Abstract
Molnupiravir (MOL) is an orally absorbed prodrug of the ribonucleoside analogue N-hydroxycytidine, which has in vitro activity against several coronaviruses, including SARS-CoV-1 and 2. It remains to be seen whether long term MOL has serious side effects. The side effects of MOL, which was the first to be allowed for oral use during the pandemic process, are not yet fully known. In this study, it was aimed to investigate the the mechanisms of possible dose-dependent damage on liver, lung, heart, and kidney tissues.
Fourty male Wistar albino rats were separated into four groups as Control, MOL10, MOL100, MOL1000. For five days, MOL (10-100-1000 mg/kg/day) was administered by oral gavaj. At the end of the five days rats were sacrificed and alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TB) levels in serum were measured. Collected tissues (liver, lung, heart, and kidney) were evaluated to the malondialdehyde (MDA), superoxide dismutase (SOD), total antioxidant state (TAS), and total oxidant state (TOS), and oxidative stress indexes (OSI) were calculated. MOL administration showed significant improvement in liver, heart, and kidney in rats (p<0.05). SOD activities which were decreased in the MOL group, while MDA level increased in the MOL groups compared to the control group in all tissue (p<0.05). ALT was increased in the MOL group compared to the control group (p<0.05). Increase of TOS and OSI is statistically significant, but TAS was decreased in the MOL group compared to the control group (p<0.05). MOL used in virus treatment is dose-dependently effective on the oxidant/antioxidant system in tissues. For this reason, the use of antioxidants may be beneficial to reduce tissue damage that may occur in the use of MOL.
Keywords
Liver, Lung, Liver, Heart, Kidney, Molnupiravir
Highlights
• MOL used in virus treatment is dose-dependently effective on the oxidant/antioxidant system in different tissues.
• It is known that changes in the oxidant/antioxidant system cause structural damage that causes loss of function in tissues.
• The use of antioxidants together with MOL may be beneficial to reduce tissue damage that may occur in the use of MOL.
Introduction
The coronavirus pandemic, called COVID-19, has entered the history page with the appearance of a severe pneumonia that did not respond to treatment and vaccines, which emerged in Wuhan, the capital of China's Hubei region, in December 2019. The virus isolated as the cause of the disease was determined as SARS-CoV2. A global epidemic was declared by the World Health Organization (WHO) in March 2020, with progressive death cases and rapidly developing infection rate. According to WHO data, COVID-19 has caused more than 590,331,311confirmed infections and 6,435,631 deaths worldwide as of 18.08. 2021 [1].
In the COVID-19 pandemic, antiviral drugs containing the active ingredients of ritonavir, lopinavir, favipiravir, and remdesivir have been used. Antiviral drugs; are drugs that help the body fight disease-causing viruses, reduce the symptoms of a viral infection, and shorten the duration of the disease. During the COVID-19 pandemic, antiviral drugs containing the active ingredients ritonavir, lopinavir, favipiravir, and remdesivir were used [2]. Molecules with antiviral effects are administered in two main ways, orally and intravenously. Until recently, remdesivir is the only antiviral effective molecule approved to prevent transmission of viruses and to treat COVID-19, and it is given to patients only by intravenous route. An antiviral drug called Molnupiravir (MOL), which is effective against the flu virus, developed by US scientists at Emory University, has been studied for a long time. Some time after the outbreak emerged, this molecule, whose effectiveness against the flu was in the testing phase, became an important option for the treatment of COVID-19. It was announced that MOL has reduced hospitalizations and deaths approximately 50% in patients by COVID-19 in a global clinical trial. [3]. As a result, MOL was approved by the UK Medicines and Healthcare Products Regulatory Authority in November 2021. It is the first antiviral effective oral drug approved for the treatment of COVID-19 [4].
MOL (C13-H19-N3-O7), a biological prodrug of NHC (β-D-N(4)-hydroxycytidine), is a novel nucleoside analogue with a prophylactic activity against multiple coronaviruses including SARS-CoV, SARS-CoV2, Middle East respiratory syndrome coronavirus (MERS-CoV), influenza virus, respiratory syncytial virus (RSV), bovine viral diarrhea virus (BVDV), hepatitis C virus (HCV) and Ebola virus (EBOV) [5]. Developed for mammals including all monkey species and humans, it is hydrolyzed in vivo with oral bioavailability. Upon oral administration, a prodrug, MOL, is metabolized to its active form, EIDD1931, and converted to the triphosphate (TP) form and is distributed into tissues, becoming the active 5′-triphosphate form. The active drug is incorporated into the genome of RNA viruses and inhibits the action of viral RNA-dependent RNA polymerase. Recent studies have shown that MOL inhibits the replication of human and bat coronaviruses, including SARS-CoV-2, in mice and human respiratory epithelial cells. It has activity against a number of RNA viruses, including SARS-CoV-2, MERS-CoV, and seasonal and pandemic influenza viruses [6].
