Abstract
Introduction: Painful diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes mellitus and a major cause of disability and poor quality of life in patients with diabetes. In addition, health care costs associated with DPN are increasing. Given the challenges and limitations of current pharmacological treatments, traditional herbal remedies present a viable option. As a result, we formulated a new topical treatment, “NeuroHelp”. This study aims to evaluate the effectiveness of the herbal medicine “NeuroHelp” in combination with oral gabapentin, and B vitamins on DPN symptoms.
Methods: We conducted a double-blinded, placebo-controlled, randomized clinical trial with parallel design on 84 type 2 diabetes patients with painful diabetic neuropathy. The 84 eligible patients were randomly allocated to one of two groups, either the intervention group (herbal medicine in combination with oral gabapentin, and B vitamins) or the placebo group (placebo in combination with oral gabapentin, and B vitamins). Topical herbal medicine ingredients were made of Citrullus colocynthis, Matricaria chamomilla, Althaea officinalis, Rheum officinale, Capparis spinosa, and Trigouella foenum graecum. A topical placebo ointment, with a similar appearance, texture and smell to the herbal medicine was made by a medicine manufacturer. Patients were evaluated for tingling feet, feet numbness, pinprick sensation, cold feet, monofilament test results, tuning fork test results, and pain using visual analogue scale (VAS) at baseline and three weeks after intervention as primary outcomes.
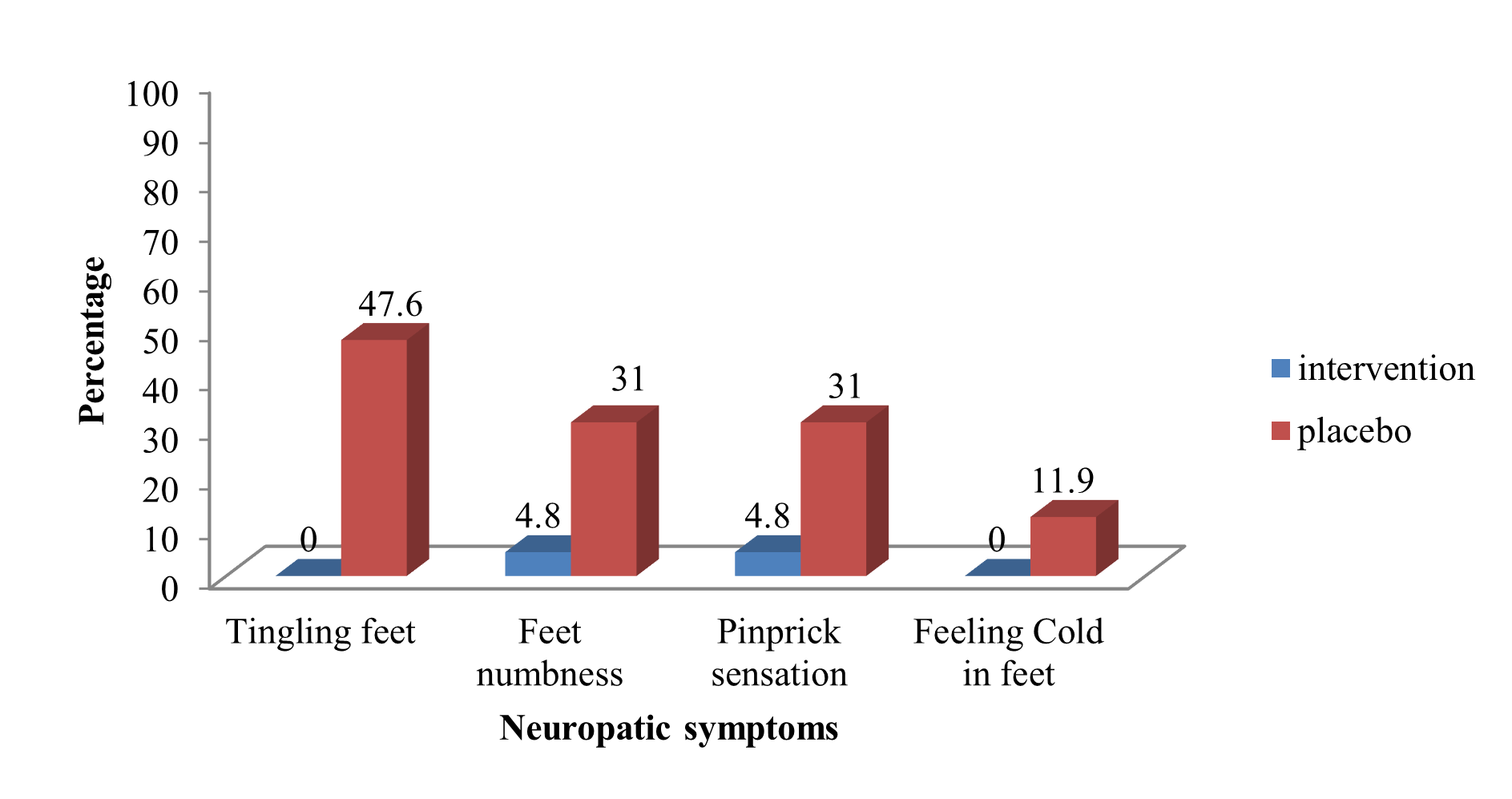
Results: The mean age of patients was 54.45 ± 8.72 years, 23.81% were male and 76.19% were female. Other baseline characteristics and diabetes relevant parameters revealed no significant differences between the intervention and placebo groups. NeuroHelp herbal medicine in combination with oral gabapentin, and B vitamins significantly reduced VAS for pain (mm) compared to placebo group after three weeks 24.0 ± 22.9 VS. 44.3 ± 33.1 (p<0.002). It also reduced diabetic neuropathy symptoms after three weeks compared to the placebo group including tingling feet 0 vs. 20 (47.60%) (p<0.001), foot numbness 2 (4.80%) vs. 13 (31%) (p<0.001), pinprick sensation 2 (4.8%) vs. 13 (31%) (p<0.001), and cold feet 0 vs. 5 (11.90%) (p=0.049).
Conclusions: Our study showed that three weeks topical application of NeuroHelp in combination with oral gabapentin, and B vitamins could improve diabetic neuropathy symptoms.
