Abstract
Background and aims: To determine the value of using Three-Dimensional Ultrasound with Power Doppler Angiography for the diagnosis and management of Ectopic Pregnancy.
Methods: Three-dimensional ultrasound was used at the time of presentation to the Emergency Department of a facility for patients who were ultimately diagnosed with having a tubal Ectopic Pregnancy. This prospective observational investigation compared the results of offline analysis with outcomes after treatment (e.g. tubal rupture), suggesting its potential for predicting that outcome.
Results: Of the 64 consecutive confirmed cases of Ectopic Pregnancy presenting to the Emergency Department of a single hospital, 39 patients were intact and 26 of them were medically treated with methotrexate. There were 9 subsequent failures of this treatment, resulting in 8 ruptures and one case with a continued rise of human Chorionic Gonadotropin. The Power Doppler Angiography quantification of the Vascularity Index correlated with those failed outcomes. STARD standards were applied to this investigation.
Conclusions: Use of Three-Dimensional Ultrasound with Power Doppler Angiography with measurement of the Vascularity Index for patients presenting with an Ectopic Pregnancy may have value for determining which patients are best treated with surgical intervention rather than the use of medical treatment.
Keywords
Ectopic Pregnancy, Methotrexate ectopic, Ultrasound, Doppler, Human chorionic gonadotropin
Introduction
Since the medical management of ectopic pregnancy (EP) was introduced by Dr. Steven Ory, and published in 1986 in the American Journal of Obstetrics and Gynecology [1], diagnostic criteria have been established to predict its successful medical treatment with methotrexate (MTX), including its maximum diameter (MaxDia), its associated human chorionic gonadotropin (hCG) level, and whether there was identified cardiac motion (CM). With 3D transvaginal sonography (3DTVS), there is yet another possible feature warranting its inclusion in the diagnostic criteria that are currently used when embarking on the medical treatment with methotrexate of a known ectopically implanted tubal pregnancy. Specifically, it is the Vascularity Index (VI) which can indicate the associated vasculature at the ectopic implantation site, which can be considered as this new diagnostic criterion. Since tubal rupture is a finite possibility of a tubal ectopic pregnancy that is medically treated, there may be significant consequences should rupture occur, including mortality [2]. The authors have used this case series to determine if the VI, which was detected with the use of 3DTVS and Power Doppler Angiography (PDA), can possibly predict the severe morbidity of a ruptured ectopic pregnancy after EP is diagnosed and medically treated.