It currently has a role as prodrug MOL, anticonnaviral agent and antiviral drug in phase-III trials for the treatment of COVID-19 patients. Developed for the treatment of influenza, MOL creates mutations that prevent replication, causing viruses to make mistakes when copying their own RNA. MOL is a prodrug of the ribonucleoside analog N-hydroxycytidine (NHC), which has orally absorbed in vitro activity. Once the NHC is incorporated into the replicating viral genome, it can base pair with cytidine (correct ribonucleotide) or adenine (wrong ribonucleotide), thus causing lethal mutagenesis of the replicating virus [7].
The effectiveness of this unique mechanism of inhibition of viral replication is due to the absence of proofreading by viral RNA-dependent RNA polymerase. Its safety depends on efficient proofreading by host DNA polymerases. MOL has been found to be mutagenic in some, but not all mammalian assays for host genetic mutations [8]. In addition, findings from mutagenesis studies suggest that MOL may cause host DNA damage. This results in termination of RNA transcription and reduces viral RNA production and viral RNA replication. Studies in animal models have suggested that longer-term treatment may result in thrombocytopenia and bone structural changes. MOL is an orally absorbed prodrug that is rapidly metabolized to NHC with little hepatic metabolism. The absence of high rates of side effects and liver damage associated with the use of MOL may be due to the absence of major hepatic metabolism and relatively short treatment duration. It remains to be seen whether long term MOL has serious side effects. Studies in animal models have suggested that longer-term treatment may result in thrombocytopenia and bone structural changes [9].
Oxidative stress is defined as the disproportion between antioxidants and the presence of free radicals and prooxidants in a biological syste [10]. Under normal physiological conditions, the balance between reactive oxygen species (ROS) and antioxidants is maintained at the cellular level. But when the redox balance is disturbed, strong oxidants (free radicals) can have harmful effects; they are often associated with the onset of various lifestyle diseases [11]. These nasty effects are caused by unregulated oxygen and nitrogen containing free radicals that eventually attack different cells and damage DNA, proteins, and lipids. Normally, ROS are involved in cell signaling (redox signaling) pathways, thiol switches, regulation of inflammatory cytokines, growth factors, etc. they play important biological roles [12-14]. Recent studies indicate that oxidative stress plays an important role in viral infections such as SARS-CoV and SARS-CoV2 infections [15,16]. Maintenance of disulfide thiol balance is an important aspect of viral entry, viral reactivity and viral fusion and can be affected by oxidative stress [17]. Various studies have suggested that excessive ROS production and a disproportionate cellular antioxidant-oxidant balance have an important function in the pathogenesis of respiratory viral infections such as SARS-CoV infections [11,15]. Additionally, recent reports have suggested that those with pre existing conditions such as diabetes, hypertension, and pulmonary, cardiac, and kidney diseases are at higher risk of developing a serious infection [18,19].
Currently, MOL is being actively evaluated for efficacy and safety in the treatment of patients not at high risk for complications, for children, and for patients known to be exposed to COVID-19 (post-exposure prophylaxis). MOL appears to be generally well tolerated; mild side effects may include headache, dizziness, gastrointestinal upset, nausea, and diarrhea. MOL is potentially teratogenic and contraindicated in pregnancy. A potential side effect of MOL use is impaired bone and cartilage growth. Therefore, it is not recommended for use in children. Total clinical experience with MOL is limited and its safety has not been fully defined [3].
The side effects of malnupiravir, which is used in the treatment of viruses and received rapid and first oral use permission during the COVID-19 pandemic period, have not been fully clarified. The aim of this study was to investigate the effects of low and high dose MOL administration on rat liver, lung, heart, and kidney tissues.
Material and Methods
Animals
The local ethics committee of Inonu University on experimental animal research approved the animal experimental protocols and the use of animals in this study (2022/7-3). The power analysis [20,21] suggested 4 groups comprised of at least 10 rats in each group [22]. Fourty male Wistar albino rats obtained from the Experimental Animals Production and Research Center, Inonu University weighed between 250-300g were randomly seperated to 4 groups as; Control, MOL10, MOL100, and MOL1000 (n=10, each). Control group rats, which were applied through an intraperitoneal (i.p.) vehicle, MOL10 group rats received 10 mg/kg/day MOL as a solution prepared in 1 mL vehicle, MOL100 group rats received 100 mg/kg/day MOL as a solution prepared in 1 mL vehicle, MOL1000 group rats received 10 mg/kg/day MOL as a solution prepared in 1 mL vehicle daily for 5 consecutive days. All rats were individually housed in a temperature-controlled (21 ± 2°C) environment with a 12h/12h light/dark cycle, and they were fed with ad libitum access to a standard laboratory chow diet. The animals' care, experimental procedures were carried out by the National Institutes of Health Animal Research Guidelines and ARRIVE guidelines [23].