Keywords
Diabetic neuropathy, Type 2 diabetes, Neuropathic pain, Clinical trial, Herbal medicine, Topical ointment, Uncontrolled blood glucose
Introduction
Painful diabetic peripheral neuropathy (DPN) is one of the most common and debilitating complications of diabetes mellitus (DM) [1-4], estimated to affect 66% of type 1 and 59% of type 2 patients with diabetes [5-8]. The prevalence of DPN has been reported between 2.4% and 78.8% in China, 5.7% in Germany and 2.4% in the United Kingdom. A study in the United States reported an overall incidence of painful DPN of 34%. Meanwhile, a study conducted in England reported an overall prevalence of painful DPN at 26.4%. However, some authors have reported prevalence as high as 77.4% [9]. In addition, diabetic painful polyneuropathy has a negative impact on patients’ quality of life, including physical pain, sleep disturbance, limitation of daily activities, polypharmacy, and depression [10]. Painful DPN is independently a major cause of disability and poor quality of life, and impacts activities of daily living [9,11].
Currently, controlling blood glucose level is the only option for preventing DPN. Treatment options for DPN patients are limited to symptomatic treatments [7]. Treatment examples include antidepressants (e.g., amitriptyline, duloxetine, and venlafaxine), anticonvulsants (e.g., gabapentin), as well as topical agents such as capsaicin cream, lidocaine patches, topical spray dinitrate isosorbide, and pain control. The effectiveness of these options is currently unsatisfactory, and long-term effects have not been studied [12,13], leaving patients without adequate pain control [14]. However, due to the lower risk of systemic side effects, patients are more likely to accept topical medicines [15].
Given the challenges and limitations associated with current pharmacological treatments, traditional herbal remedies present a viable option. Traditional medicine practitioners used ancient formulas to improve diabetic neuropathy symptoms and control blood glucose, which they claimed to be effective for all patients with diabetes [16]. In seeking to formulate a new topical medicine, based on traditional medicine formularies, we expanded an ancient formula. Our new herbal medicine, named NeuroHelp, consists of six herbs (Citrullus colocynthis, Matricaria chamomilla, Althaea officinalis, Rheum officinale, Capparis spinosa, and Trigouella foenum graecum). The aim of this study was to evaluate the effectiveness of this herbal medicine on the symptoms of DPN including foot tingling, foot numbness, cold feet, and pain.
Painful diabetic peripheral neuropathy (DPN) is one of the most common and debilitating complications of diabetes mellitus (DM) [1-4], estimated to affect 66% of type 1 and 59% of type 2 patients with diabetes [5-8]. The prevalence of DPN has been reported between 2.4% and 78.8% in China, 5.7% in Germany and 2.4% in the United Kingdom. A study in the United States reported an overall incidence of painful DPN of 34%. Meanwhile, a study conducted in England reported an overall prevalence of painful DPN at 26.4%. However, some authors have reported prevalence as high as 77.4% [9]. In addition, diabetic painful polyneuropathy has a negative impact on patients’ quality of life, including physical pain, sleep disturbance, limitation of daily activities, polypharmacy, and depression [10]. Painful DPN is independently a major cause of disability and poor quality of life, and impacts activities of daily living [9,11].
Currently, controlling blood glucose level is the only option for preventing DPN. Treatment options for DPN patients are limited to symptomatic treatments [7]. Treatment examples include antidepressants (e.g., amitriptyline, duloxetine, and venlafaxine), anticonvulsants (e.g., gabapentin), as well as topical agents such as capsaicin cream, lidocaine patches, topical spray dinitrate isosorbide, and pain control. The effectiveness of these options is currently unsatisfactory, and long-term effects have not been studied [12,13], leaving patients without adequate pain control [14]. However, due to the lower risk of systemic side effects, patients are more likely to accept topical medicines [15].
Given the challenges and limitations associated with current pharmacological treatments, traditional herbal remedies present a viable option. Traditional medicine practitioners used ancient formulas to improve diabetic neuropathy symptoms and control blood glucose, which they claimed to be effective for all patients with diabetes [16]. In seeking to formulate a new topical medicine, based on traditional medicine formularies, we expanded an ancient formula. Our new herbal medicine, named NeuroHelp, consists of six herbs (Citrullus colocynthis, Matricaria chamomilla, Althaea officinalis, Rheum officinale, Capparis spinosa, and Trigouella foenum graecum). The aim of this study was to evaluate the effectiveness of this herbal medicine on the symptoms of DPN including foot tingling, foot numbness, cold feet, and pain.
Method and Materials
Study design
This double-blinded, placebo-controlled, randomized clinical trial was performed between January and May 2019 in the Diabetes Clinic in Dezyani Polyclinic, Gorgan, Iran. The parallel design was used, and statistical analysis was based on the intention to treat analysis. This study was conducted in accordance with the Declaration of Helsinki, and was approved by the local Institutional Review Board and the ethics committee of Golestan University of Medical Sciences (IR.GOUMS.REC.1397.001). The study was registered at the Iranian registry of clinical trials, http://www.irct.ir (identifier- IRCT20170422033566N2).
Study sample
Eighty-four type 2 diabetes mellitus (T2DM) male and female patients with a clinical diagnosis of painful DPN based on patients’ complaints were referred from Gorgan Diabetes Clinic in Dezyani Polyclinic and included in our study. We included T2DM patients with uncontrolled blood glucose, patients between 30-70 years old who had DPN and daily pain for more than three months with a pain score of at least four based on the visual analog scale (VAS) scoring system. To diagnose DPN, a thorough examination was conducted by physicians (ONN, MZ). Patients who did not meet the diagnostic criteria or who had other causes of pain were excluded from the study. Neurological symptoms affecting organs other than the peripheral nerves were not considered in this study.
The sample size was calculated using GPower 3.1 based on a previous study [1]. Considering a two-sided P<0.05, power of 0.80, and a 20% attrition rate, the required sample size to compare the mean pain scores after treatment between the intervention and control groups was determined to be 44 patients per group.
The exclusion criteria were prior or current history of anticonvulsants use, alcohol abuse, other reasons for neuropathy, renal dysfunction (serum creatinine >1.5), liver dysfunction (ALT>40 mg/dl, AST>40 mg/dl, total bilirubin>1 mg/dl, disrupted alkaline phosphatase), pregnancy, lactation, foot ulcer or foot infections, and complete patient unconsciousness. DPN was confirmed by assessing the Neuropathic Symptom Score (NSS), Neuropathic Disability Score (NDS), endocrinologists’ evaluations and clinical examinations [17-19]. The use of any drugs for the treatment of neuropathic pain was discontinued two weeks before entering the study. Insulin therapy and oral glucose-lowering medications were continued throughout the study.
