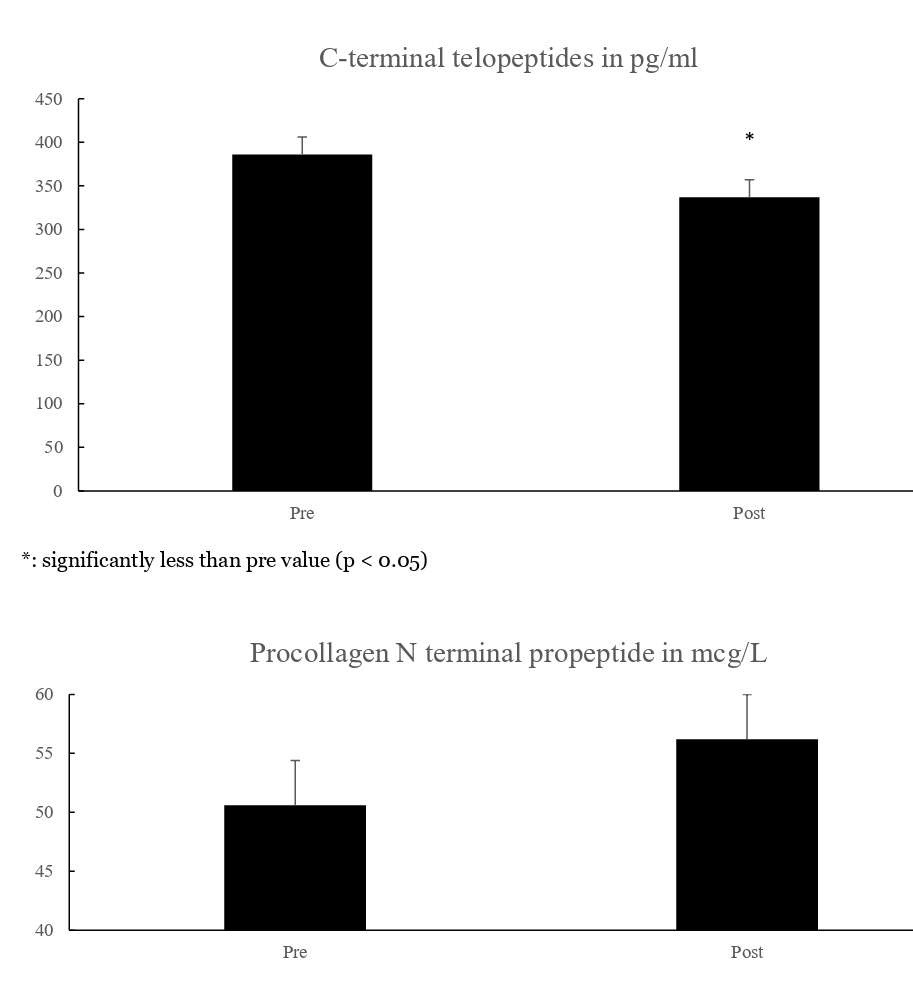
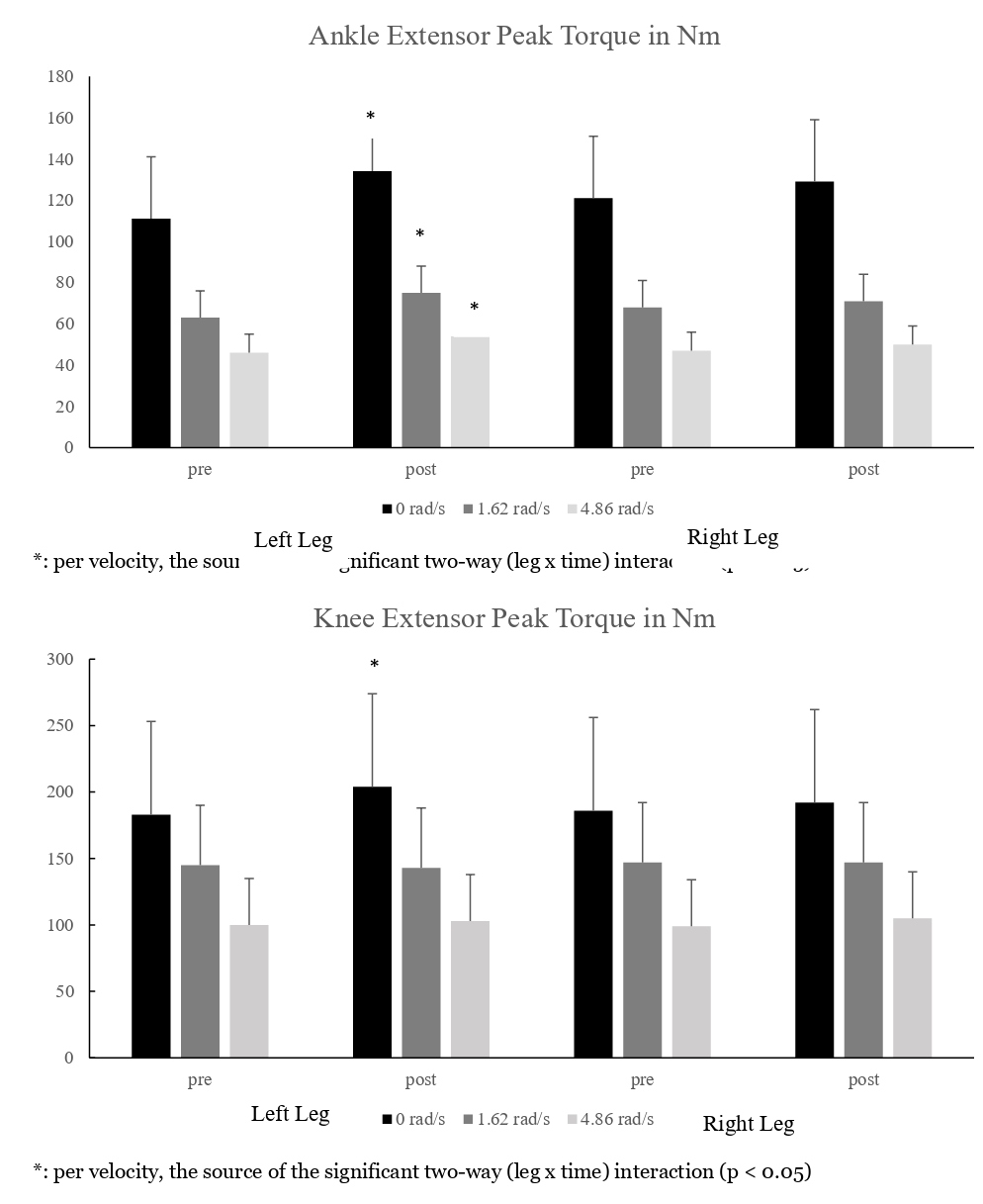
Such a paradigm was examined for bone changes [26]. Ambulatory subjects (n=13) did 30 IET workouts with their left leg, while their right served as an untreated control. Workouts, with 3.4 kg added to the carriage and maintained throughout the study, entailed three 60-second sets each of hip extension, knee extension and ankle extension exercises. Sets were separated by 90-second rests. Pictures of the exercises appear in Figure 2. Per exercise and set, subjects did tonic actions in rapid fashion, which is the IET repetition version that assures large impact forces. This allows high strain magnitudes, rates and frequencies to occur with each workout. Before and after the 30 workouts, subjects underwent strength tests (knee and ankle extensors of both legs), DXA scans (hip, knee and ankles of both legs), and blood draws. After 30 workouts there were significant left leg gains in peak torque, as well as calcaneal BMC (+29%) and BMD (+33%). Significant BMC and BMD gains were confined to the calcaneus; higher anatomical sites saw smaller interleg and -time differences [26]. Data that depict changes in calcaneal BMC and BMD, bone marker and peak torque values appear in Figures 3-5 respectively [26]. Results show promise for space flight, as calcanei demineralize in microgravity from limited weight bearing and heel strike activity [12,14,26,56]. Perhaps with different exercises, significant BMC and BMD gains to other regions, such as the hip, may occur. Significant bone resorption losses occurred after 30 workouts. With insignificant dietary changes throughout the study, BMC and BMD gains were attributed to high strain magnitudes, rates, frequencies and impact forces imparted to the left leg’s calcaneus, that in turn remodeled while bone resorption and osteoclast activity were significantly suppressed [26].