Materials and Methods
Table 1 reveals the patient flow of this prospective observational investigation, which was performed in collaboration with the Emergency, Radiology and Obstetrics/ Gynecology departments. The study was approved by the Advocate IRB (#6422), with the appropriate patient consents obtained for this investigation. It was presented as a poster at the Annual Meeting of the Central Association of Obstetricians and Gynecologists (CAOG) on October 17-20, 2018 in Minneapolis, Minnesota.
Table 1.Table 1. Patient flow.
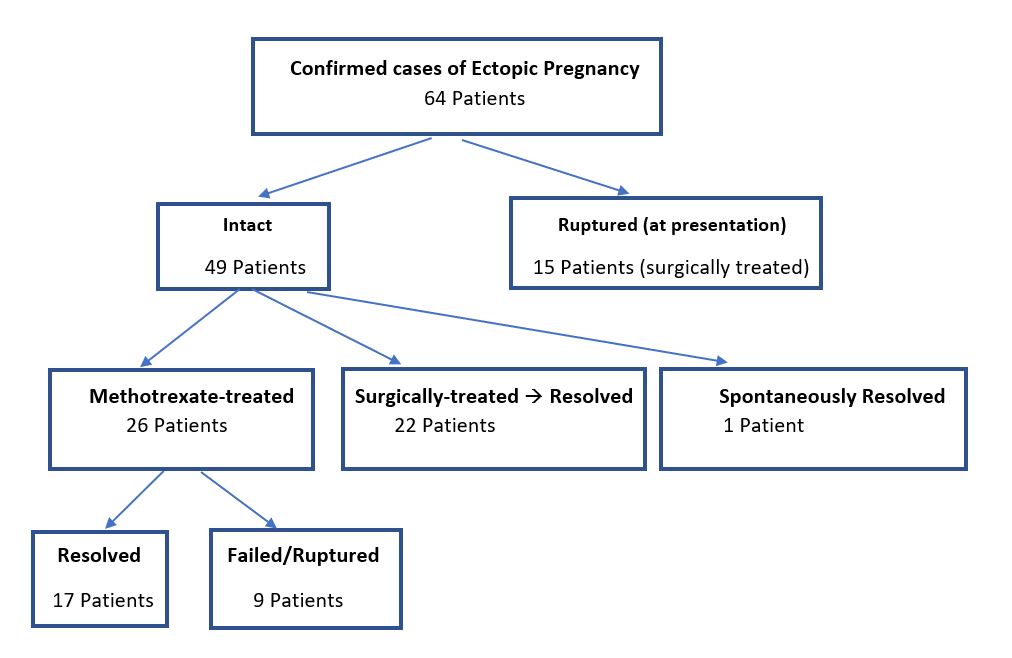
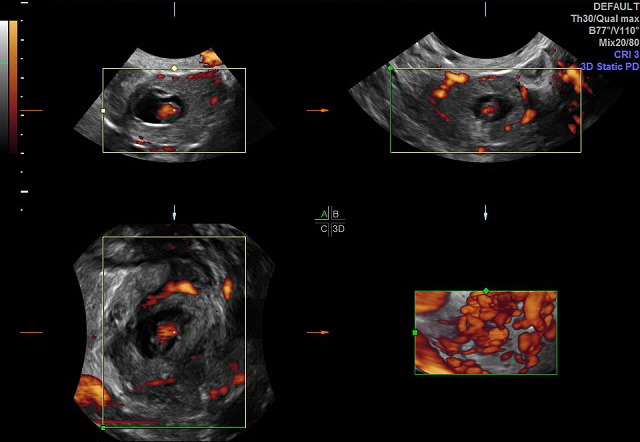
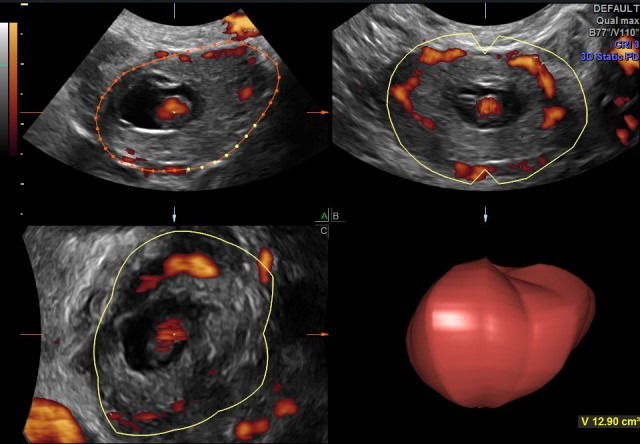
All patients consecutively presenting to the Emergency Department (ED) of Advocate Illinois Masonic Medical Center who were diagnosed with an EP (each of which with tubal implantation), from May 1, 2016 through December 31, 2019. Each patient who presented with bleeding and adnexal pain was considered for this diagnosis of EP, for whom ultrasound was always performed to confirm the diagnosis. Each patient underwent 3DTVS, using a Logiq E9 (GE Healthcare Ultrasound, Milwaukee, WI, USA) ultrasound machine, equipped with a multifrequency (5-12 MHz) endovaginal probe. Initial 2D TVS identified the EP location, MaxDia and the presence or not of CM. Power Doppler Angiography (PDA) was used when acquiring the 3D volume for each EP. The 3DTVS/PDA was obtained for each patient in the Department of Radiology, according to the predetermined protocol scan settings of pulse repetition frequency (PRF) = 1.4, color gain = 18, color frequency = 42, and power threshold = 80%. The EP volumes were stored for subsequent offline analysis by the author CMF, using the GE 4D View program (Version 10.5 GE Healthcare, Austria), with Virtual Organ Computer-Aided analysis (VOCAL) in manual mode, to ascertain the associated volume and VI for each EP (see Figures 1-3).
Figure 1. Ectopic pregnancy three-dimensional ultrasound volume acquisition with Power Doppler Angiography (PDA).
Figure 2. Example ectopic pregnancy volume using Virtual Organ Computer-aided Analysis (VOCAL).
Figure 3. Histogram used, to obtain the Vascularity Index (VI) of the ectopic pregnancy.