Randomization
A complete list of randomized blocks was provided to the researchers by the epidemiologist. Group allocation was concealed prior to the treatment. Study medication was administered by a nurse who was blinded to which treatment was used. Patients, study staff, and investigators were blinded to treatment assignments.
After obtaining written informed consent from all participants, patients were randomized to receive NeuroHelp herbal medicine + standard treatment (gabapentin 300 mg, vitamin B1 100-300 mg and vitamin B complex) or placebo + standard therapy (gabapentin 300 mg, vitamin B1 100-300 mg and vitamin B complex), with using a randomized block design. The treatment allocation sequence was carried out based on a block size of six generated with Microsoft Excel.
Interventions
The NeuroHelp topical herbal mixture was made by combining six herbs including Citrullus colocynthis (150-100mg), Matricaria chamomilla (10 g), Althaea officinalis (10 g), Rheum officinale (2 g), Capparis spinosa (5 g), and Trigouella foenum graecum (10 g), utilizing a solvent for traditional medicine.
A topical placebo ointment, with similar appearance, texture and smell to NeuroHelp, was made by a medicine manufacturer. Patients were instructed to either apply 2-5 cc of their assigned treatment to the plantar surface of their feet for three weeks or pour 10-14 cc into a non-metallic container and place their feet in the container for 15 minutes.
Confounding variables
Baseline clinical characteristics of the patients including age, sex, duration of diabetes, treatment type, as well as comprehensive metabolic panel including fasting blood sugar (FBS), 2 hours postprandial blood glucose, glycated hemoglobin (HbA1c), systolic and diastolic blood pressure (SBP, DBP), cholesterol, triglycerides (TG), low density lipoprotein (LDL), and high-density lipoprotein (HDL) were assessed at the beginning of the study. HbA1C higher than 7% was considered uncontrolled. None of our patients had FBS below 110 or blood sugar two hours after a meal below 180.
Endpoints
The primary endpoint of study was measuring VAS scores of DPN after three weeks of using NeuroHelp. The VAS consists of a 100 mm line with one end representing no pain and the other end indicating maximum pain [20].
Patients were evaluated in terms of tingling feet, feet numbness, pinprick sensation, cold feet, pain, monofilament test results, tuning fork test results at the baseline and three weeks after intervention as primary outcomes. During these three weeks, patients were visited every week so that if any complications were observed, the patients would be excluded from the study.
The pain was assessed using a visual analog scale. The monofilament test was used for assessing peripheral neuropathy by examining nine plantar sites (first, third, and fifth metatarsal heads, distal great toe, third toe, fifth toe, medial foot, lateral foot, and heel).
Statistical analysis
Data analysis was performed using SPSS V.22 software. Descriptive data were reported as mean (SD) or frequency (percentage) as appropriate. After confirming the normal distribution of the data with Kolmogorov-Smirnov test, statistical comparisons of primary characteristics and outcomes between the intervention and placebo groups were conducted using the independent t-test and Chi-squared test. Repeated measure analysis was used to assess pain trend at baseline and three weeks after the intervention. Effectiveness analysis was performed on intention to treat (ITT) analysis. Two-sided P<0.05 was considered significant.
Results
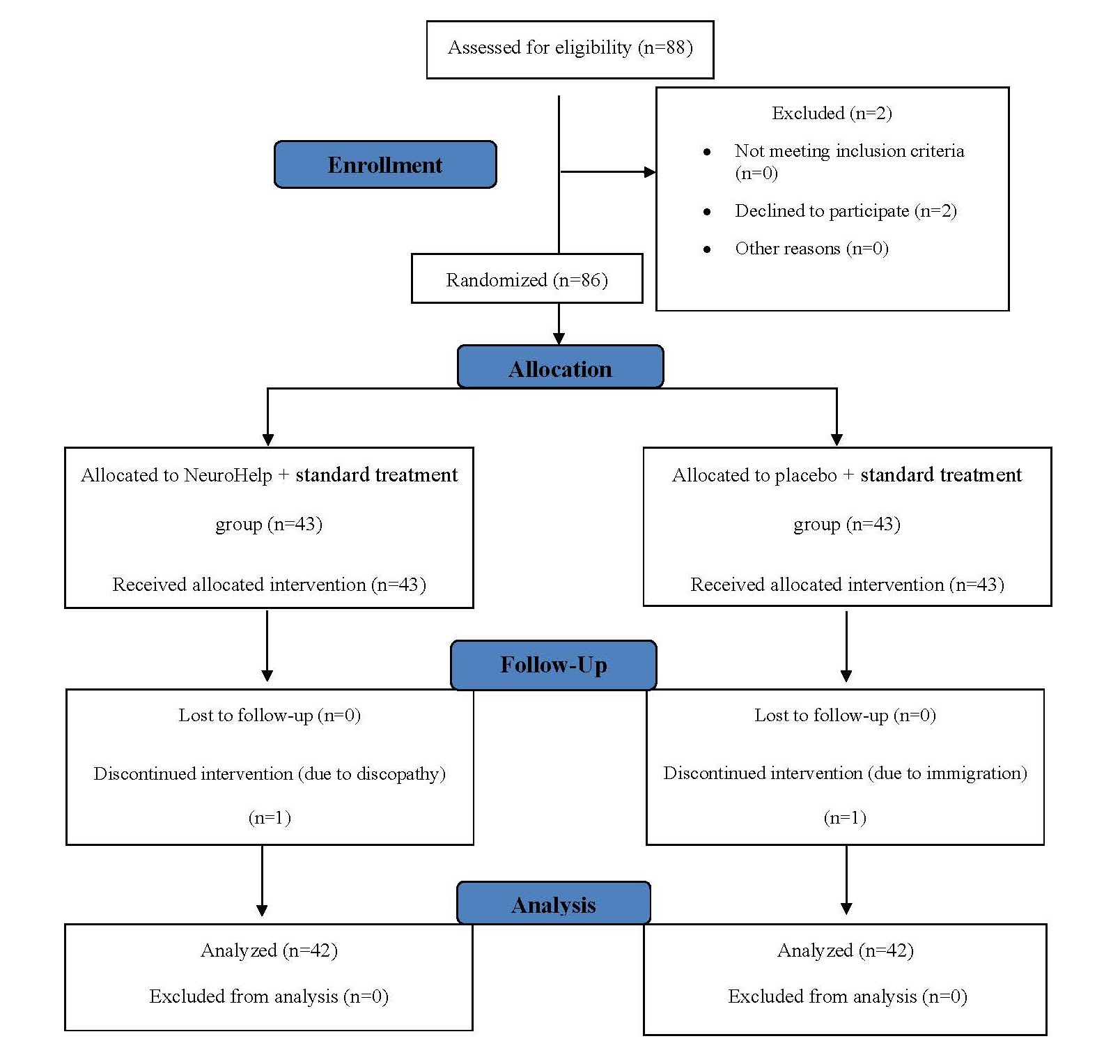
A total of 84 patients with DPN were randomly assigned to the intervention group (n=42) or the placebo group (n=42). Figure 1 shows the allocation decision tree; baseline characteristics are presented in Table 1. The mean age of patients was 54.45 ± 8.72 years and 76.19% were female. Other baseline characteristics revealed no significant differences between the intervention and placebo groups.