Figure 2: IET hip, knee and ankle extension exercises appear at the top, middle and bottom rows respectively.
Figure 2: IET hip, knee and ankle extension exercises appear at the top, middle and bottom rows respectively.
Figure 3: Calcaneal BMC and BMD values (mean ± sem).
Figure 4: Bone marker values (mean ± sem).
Figure 5: Peak torque values (mean ± sem).
A follow-up study with the same data set examined IETbased workout variables as correlates to calcaneal BMC and BMD gains [56]. Peak force, peak acceleration, impulse and dwell times were examined for their relationship to the changes (Δ) in BMC and BMD values. Dwell times were defined as the elapsed time between the end of a repetition’s eccentric phase, and the start of the subsequent concentric phase [56]. Pearson coefficients assessed correlations between performance and criterion variables. For hip extension data, only dwell times had significant, albeit inverse, correlations with calcaneal ΔBMC and ΔBMD values. In similar fashion, ankle extension data only saw dwell times had a significant, albeit inverse, correlation to calcaneal ΔBMC as the criterion. Inverse correlations infer shorter dwell times evoked more osteogenesis. Dwell time values for the hip extension exercise had a unique outcome, namely subjects who produced values less than 26 milliseconds saw positive calcaneal ΔBMC and ΔBMD changes, while those which exceeded that time frame incurred calcaneal ΔBMC and ΔBMD losses [56].
Conclusions
Mechanotransduction stimuli received by bone, which lead to the subsequent expression of strains and skeletal remodeling, is far from being fully understood [2]. Exercise’s impact on BMC and BMD has produced mixed results due to limited delivery of one or more strain feature [25]. Results from this paper suggest all three strain features should be high when mechanical loads are imparted to bones in order to best evoke osteogenesis. The IET’s design replaces heavy loads with rapid changes in carriage direction to provide high strain magnitudes, rates, frequencies and impact forces to bones. Despite promising results from ambulatory subjects [26,56], similar research is needed with disuse conditions. Only when the large calcaneal BMC and BMD gains are replicated in disuse models will the IET gain greater acceptance as exercise hardware used to treat BMC and BMD losses.
Conflict of Interest
The authors have no conflicts of interest to report.
References
2. Battafarano G, Rossi M, Marampon F, Minisola S, Del Fattore A. Bone control and muscle function. Int J Molec Sci. 2020;21:1178.
3. Hsieh F, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo: The strain threshold for an osteogenic response varies with location. J Bone Miner Res. 2001;16: 2291-2297.
4. Kameo Y, Adachi T. Interstitial fluid flow in canaliculi as a mechanical stimulus for cancellous bone remodeling: In silico validation. Biomech Model Mechanobiol. 2014;13:851-860.
5. Bezodis IN, Kerwin DG, Salo AI. Lower-limb mechanics during the support phase of maximum-velocity sprint running. Med Sci Sports Exerc. 2008;40:707-715.
6. Duncan CS, Blimkie CJ, Cowell CT, Burke ST, Briody JN, Howman-Giles R. Bone mineral density in adolescent female athletes: relationship to exercise type and muscle strength. Med Sci Sports Exerc. 2002;34:286-294.
7. Heikkinen R, Vihriälä E, Vainionpää A, Korpelainenace R, Jämsä T. Acceleration slope of exercise-induced impacts is a determinant of changes in bone density. J Biomech. 2007; 40:2967-2974.
8. Pavei G, Cazzola D, La Torre A, Minetti AE. The biomechanics of race walking: literature overview and new insights. Eur J Sports Sci. 2014;14:661-670.
9. Englund U, Littbrand H, Sondell A, Pettersson U, Bucht G. A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos Int. 2005;16:1117-1123.
10. Vainionpää A, Korpelainen R, Sievänen H, Vihriälä E, Leppäluoto J, Jämsä T. Effect on impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone. 2007;40:604-611.
11. NASA Human Research Roadmap. https://human researchroadmap.nasa.gov/Gaps/. Accessed December 8th, 2020.
12. Orwoll ES, Adler RA, Amin S, Binkley N, Lewiecki EM, Petak SM, et al. Skeletal health in long-duration astronauts: nature, assessment and management recommendations from the NASA Bone Summit. J Bone Min Res. 2013;28:1243-1255.