Vascularity Index calculation
The VI calculations were automatically made using this program for the offline analysis. The 180° EP volumes obtained from each scan performed in the ED were incrementally rotated every 15°, manually tracing the resultant 12 EP volume slices, and carefully including the imaged adnexal EP in the acquired volume of each slice. This procedure was followed in order to have the consistent calculation of the VI from the obtained histogram. This VI is the proportion of color-filled voxels, representing the vascular flow using PDA, among the total voxels within that created EP volume of interest, defined by this manual image processing. The calculated VI of each slice of the imaged volume was then averaged for the total slices included for each imaged EP. The 3DTVS/PDA was performed with this described methodology, in order to quantify the clinically impactful vascular flow surrounding each tubal EP. Though other vascular indices are produced from the histogram, such as the Flow Index (FI) and Vascular Flow Index (VFI), the VI appeared to be most clinically important. The amount of time spent on this offline EP analysis was approximately 5 minutes per case, and was performed by a single operator (CMF) in a blinded fashion. The total number of consecutive subjects included in this retrospective investigation was limited by the approved IRB application.
Results
Of the 64 consecutive confirmed cases of EP that presented to the Emergency Department (ED) during the course of this investigation, and who had a diagnostic 3DTVS performed, 49 were intact and 15 were found to be ruptured on presentation. Each of the 26 intact EP patients were administered MTX (50 mg/m2), and the rest of those patients and the 15 ruptured EP patients proceeded to a surgical intervention. Considering the associated diagnostic testing of those ruptured patients, 7 had a MaxDia >4 cm., 3 had an hCG >5,000 mIU, and 1 showed CM. Of the 8 ruptured EPs subsequent to MTX administration, 7 represented cases of at least one prior single-dose MTX treatment in this dataset. One patient received a second MTX dose. An additional case of MTX failure included one which was deemed a failure due to a continued rise of hCG (an additional MTX dose was not administered). Of those 9 cases of MTX failure, 6 were previously found to have initially had a VI of >9.5 (67%). Of all of the 23 ruptures, 6 had an initial elevated VI, but without any severe markers (e.g. hCG >5,000, or MaxDia >4 cm, or presence of CM). When an elevated VI was found (i.e. > 9.5), 9 of the 26 cases were subsequently found to fail after MTX treatment (35%). Table 2 reveals these details which are described here regarding the biomarkers collected in this investigation.
In comparison with the other established biomarkers used for the diagnosis of EP at presentation (i.e. MaxDia, hCG and CM), VI was more predictive of EP follow-up status. For example, of the 15 ruptured EPs at presentation, 7 (47%) had a MaxDia over 4 cm, though 8 had a VI >9.5 (53%). Also, of those 15 ruptured EPs, only 3 had an hCG >5,000 (20%), yet 8 had a VI >9.5 (53%), and only 1 had positive CM (7%). The prediction of EP outcome after MTX treatment with VI measurement is stated above. The sensitivity of VI for the prediction of rupture occurring after administration of MTX was determined to be 67%.
The MaxDia of the tubal segment containing the EP was compared with its acquired EP volume, as measured sonographically with 3DTVS, to see if the volume was found to have a distinct advantage over the linear MaxDia. However, no advantage was found, even when looking at the few examples of discordant measures (e.g. MaxDia of 5 cm and a volume of 35.1 cm2). The acquired volume of the ectopically implanted gestation did not perform any better than the linear measurement of MaxDia, in terms of predicting outcome of the EP.