Figure 1. Flowchart of patients' admission process and follow-up during the study to evaluate the efficacy of herbal medicine (NeuroHelp) plus standard treatment on the treatment of diabetic neuropathy.
|
Characteristics |
Intervention group (NeuroHelp+current treatment) (n=42) |
Placebo group (Placebo+current treatment) (n=42) |
P value |
|
Sex, No. (%) Male Female |
11 (26.20) 31 (73.80) |
9 (21.40) 33 (78.60) |
0.798 |
|
Age, mean (SD), y |
56.48 (8.42) |
56.43 (9.11) |
0.980 |
|
Duration of diabetes mean (SD), y |
10.78 (6.42) |
11.88 (8.49) |
0.512 |
|
FBS mean (SD) |
177.11(62.77) |
191.39 (70.52) |
0.334 |
|
2hPG mean (SD) |
268.10 (86.96) |
291.94 (87.32) |
0.09 |
|
HbA1C mean (SD) |
8.48 (1.31) |
8.16 (1.91) |
0.406 |
|
Systolic BP mean (SD) |
113.11 (22.40) |
119.68 (16.63) |
0.174 |
|
Diastolic BP mean (SD) |
70.28 (9.10) |
72.90 (6.92) |
0.185 |
|
Treatment type No. (%) Insulin Injection Oral medication Both none |
5 (11.90) 20 (47.60) 16 (38.10) 1 (2.4) |
4 (9.50) 26 (61.90) 6 (14.30) 1 (2.40) |
0.162 |
|
Cholesterol mean (SD) |
148.59 (45.08) |
171.88 (48.63) |
0.036 |
|
TG mean (SD) |
158.97 (62.69) |
168.06 (97.94) |
0.641 |
|
LDL mean (SD) |
86.37 (29.76) |
100.23 (31.67) |
0.059 |
|
HDL mean (SD) |
40.95 (8.32) |
48.38 (11.64) |
0.003 |
|
Data presented as n (%) or mean (SD), FBS: Fasting Blood Sugar; 2hPG: 2-hour Postprandial Blood Glucose; HbA1C: Hemoglobin A1c; BP: Blood Pressure; TG: Triglycerides; LDL: Low Density Lipoprotein; HDL: High-Density Lipoprotein |
|||
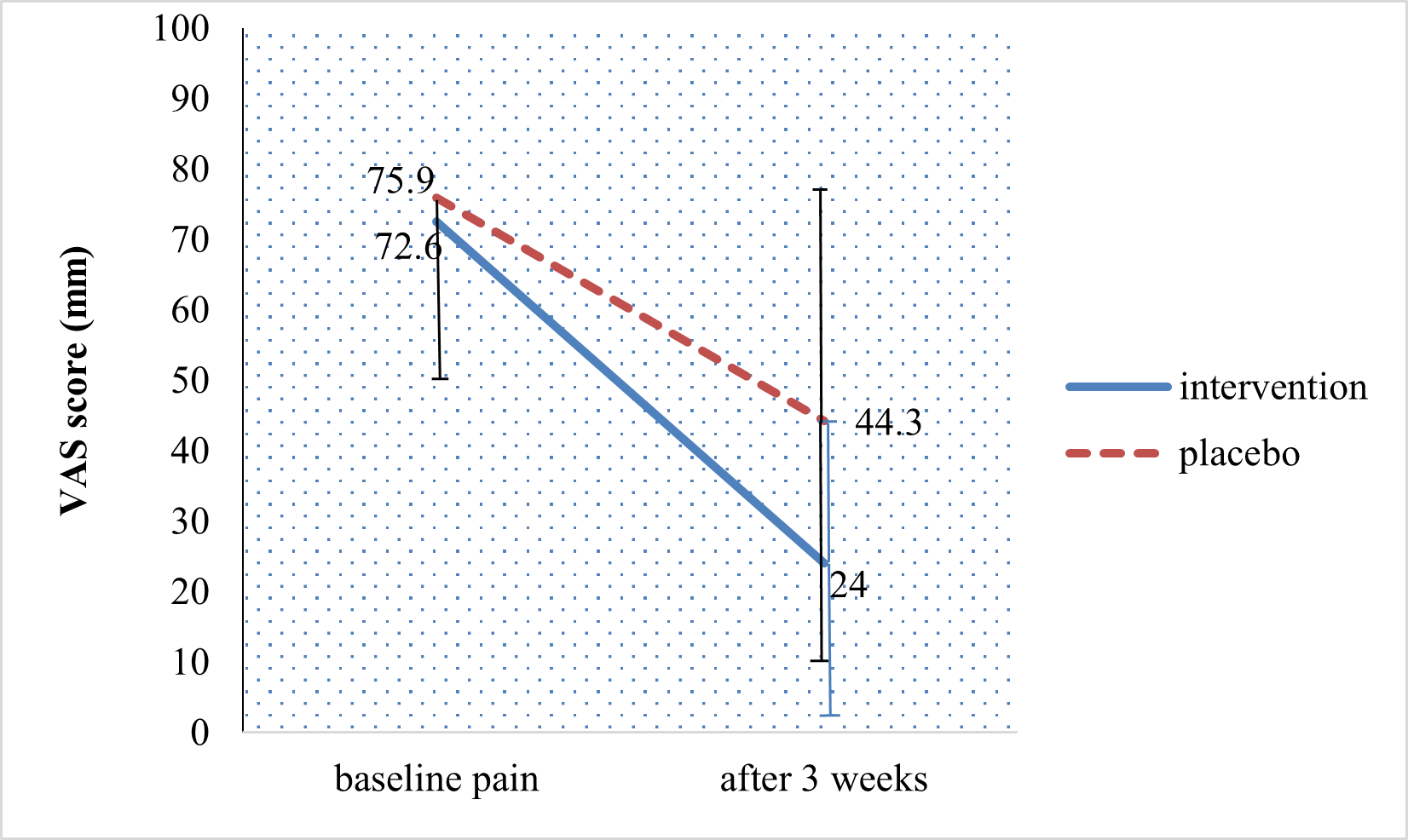
The trend of mean pain reduction before and three weeks after treatment with herbal medicine plus standard treatment is shown in Figure 2. Pain was measured on a VAS and the statistical significance of the reduction of VAS scores in the intervention group was confirmed through the ANOVA test (p = 0.022). In the intervention group, the VAS score reduced from 72.6 to 24 mm, while in the placebo group, the VAS score changed from 75.9 to 44.3 mm. NeuroHelp herbal medicine in combination with oral gabapentin, and B vitamins significantly reduced VAS for pain (mm) compared to placebo group after three weeks 24.0 ± 22.9 versus 44.3 ± 33.1 (p<0.002). Two-month follow-up after completion of the study revealed no complications in the treatment group.