13. Holick MF. Perspective on the impact of weightlessness on calcium and bone metabolism. Bone. 1998; 22: 105S-111S.
14. Sibonga JD, Cavanagh PR, Lang TF, LeBlanc AD, Schneider VS, Shackelford LC, et al. Adaptation of the skeletal system during long-duration space flight. Clin Rev Miner Metab. 2007;5:249-261.
15. Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz-Snyder L, Zwart SR. Benefits for bone from resistive exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J Bone Miner Res. 2012;27:1896-1906.
16. Sutterfield SL, Alexander AM, Hammer SM, Didier KD, Caldwell JT, Barstow TJ, et al. Prediction of planetary mission task performance for long-duration spaceflight. Med Sci Sports Exerc. 2019;51:1662-1670.
17. Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437-444.
18. Jones BH, Thacker SB, Gilchrist J, Kimsey CD, Sosin DM. Prevention of Lower Extremity Stress Fractures in Athletes and Soldiers: A Systematic Review. Epidem Rev. 2002;24:228-247.
19. Kowal D. Nature and causes of injuries in women resulting from an endurance training program. Am J Sports Med. 1980;8:265-269.
20. Shaffer RA, Brodine SK, Ito SI. Epidemiology of illness and injury among U.S. Navy and Marine Corps female training population. Mil Med. 1999;164:17-21.
21. Vainionpää A, Korpelainen R, Sievänen H, Vihriälä E, Leppäluoto J, Jämsä T. Effect on impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone. 2007;40:604-611.
22. Farahmand BY, Michaëlsson K, Ahlbom A, Ljunghall S, Baron JA. Survival after hip fracture. Osteoporo Int. 2005;16:1583-1590.
23. Koh GC, Tai BC, Ang LW, Heng D, Yuan JM, Koh WP. All-cause and cause-specific mortality after hip fracture among Chinese women and men. Osteoporo Int. 2013;24:1981-1989.
24. Kalkhoven JT, Watsford ML, Coutts AJ, Edwards WB, Impellizzeri FM. Training load and injury: causal pathways and future directions. Sports Med. 2020; https://doi.org/10.1007/s40279-020-01413-6.
25. Nguyen VH, Loethen J, LaFontaine T. Resistance training and dietary supplementation for persons with reduced bone mineral density. Stren Cond J. 2008;30(5):28-31.
26. Caruso JF, Voor M, Jaggers J, Symons TB, Stith J, Bai L, et al. Musculoskeletal outcomes from chronic highspeed, high-impulse resistance exercise. Int J Sports Med. 2018;39:791-801.
27. Lang T, LeBlanc AD, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration space flight. J Bone Miner Res. 2004;19:1006-1012.
28. Vainionpää A, Korpelainen R, Leppäluoto J, Jämsä T. Effects of high-impact exercise on bone mineral density: A randomized controlled trial in premenopausal women. Osteoporos Int. 2005; 16:191-197.
29. Cullen DM, Smith RT, Akhter MP. Bone-loading response varies with strain magnitude and cycle number. J Appl Physiol. 2001;91:1971-1976.
30. Lanyon LE, Rubin CT. Static vs dynamic loads as an influence on bone remodeling. J Biomech. 1984;17:897- 905.
31. Rubin CT, Lanyon LE. Regulation of bone mass by mechanical strain magnitude. Calcified Tissue Int. 1985;37:411-417.
32. Judex S, Zernicke RF. High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J Appl Physiol. 2000;88:2183- 2191.
33. Sugiyama T, Price JS, Lanyon LE. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone. 2012;46:314-321.
34. Turner CH, Forwood MR, Rho JY, Yoshikawa T. Mechanical thresholds for lamellar and woven bone formation. J Bone Miner Res. 1994;9:878-897.
35. Turner CH, Owan I, Takano Y. Mechanotransduction in bone: role of strain rate. Am J Physiol Endocrinol Metab. 1995;269:442.