|
Case# |
Maximum Diameter (cm) |
Human Chorionic Gondotropin (mIU) |
Cardiac Motion (Y/N) |
Vascularity Index (ratio) |
Treatment |
Outcome |
Elevated Biomarker |
|
1 |
1.76 |
442 |
N |
2.5 |
MTX |
|
|
|
2 |
3.8 |
58 |
N |
3.9 |
MTX |
|
|
|
3 |
2.9 |
471 |
N |
6.8 |
Surgery |
|
|
|
4 |
1.9 |
1,500 |
N |
1.2 |
MTX |
|
|
|
5 |
3.8 |
13,296 |
Y |
13.6 |
Surgery |
|
√ |
|
6 |
1.1 |
3,146 |
N |
27.1 |
MTX |
Subsequent MTX failure (Rupture) |
√ |
|
7 |
2.5 |
281 |
N |
5.6 |
MTX |
|
|
|
8 |
3.7 |
5,664 |
N |
10.7 |
Surgery |
Ruptured |
√ |
|
9 |
2.6 |
1,371 |
N |
2.3 |
MTX |
|
|
|
10 |
3 |
1,418 |
N |
6 |
Surgery |
|
|
|
11 |
2.9 |
8 |
N |
1.9 |
No Rx |
|
|
|
12 |
2.2 |
170 |
N |
4.7 |
MTX |
|
|
|
13 |
1.5 |
581 |
N |
3.3 |
MTX |
|
|
|
14 |
3.2 |
158 |
N |
5 |
MTX |
|
|
|
15 |
5 |
2,610 |
N |
2.8 |
MTX |
Subsequent MTX failure (Rupture) |
√ |
|
16 |
2.9 |
910 |
N |
2.5 |
MTX |
|
|
|
17 |
3.1 |
286 |
N |
5.6 |
MTX |
|
|
|
18 |
6.3 |
3,289 |
N |
4.1 |
MTX |
Subsequent MTX failure (Rupture) |
√ |
|
19 |
4.3 |
865 |
N |
1.2 |
Surgery |
|
√ |
|
20 |
1.4 |
196 |
N |
6.2 |
Surgery |
|
|
|
21 |
2.9 |
6,270 |
N |
12.5 |
Surgery |
|
√ |
|
22 |
2.4 |
306 |
N |
10.6 |
Surgery |
|
√ |
|
23 |
2.5 |
470 |
N |
8 |
Surgery |
|
|
|
24 |
2.7 |
627 |
N |
2.9 |
MTX |
|
|
|
25 |
4.8 |
75 |
N |
10.7 |
Surgery |
|
√ |
|
26 |
8.1 |
2,097 |
N |
0.8 |
Surgery |
Ruptured |
√ |
|
27 |
2.2 |
5,767 |
N |
33.2 |
Surgery |
|
√ |
|
28 |
4.5 |
1,500 |
N |
17.1 |
Surgery |
Ruptured |
√ |
|
29 |
3.3 |
363 |
Y |
4.6 |
MTX |
|
√ |
|
30 |
1.6 |
5,522 |
N |
46.4 |
MTX |
MTX Failure (rising hCG) |
√ |
|
31 |
2.5 |
400 |
N |
4.2 |
MTX |
|
|
|
32 |
5.3 |
30,942 |
N |
5.5 |
Surgery |
|
√ |
|
33 |
2.2 |
38,000 |
N |
3.9 |
Surgery |
|
√ |
|
34 |
3.7 |
5,664 |
y |
11.8 |
Surgery |
Ruptured |
√ |
|
35 |
2.5 |
9,469 |
Y |
23.4 |
Surgery |
|
√ |
|
36 |
1.8 |
460 |
N |
1.5 |
MTX |
Subsequent MTX failure (Rupture) |
|
|
37 |
2.1 |
5,392 |
N |
22.7 |
MTX |
Subsequent MTX failure (Rupture) |
√ |
|
38 |
3.2 |
15,891 |
N |
3.9 |
Surgery |
|
√ |
|
39 |
4.4 |
11,265 |
Y |
9 |
Surgery |
|
√ |
|
40 |
6.1 |
205 |
N |
5 |
Surgery |
|
√ |
|
41 |
6.9 |
492 |
N |
3.6 |
Surgery |
|
√ |
|
42 |
2.4 |
76 |
N |
3.4 |
MTX |
|
|
|
43 |
3.7 |
38,000 |
N |
10.8 |
Surgery |
|
√ |
|
44 |
2.6 |
2,066 |
N |
15.3 |
Surgery |
Ruptured |
√ |
|
45 |
3.2 |
31,477 |
N |
13.6 |
Surgery |
|
√ |
|
46 |
1.8 |
475 |
N |
10.6 |
MTX |
|
√ |
|
47 |
2.1 |
702 |
N |
12.4 |
Surgery |
|
√ |
|
48 |
4.5 |
897 |
N |
4.6 |
Surgery |
Ruptured |
√ |
|
49 |
3.6 |
1,461 |
N |
11.2 |
Surgery |
Ruptured |
√ |
|
50 |
3.2 |
3,458 |
N |
4.5 |
MTX |
|
|
|
51 |
6.9 |
65 |
N |
1.5 |
Surgery |
Ruptured |
√ |
|
52 |
3 |
654 |
N |
3.9 |
Surgery |
Ruptured |
|
|
53 |
2.5 |
1,709 |
N |
12.5 |
MTX |
Subsequent MTX failure (Rupture) |
√ |
|
54 |
9 |
948 |
N |
5.3 |
Surgery |
Ruptured |
√ |
|
55 |
4.8 |
44 |
N |
11.7 |
Surgery |
Ruptured |
√ |
|
56 |
3.6 |
32,705 |
N |
12.4 |
Surgery |
Ruptured |
√ |
|
57 |
3.3 |
825 |
N |
21.9 |
MTX |
Subsequent MTX failure (Rupture) |
√ |
|
58 |
1.8 |
425 |
N |
9.7 |
Surgery |
Ruptured |
√ |
|
59 |
2.8 |
799 |
N |
6.1 |
MTX |
|
|
|
60 |
2.3 |
2,515 |
N |
9.7 |
MTX |
Subsequent MTX failure (Rupture) |
√ |
|
61 |
4.3 |
1,615 |
Y |
13.9 |
Surgery |
|
√ |
|
62 |
2.7 |
18,033 |
N |
21.2 |
Surgery |
|
√ |
|
63 |
3.9 |
853 |
N |
4.8 |
Surgery |
Ruptured |
|
|
64 |
5.7 |
3,200 |
N |
10.3 |