Figure 2. Pain trend in patients with neuropathic diabetes with using repeated measures ANOVA test (P=0.022). Data presented as mean (SD).
Table 2 reveals the relevant parameters of patients' diabetes, comparing the intervention group and the placebo group. The mean duration of diabetes in the placebo and intervention groups was 11.88 ± 8.49 and 10.78 ± 6.42 years, respectively, which made the two groups comparable in terms of prognostic factors.
|
Characteristics |
Intervention group (NeuroHelp+current treatment) (n=42) |
Placebo group (Placebo+current treatment) (n=42) |
P value |
|
Tingling feet No. (%) no mild-moderate severe |
4 (9.50) 12 (28.60) 26 (61.9) |
2 (4.80) 17 (40.50) 23 (54.80) |
0.425 |
|
Feet numbness No. (%) no mild-moderate severe |
12 (28.60) 7 (16.70) 23 (54.80) |
8 (19.00) 19 (45.20) 15 (35.70) |
0.018 |
|
Pinprick sensation No. (%) no mild-moderate severe |
10 (23.80) 11 (26.20) 21 (50.00) |
8 (19.00) 21 (50.00) 13 (31.00) |
0.073 |
|
Cold feet No. (%) no mild-moderate severe |
11 (26.20) 14 (33.30) 17 (40.50) |
16 (38.10) 20 (47.60) 6 (14.30) |
0.027 |
|
Neuropathic pain VAS score in mm mean (SD) |
72.6 (22.5) |
75.9 (26.1) |
0.09 |
|
Monofilament test Normal Abnormal In one foot In both feet |
19 (45.20)
9 (21.40) 14 (33.30) |
12 (28.60)
12 (28.60) 18 (42.90) |
0.285 |
|
Tuning fork Test Normal Abnormal In one foot In both feet |
30 (71.40)
5 (11.90) 7 (16.70) |
34 (82.90)
3 (7.30) 4 (9.80) |
0.46 |
|
Data presented as n (%) or mean (SD) |
|||
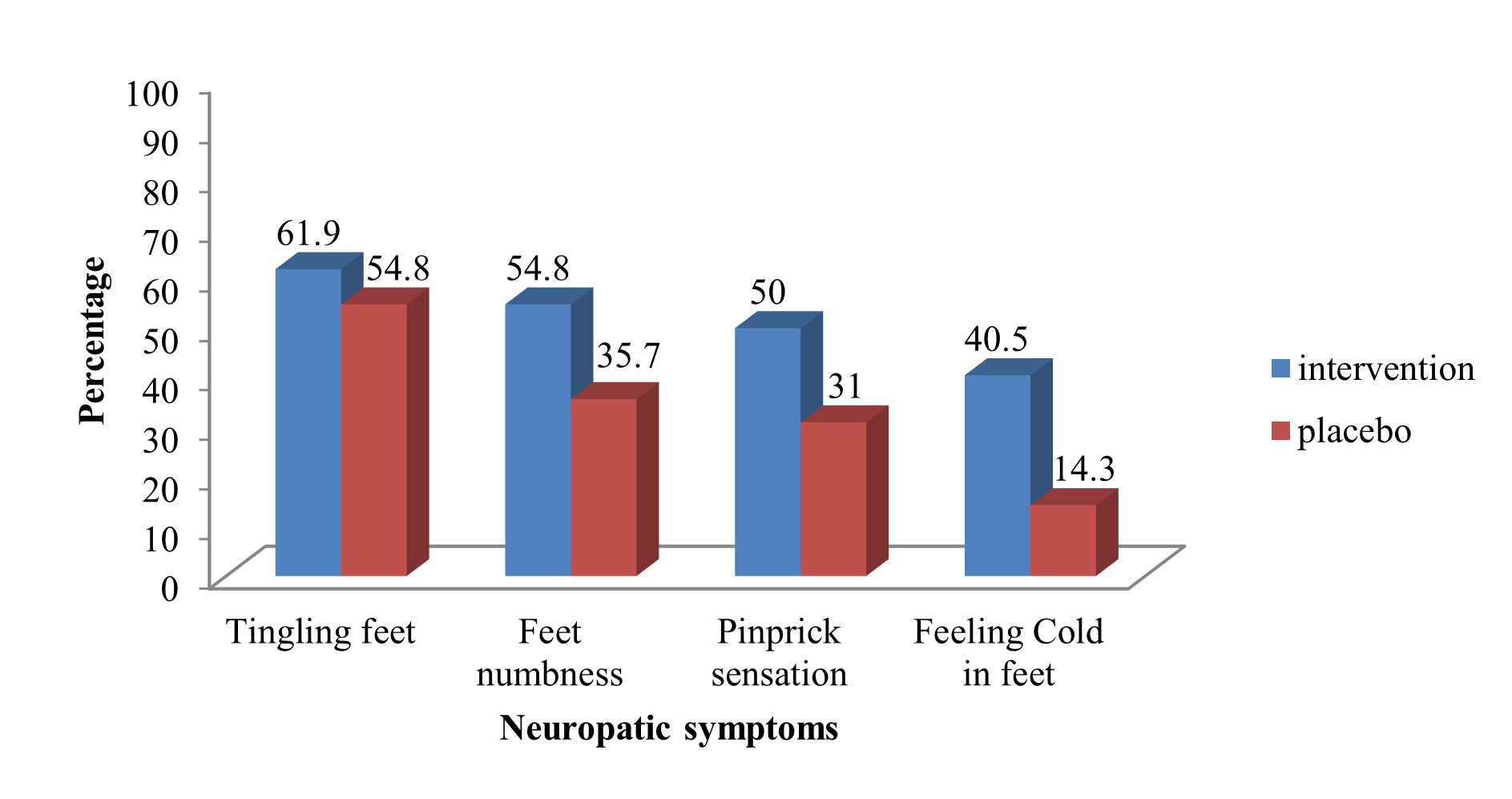
The monofilament test result was similar between the intervention and control groups before the administration of NeuroHelp. However, improvement in the results of the monofilament test and other neurological symptoms (tingling feet, feet numbness, pinprick sensation, cold feet, and pain) was reported after three weeks in the intervention group (p <0.01). There was no significant difference in the tuning fork test results before and three weeks after the administration of NeuroHelp in the intervention and placebo groups (p = 0.546). Patients reported higher satisfaction with treatment with NeuroHelp compared to the group that received the placebo, 36 (85.7%) vs. 8 (19%) (Table 3). Distribution of severe neuropathy symptoms (tingling feet, feet numbness, pinprick sensation, and cold feet) in diabetes patients at baseline and after three weeks is demonstrated in Figures 3 and 4.