36. D’Lima DD, Fregly BJ, Colwell CW. Implantable sensor technology: measuring bone and joint biomechanics of daily life in vivo. Arthritis Res Ther. 2013;15:203.
37. Coyle S. An exercise device to stimulate bone remodeling for the reduction of hip fractures in aging women. Electronic These and Dissertations. Master of Engineering, University of Louisville, 2020.
38. Osborne A. Biomechanical testing of an exercise for strengthening the proximal femur. Electronic Theses and Dissertations. Master of Engineering Thesis, University of Louisville, 2017.
39. Mustafy T, Londono I, Moldovan F, Villemure I. Isolated cyclic loading during adolescence improves tibial bone microstructure and strength at adulthood. J Bone Min Res Plus. 2020;4:1-6.
40. Ahola R, Korpelainen R, Vainionpää A, Jämsä T. Daily impact score in long-term acceleration measurements of exercise. J Biomech. 2010;43:1690-1694.
41. Cavanaugh DJ, Cann CE. Brisk walking does not stop bone loss in postmenopausal women. Bone. 1988;9:201- 204.
42. Fratini A, Bonci T, Bull AMJ. Whole body vibration treatments in postmenopausal women can improve bone mineral density: results of a stimulus focused metaanalysis. PLoS One. 2016;11:e0166774.
43. Sá-Caputo DC, Dionello CF, Frederico EHFF, Paineiras-Domingos LL, Sousa-Goncalves CR, Morel DS, et al. Whole-body vibration exercise improves functional parameters in patients with osteogenesis imperfecta: A systematic review with a suitable approach. Afr J Tradit Complement Altern Med. 2017;14:199-208.
44. Zehnacker CH, Bemis-Daugherty A. Effect of weighted exercises on bone mineral density in postmenopausal women a systematic review. J Geriat Phys Ther. 2007;30:79-88.
45. Cussler EC, Going SB, Houtkooper LB, Stanford VA, Blew RM, Flint-Wagner HG, et al. Exercise frequency and calcium intake predict 4-year bone changes in postmenopausal women. Osteoporos Int. 2005;16:2129- 2141.
46. de Kam D, Smulders E, Weerdesteyn V, Smits- Engelsman BCM. Exercise interventions to reduce fallrelated fractures and their risk factors in individuals with low bone density: A systematic review of randomized controlled trails. Osteoporos Int. 2009;20:2111-2125.
47. Engelke K, Kemmler W, Lauber D, Beeskow C, Pintag R, Kalender WA. Exercise maintains bone density at spine and hip EFOPS: A 3-year longitudinal study in early postmenopausal women. Osteoporos Int. 2006;17:133-142.
48. Almstedt HC, Grote S, Korte JR, Perez Beaudion S, Shoepe TC, Strand S, et al. Combined aerobic and resistance training improves bone health of female cancer survivors. Bone Rep. 2016;5:274-279.
49. English KL, Downs M, Goetchius E, Buxton R, Ryder JW, Ploutz-Snyder R, et al. High intensity training during space flight: results from the NASA Sprint Study. Microgravity 2020; 6(21).
50. Hamaguchi K, Kurihara T, Fujimoto M, Iemitsu M, Sato K, Hamaoka T, et al. The effects of low-repetition and light-load power training on bone mineral density in postmenopausal women with sarcopenia: A pilot study.BMC Geriatrics. 2017;17:102.
51. Ducher G, Courteix D, Même S, Magni C, Viala JF, Benhamou CL. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: A quantitative magnetic resonance imaging study in tennis players. Bone. 2005;37:457-466.
52. Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, et al. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: Results from the LTBR study. Bone. 2005;36:1019-1029.
53. Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson N, et al. High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone. 2008;43:169-176.
54. Lanyon LE. The success and failure of the adaptive response to functional load-bearing in averting bone fracture. Bone. 1992;13:S17-S21.
55. Petersen BA, Hastings B, Gottschall JS. Low load, high repetition resistance training program increases bone mineral density in untrained adults. J Sports Med Phys Fitness. 2017;57: 70-76.
56. Davison SW, Chen L, Gray D, McEnroe B, O’Brien I, Kozerski A, Caruso J. Exercise-based correlates to calcaneal osteogenesis produced by a chronic training intervention. Bone. 2019;128:115049.