Surgery |
Ruptured |
√ |
|
Biomarkers √ if exceeded risk levels: Maximum Diameter > 4 cm Human chorionic gonadotropin > 5,000 mIU Cardiac Motion present Vascularity Index > 9.5 |
|||||||
Discussion
A finite risk of tubal rupture exists with ectopic pregnancy even after medical treatment with methotrexate, and mortality and morbidity can thus occur. The most recent statistics in the U.S. have been estimated to be about 20 deaths per year resulting from EP [3]. If such a result can be predicted and thereby avoided, the clinical value of VI may be important. The authors describe a few cases of tubal rupture that occurred after initiation of medical management of EP with MTX, in the presence of a described vasculature surrounding the tube, and identified with 3D ultrasound with PDA, and quantified with a VI. Such increased vascularity (i.e. VI >9.5) was seen in 41% of our cases of EP, and in 14% of those cases, no other elevated biomarker was identified (e.g. hCG, MaxDia and CM). Others have identified this sonographic feature, and they have applied a grading system to it [4]. This vasculature surrounding the fallopian tube at the ectopic implantation site can be referred to as a “ring of fire” [5].
While the success rate of MTX-treated EP varies, ranging from 72% to over 90% [6-9], depending on a variety of variables, an average of 90% seems to be a reasonable threshold to use (or 10% failure). Our experience revealed an 82% MTX success rate. In contrast to this, a three-fold tubal rupture rate was seen after MTX treatment, if the VI was >9.5 (67% of MTX failure). From the data presented, if VI was routinely identified when diagnosing EP in a generalized setting, and the option of using surgical management were elected when it is >9.5, catastrophic tubal rupture can be possibly avoided. Naturally, the medical treatment of EP has the benefit of its ease of use, decreased cost and patient acceptance. However, every effort should be made to avoid the possible morbidities associated with its negative outcome (e.g. tubal rupture following MTXtreatment), should there be a technology that can be used to predict it. In comparison of the 9 cases of MTX-failure in this dataset, 6 cases had an elevated VI, and only 2 of them had an elevated hCG, and only 2 had a MaxDia > 4 cm. With regard to the prediction of EP MTX-treatment outcome, there has been no consistent demonstrated value of hCG (or serial hCGs) or sonographic size (i.e. MaxDia) to predict this [10-14].
The limitations in this study include the relatively low number of EPs with a 3DTVS analysis, from which no definitive conclusion can be made. The strength relates to the novelty of the diagnostic criterion of VI for EP, and the described relative ease by which it can be accomplished. This study presents a novel diagnostic measure (VI) which may predict the possible failure of the medical treatment of EP, and the possible tubal rupture which can occur. The authors show some examples of the clinical benefit of using 3DTVS/PDA diagnostically for EP, so that the gynecologic community (and their patients) can benefit from this pilot study, hoping that others who have the necessary sonographic skills, will investigate this further in a properly controlled fashion. In this way, it may be possible that some of the morbidities associated with EP can be prevented.