|
Characteristics |
Intervention group (NeuroHelp+ current treatment) (n=42) |
Placebo group |
P value |
|
Tingling feet No (%) No Mild-moderate Severe |
16 (38.10) 26 (61.90) 0 |
5 (11.90) 17 (40.50) 20 (47.60) |
0.001 |
|
Feet numbness No (%) No Mild-moderate Severe |
22 (52.40) 18 (42.90) 2 (4.80) |
8 (19) 21(50) 13 (31) |
0.001 |
|
Pinprick sensation No. (%) No Mild-moderate Severe |
26 (61.90) 14 (33.30) 2 (4.8) |
11(26.20) 18 (42.90) 13 (31) |
0.001 |
|
Cold feet No. (%) No Mild-moderate Severe |
21 (50) 21 (50) 0 |
22 (52.40) 15 (35.70) 5 (11.90) |
0.049 |
|
Neuropathic pain VAS score in mm mean (SD) |
24.0 (22.9) |
44.3 (33.1) |
0.002 |
|
Monofilament test Normal Abnormal In one foot In both feet |
27 (73)
8 (21.60) 2 (5.40) |
12 (31.60)
9 (23.70) 17 (44.70) |
0.001 |
|
Tuning fork Test Normal Abnormal In one foot In both feet |
34 (91.90)
1 (2.70) 2 (5.40) |
37 (92.50)
0 3 (7.50) |
0.546 |
|
Patients' satisfaction (%) |
36 (85.7) |
8 (19.00) |
0.001 |
|
X2-test was used to compare neuropathic symptoms in two groups. Independent t-test was used to compare neuropathic pain in two groups. |
|||
Figure 3. Distribution of severe neuropathy symptoms in diabetes patients at baseline.
Figure 4: Distribution of severe neuropathy symptoms in diabetes patients after 3 weeks.
The effect of NeuroHelp on feet numbness, three weeks post-intervention, adjusted for baseline feet numbness, was statistically significant (β=1.15, 95% CI=0.304–1.997, p=0.008) based on ordinary logistic regression analysis.
The effect of NeuroHelp on feeling cold in the feet, three weeks post-intervention, adjusted for baseline feeling cold in the feet, was statistically significant (β=2.20, 95% CI=1.11–3.30, p=0.001) based on ordinary logistic regression analysis.
Discussion and Conclusions
The main aim of this study was to evaluate the effect of the herbal medicine “NeuroHelp” on DPN symptoms. To our knowledge, this is the first study that has investigated the combined effects of several herbal medicines.
The potential use and the mechanism of our topical herbal medicine compounds are as follows: Citrullus colocynthis extract has many therapeutic properties and is one of the important herbal medicine treatments in diabetes; its effective substance is colocynthin. There are many effective substances in Matricaria chamomilla and it has many therapeutic effects; the sedative effect was the most important effect in our study. Anti-inflammatory and pain-relieving effects of Althaea officinalis were a priority for us in our study. Rhynoticin, the bitter compound found in Rheum officinale, serves as the primary active substance, and its analgesic properties were of particular significance to us. Cappari spinosa has many properties that have been noted since ancient times and is widely used in Italian cuisine; it is especially useful in improving liver function. In our study, its analgesic, healing and emollient properties were important. Trigouella foenum graecum was used in ancient Egypt for its therapeutic properties and has been shown to reduce blood glucose. In addition it helps with healing wounds, reducing inflammation, and skin inflammation, which were important factors for us in this study [16].
Our findings showed that after three weeks, all neuropathy symptoms in diabetes patients were improved except for the results of tuning fork test. Among 42 patients in the intervention group, 12 patients had changes and weakness in response to diapason, of which nine improved at the end of three weeks and three did not recover. Among the 42 patients in the placebo group, seven patients had tuning fork test abnormalities, and four showed improvement by the end of three weeks.
DPN is a chronic, debilitating pathological condition that is characterized by nerve damage. Herbal remedies have the potential to reduce disabilities associated with DPN. Previous studies have shown that the administration of herbal drugs, whether plant extracts containing active herbal substances or herbal compounds isolated in safe concentrations, had a promising positive effect in the treatment of diabetic neuropathy. They primarily have antioxidant, anti-inflammatory, analgesic, and neuro protective effects [21-23].
The results of the Heydari study confirmed the therapeutic effects of bitter apple (Citrullus colocynthis), which is one of the ingredients of NeuroHelp, on reducing pain in patients with DPN. Based on Heydari’s study, C. colocynthis fruit extract decreased pain in patients with DPN [5]. Talaaie et al. aimed to determine sensory neuropathy in patients with diabetes using monofilament test [24], and reported that 15.6% of patients with diabetes had neuropathy of which 27.8% of cases were severe, 41.8% moderate, 12.8% mild. 17.6% had no neuropathy. Their study suggests that all patients with diabetes should be tested for diabetic foot ulcers. A monofilament test should be performed for diabetic neuropathy patients regardless of symptoms of neuropathy [24].
Rostami et al. reported that topical Citrullus colocynthis (bitter apple) extract oil did not improve chemotherapy induced peripheral neuropathy (CIPN) symptoms including tingling, burning, numbness, sensitivity to temperature, muscle weakness, or pain in the hands and feet compared with placebo. One of the reasons that the effectiveness of the topical Citrullus colocynthis was not reported in this study was the small sample size [25].
The dissemination of adverse findings from studies concerning herbal medicines can be used to guide the improvement of the efficacy, safety and quality of these remedies with the ultimate goal that their effectiveness can achieve comparable rates to pharmaceutical drugs [26-28].
In 2016, a meta-analysis of 16 clinical trials by Pang et al. showed that Huangqi Guizhi Wuwu Decoction (HGWWD), an old Chinese prescription that includes Radix astragali seu Hedysari, Cinnamomi ramulus, Radix Paeoniae Alba, Rhizoma Zingiberis Recens and Fructus Jujubae, may have a significant therapeutic effect in the treatment of diabetic peripheral neuropathy [29]. However, methodologically, the quality of their randomized controlled trials was generally low, and larger randomized clinical trials (RCTs) should be conducted for more reliable results. Nonetheless, their findings suggest that herbal remedies may be appropriate supplements to improve diabetic neuropathy.
Prior to this study, the authors conducted an experimental evaluation to assess the impact of drug combinations on ameliorating diabetic neuropathic complications. This involved a cohort of 15 patients who received treatment with the NeuroHelp herbal medicine. The evaluation utilized nerve conduction velocity (NCV) and electrodiagnostic testing. After a three-week duration, no significant changes were observed in the electrodiagnostic results. These findings may be attributable to operator-dependent factors during testing, variability in probe placement, or the short term follow-up period. Additionally, the results suggested that while electrodiagnostic tests can be useful, they may not always detect subtle improvements, highlighting the importance of a thorough physical examination. This is consistent with the findings of Boostani et al., who highlighted the limitations of relying solely on electrodiagnostic testing and stressed the importance of the patient’s medical history and a through neurological examination in diagnosing diabetic neuropathy. The authors emphasized that physicians should prioritize clinical examination over costly electrophysiological investigations for diagnosing diabetic neuropathy [30]. In a 2014 study, Baraz et al. used EMG-NCV as the gold standard to evaluate the sensitivity and specificity of the monofilament test in comparison. Their findings demonstrated that the monofilament test results were comparable to those of EMG-NCV, while being considerably easier to perform [31].
NeuroHelp herbal medicine may have systemic effects, such as lowering blood sugar, which warrants follow up confirmatory studies. In addition, NeuroHelp encouraged patients to be more attentive to their daily foot care regimen. The requirement for daily foot care when using this medication, effectively helped mitigate the risk of diabetic foot-related complications [16]. The key advantages of topical drug like NeuroHelp compared to oral drugs, are the rapid onset of action and fewer systemic side effects.
Study results lead us to the conclude that adding NeuroHelp herbal medicine to the standard treatment regimen of diabetic neuropathy (gabapentin and B vitamins) is effective in managing diabetic peripheral neuropathy. Additionally, it has demonstrated benefits in alleviating neuropathic pain, tingling sensations in the feet, foot numbness, and pinprick sensations among diabetic patients.
Our study presents an advantage in utilizing monofilament testing as the primary method for neuropathy assessment, as opposed to relying on the tuning fork test, since pre and post NeuroHelp tuning fork test results did not demonstrate any significant changes. The established efficacy of the monofilament test in evaluating neuropathy among diabetic individuals has been corroborated in prior research [32].
Despite this, our study has some limitations. One is the short follow-up period in our study. To comprehensively assess the effects of Citrullus colocynthis on patients' blood glucose levels, particularly when applied topically, a three-month follow-up would have been necessary to monitor its impact on HbA1C. Achieving this would have required a more extensive and detailed study design with a larger budget allocation. Furthermore, for a thorough evaluation of the drug's efficacy and to conduct EMG-NCV testing, a minimum of a three-month follow-up period would have been essential to confirm safety and efficacy of the treatment. Regrettably, our study did not extend long enough to evaluate potential side effects associated with prolonged use over a three-month period.
This study has limited power due to the small sample size. Future studies could build upon our findings by including larger sample size in each group and extending the follow-up period to three to six months after herbal drug administration, while also monitoring potential effects on blood glucose and lipid profile indices.
Declaration
Ethics approval and consent to participate
This study was conducted in accordance with the with the Declaration of Helsinki and HIPPA and published by the National Institutes of Health and was approved by the ethics committee of Golestan University of Medical Sciences with approval No. IR.GOUMS.REC.1397.001. The study was also registered on the Iranian Registry of Clinical Trials website (http://www.IRCT.ir, ID: IRCT20170422033566N2).
Availability of data and materials
The data used and analyzed during the current study are included in this published article.
Conflicts of Interests
The authors declare that they have no conflict of interests.
Funding statement
Not applicable. The study was personally funded by the principal investigator.
Authors’ contributions
ONN, MM, FS, MZ, SV, and SG designed the experiments, performed the experiments, contributed to the data collection and analysis, wrote the manuscript, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Dr. Reza Ghaffarzadegan, Mr. Masoud Mohebbi, Dr. Gholamreza Roshandel, PhD (epidemiologist) and the laboratory of Faraz Nika Teb Kavous Company for manufacturing NeuroHelp herbal medicine and the placebo, as well as the staff of Gorgan Diabetes Clinic in Dezyani Polyclinic.
References
2. Garrod K, McCabe S, Lee D. Prevention of ulceration and amputation, by neurolysis of peripheral nerves in diabetics with neuropathy and nerve compression: decision-tree utility analysis. J Diabetes Metab. 2014;5(1):5.