Conclusion
3DTVS offers the ability to detect a clinical measure (i.e., vascularity index), which can help to predict the possible failure of the medical treatment of ectopic pregnancy. This can be important for the prevention of the finite risk of medical failure, and the associated risk of intraabdominal hemorrhage resulting from the rupture of an ectopic pregnancy. This capability of sonographic diagnosis is widely available and should be recognized as a valuable tool. The demonstration of its utility in this case series can be clinically useful, though it needs to be verified by others in different practice settings before becoming routinely used for this purpose.
Conflict of Interest
The authors have no conflicts of interest, and there has been no funding for this investigation.
Acknowledgements
Bernardo Bello. RDMS, Mary Stopper, RDMS, lead sonographers, who managed the performance of the sonograms and data collection.
Abbreviations
EP: Ectopic Pregnancy; MTX: Methotrexate; MaxDia: Maximum Diameter; 3DTVS: Three-Dimensional Transvaginal Sonography; CM: Cardiac Motion; hCG: Human Chorionic Gonadotropin; VI: Vascularity Index; PDA: Power Doppler Angiography; IRB: Institutional Review Board; CAOG: Central Association of Obstetrician Gynecologists; ED: Emergency Department; VOCAL: Virtual Organ Computer-aided Analysis.
References
2. Creanga AA, Shapiro-Mendoza CK, Bish CL, Zane S, Berg CJ, Callaghan WM. Trends in ectopic pregnancy mortality in the United States: 1980–2007. Obstetrics & Gynecology. 2011 Apr 1;117(4):837-43.
3. Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011–2013. Obstetrics &Gynecology.2017 Aug;130(2):366-73.
4. Aydoğmuş S, Aydoğmuş H, Gençdal S, Kelekçi S. Density of tubal ring vascularization: A new marker for prediction of success of medical treatment in tubal ectopic pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2017 Oct 1; 217:113-8.
5. Coulier B, Malbecq S, Brinon PE, Ramboux A. MDCT diagnosis of ruptured tubal pregnancy with massive hemoperitoneum. Emergency Radiology. 2008 May;15(3):179-82.
6. Levin G, Dior UP, Shushan A, Gilad R, Benshushan A, Rottenstreich A. Success rate of methotrexate treatment for recurrent vs. primary ectopic pregnancy: a case-control study. Journal of Obstetrics and Gynaecology. 2020 May 18;40(4):507-11.
7. Sendy F, AlShehri E, AlAjmi A, Bamanie E, Appani S, Shams T. Failure rate of single dose methotrexate in managment of ectopic pregnancy. Obstetrics and Gynecology International. 2015 Mar 12;2015:902426.
8. Cohen A, Zakar L, Gil Y, Amer-Alshiek J, Bibi G, Almog B, Levin I. Methotrexate success rates in progressing ectopic pregnancies: a reappraisal. American Journal of Obstetrics and Gynecology. 2014 Aug 1;211(2):128-e1-5.
9. Lipscomb GH. Medical management of ectopic pregnancy. Clinical Obstetrics And Gynecology. 2012 Jun 1;55(2):424-32.
10. Kim J, Jung YM, Lee DY, Jee BC. Pretreatment serum human chorionic gonadotropin cutoff value for medical treatment success with single-dose and multi-dose regimen of methotrexate in tubal ectopic pregnancy. Obstet Gynecol Sci 2017;60(1):79-86.
11. Lee JH, Kim S, Lee I, Yun J, Yun BH, Choi YS, et al. A risk prediction model for medical treatment failure in tubal pregnancy. European Journal Of Obstetrics & Gynecology And Reproductive Biology. 2018 Jun 1;225:148-54.
12. Karadeniz RS, Tasci Y, Altay M, Akkus M, Akkurt O, Gelisen O. Tubal rupture in ectopic pregnancy: is it predictable? Minerva Ginecol 2015;67(1):13-9.
13. Nadim B, Lu C, Infante F, Reid S, Condous G. Relationship between ultrasonographic and biochemical markers of tubal ectopic pregnancy and success of subsequent management. J Ultrasound Med 2018;37(12):2899-2907.
14. Bonin L, Pedreiro C, Moret S, Chene G, Gaucherand P, Lamblin G. Predictive factors for the methotrexate treatment outcome in ectopic pregnancy: a comparative study of 400 cases. Eur J Obstet Gynecol Reprod Biol 2017;208:23-30.