3. Kiani J, Nasrollahi SA, Esna-Ashari F, Fallah P, Sajedi F. Amitriptyline 2% cream vs. capsaicin 0.75% cream in the treatment of painful diabetic neuropathy (Double blind, randomized clinical trial of efficacy and safety). Iranian Journal of Pharmaceutical Research: IJPR. 2015;14(4):1263.
4. Abu-Shennar JA, Bayraktar N, Bebis H. Prevalence and Characteristics of Painful Diabetic Peripheral Neuropathy in Adult Patients with Type 2 Diabetes Mellitus, And Determine the Effects of This Pain On Their Quality of Life. J Hormonal Disord and Endocr Res. 2021;1:103.
5. Heydari M, Homayouni K, Hashempur MH, Shams M. Topical C itrullus colocynthis (bitter apple) extract oil in painful diabetic neuropathy: A double‐blind randomized placebo‐controlled clinical trial: Journal of Diabetes. 2016;8(2):246-52.
6. Dyck PJ, Kratz K, Karnes J, Litchy WJ, Klein R, Pach J, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population‐based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817.
7. Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Medicine. 2008;9(6):660-74.
8. Ye D, Fairchild TJ, Vo L, Drummond PD. Painful diabetic peripheral neuropathy: Role of oxidative stress and central sensitisation. Diabetic Medicine. 2022;39(1):e14729.
9. Román-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. Journal of Diabetes Research. 2016;2016(1):3425617.
10. Boyd A, Casselini C, Vinik E, Vinik A. Quality of life and objective measures of diabetic neuropathy in a prospective placebo-controlled trial of ruboxistaurin and topiramate. Journal of Diabetes Science and Technology. 2011;5(3):714-22.
11. Mehra P, Sharma B, Baig H, Raveendar C, Prasad R, Rao MP, et al. Efficacy of homoeopathic treatment for diabetic distal symmetric polyneuropathy: A multicentric randomised double-blind placebo-controlled clinical trial. Explore. 2021;17(5):417-23.
12. Wong M-c, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335(7610):87.
13. Miralles-García JM, de Pablos-Velasco P, Cabrerizo L, Pérez M, López-Gómez V, Nutrición SEdEy. Prevalence of distal diabetic polyneuropathy using quantitative sensory methods in a population with diabetes of more than 10 years’ disease duration. Endocrinología y Nutrición. 2010;57(9):414-20.
14. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double‐blind, crossover, placebo-controlled trial. Pain Practice. 2013;13(6):497-503.
15. Jorge LL, Feres CC, Teles VE. Topical preparations for pain relief: efficacy and patient adherence. Journal of Pain Research. 2010:11-24.
16. Aghili Khorasani MH. Makhzan Al-Advieh. 2, editor. Tehran: Tehran University; 1913 1390.
17. Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical amitriptyline and ketamine in neuropathic pain syndromes: an open-label study. The Journal of Pain. 2005;6(10):644-9.
18. Uzaraga I, Gerbis B, Holwerda E, Gillis D, Wai E. Topical amitriptyline, ketamine, and lidocaine in neuropathic pain caused by radiation skin reaction: a pilot study. Supportive Care in Cancer. 2012;20:1515-24.
19. Lynch ME, Clark AJ, Sawynok J, Sullivan MJ. Topical 2% amitriptyline and 1% ketamine in neuropathic pain syndromes: a randomized, double-blind, placebo-controlled trial. The Journal of the American Society of Anesthesiologists. 2005;103(1):140-6.
20. Streiner DL, Norman GR, Cairney J. Health measurement scales: a practical guide to their development and use: Oxford University Press; 2024.
21. Kale MB, Bajaj K, Umare M, Wankhede NL, Taksande BG, Umekar MJ, et al. Exercise and nutraceuticals: eminent approach for diabetic neuropathy. Current Molecular Pharmacology. 2022;15(1):108-28.
22. Arora K, Tomar PC, Mohan V. Diabetic neuropathy: an insight on the transition from synthetic drugs to herbal therapies. Journal of Diabetes & Metabolic Disorders. 2021;20(2):1773-84.
23. Shi Y, Liu L, Sun X, Jiao J. Efficacy and safety of acupuncture combined Chinese herbal medicine for diabetic peripheral neuropathy: A protocol for systematic review and meta-analysis. Medicine. 2021;100(50):e28086.
24. Talaee R, Doroudgar A, Muosavi SG, Abdevali N. Detection of sensory neuropathy in diabetic patients using 5.07/10g monofilament. Journal of Dermatology and Cosmetic. 2011;2(3):158-65.
25. Rostami N, Mosavat SH, Heydarirad G, Arbab Tafti R, Heydari M. Efficacy of topical Citrullus colocynthis (bitter apple) extract oil in chemotherapy‐induced peripheral neuropathy: A pilot double‐blind randomized placebo‐controlled clinical trial. Phytotherapy Research. 2019;33(10):2685-91.
26. Andrew R, Izzo AA. Principles of pharmacological research of nutraceuticals. British Journal of Pharmacology. 2017;174(11):1177.
27. Hunter PM, Hegele RA. Functional foods and dietary supplements for the management of dyslipidaemia. Nature Reviews Endocrinology. 2017;13(5):278-88.
28. Minuz P, Velo G, Violi F, Ferro A. Are nutraceuticals the modern panacea? From myth to science. British Journal of Clinical Pharmacology. 2017;83(1):5.
29. Pang B, Zhao T-y, Zhao L-h, Wan F, Ye R, Zhou Q, et al. Huangqi Guizhi Wuwu Decoction for treating diabetic peripheral neuropathy: a meta-analysis of 16 randomized controlled trials. Neural Regeneration Research. 2016;11(8):1347-58.
30. Boostani R, Afkhamizade M, Shahri B, Ahmadi S. Evaluation of the frequency of polyneuropathy based upon the clinical and paraclinical findings in diabetic patients. Medical Journal of Mashhad University of Medical Sciences. 2012;55(2):81-7.
31. Baraz S, Zarea K, Shahbazian HB, Latifi SM. Comparison of the accuracy of monofilament testing at various points of feet in peripheral diabetic neuropathy screening. Journal of Diabetes & Metabolic Disorders. 2014;13:1-7.
32. Forouzandeh F, Aziz Ahari A, Abolhasani F, Larijani B. evaluation of foot neurovascular status in diabetic patients referred to diabetes clinic of dr. shariati hospital, 2003-2004. Iranian Journal of Diabetes and Lipid Disorders. 2005;4(4):43-51.