Molnupiravir administration
Rats in the MOL groups were administrationed with MOL (sold as Covinavir) for 5 days from the beginning of the study [4]. A daily dose of freshly prepared 10-100-1000 mg/kg/day MOL as a solution prepared in 1 mL 5% Carbxymethyl Cellulose Sodiom Salt (CAS:9004-32-4) solution were given at the same time every day (10:00-11:00 am) to each animal by oral gavage.
Termination of experiment and collection tissues
At the end of the required period to (5 days), the rats were sacrificed under anesthesia (ketamine/xylazine, 80/12 mg/kg), blood samples were collected, and liver, lung, heart, and kidney tissues split for biochemical analysis. The tisues were stored at -80°C under suitable conditions until the day of the biochemical analysis. The blood samples were collected in gel sterile tubes. The blood samples were centrifuged at 3500 rpm for 10 min, and then separated into serums used for ELISA.
Biochemical analyses
Two hundred milligrams of frozen tissue specimens were cut into pieces on dry ice and homogenised in PBS buffer (1:9, w/v) using a grinders homogeniser for approximately 5 min. Malondoaldehyde (MDA), which is considered as an indicator of lipid peroxidation, were analyzed from the prepared homegenate. The homogenate was centrifuged at 3500g for approximately 45 minutes to remove supernatant for the evaluation of antioxidant enzyme activities, total antioxidant status (TAS), and total oxidant status (TOS) analyses.
MDA levels in homogenate were measured with the thiobarbituric acid reaction by the method of Esterbauer and Cheeseman [24]. The quantification of thiobarbituric acid reactive substances was determined by comparing the absorption with a standard curve of MDA equivalents generated by acid catalyzed hydrolysis of 1,1,3,3 tetramethoxypropane. The values of MDA were expressed as nanomoles per gram wet tissue.
Antioxidant enzyme activities; Total (Cu–Zn and Mn) Superoxide dismutase (SOD) (EC 1.15.1.1) activity were evaluated as described by Sun et al. [25]. The principle of the method is based on the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine–xanthine oxidase system as a superoxide generator. Activity was assessed in the ethanol phase of the supernatant after 1.0 mL ethanol/chloroform mixture (5/3, v/v) was added to the same volume of sample and centrifuged at 4000g. One unit of SOD was defined as the amount of enzyme causing 50% inhibition in the NBT reduction rate. SOD activity was also expressed as units per milligram protein.
TAS and TOS were measured using Biotek HT Snynergy Gen 5 software, immino plate reader and TAS- TOS kit sets (Rel Assay Diagnostics kit, Mega Tip, Gaziantep, Turkey). Results were calibrated with trolox solution, which is the standard antioxidant and vitamin E analog used for TAS measurement tests. Results were calculated in mmol Trolox Equivalent/ g tissue [26,27]. TOS measurement tests were calibrated with hydrogen peroxide and the results were expressed as μmol H2O2 equivalent/ g tisue [28]. Oxidative stress index (OSI), an indicator parameter of the degree of oxidative stress, was calculated according to TOS/TAS results. Percent ratio of total peroxide level to TAS level was accepted as OSI [29]. To perform the calculation, the result unit of TAS, mmol Trolox equivalent/ g tisue, was changed to μmol Trolox equivalent/ g tissue and the OSI value was calculated using the formula below
OSI = ((Total peroxide, μmol/g tisue)/(TAS, μmol Troloxequivalent/ g tisue)×100).
Serum AST, ALT, and TB levels were measured using an ELISA kit for rat (SunRed Biotechnology Company, Shanghai, China) according to the manufacturer's instructions and results are expressed in U/L and nmol/mL.
Statistical analysis
In data analysis, first, controls were made to prevent missing and erroneous data and excessive variable/outlier problems, and corrections were made if necessary. Quantitative data were summarized as mean ± standard deviation, and qualitative data were summarized as numbers (percentage). In order to be able to compare the examined variables between groups with one-way analysis of variance, conformity to normal distribution and homogeneity of variances (Levene test) were checked. When these assumptions were met, the difference between the group means, one-way analysis of variance and multiple comparisons were made with the Tukey HSD test when the variances were homogeneous, and with the Tamhane T2 test when they were not. In cases where the normality assumptions were not met, the Kruskal Wallis H test was used, and the Conover test was used for multiple comparisons. A value of p<0.05 was considered statistically significant [22].
Results
Liver tissue
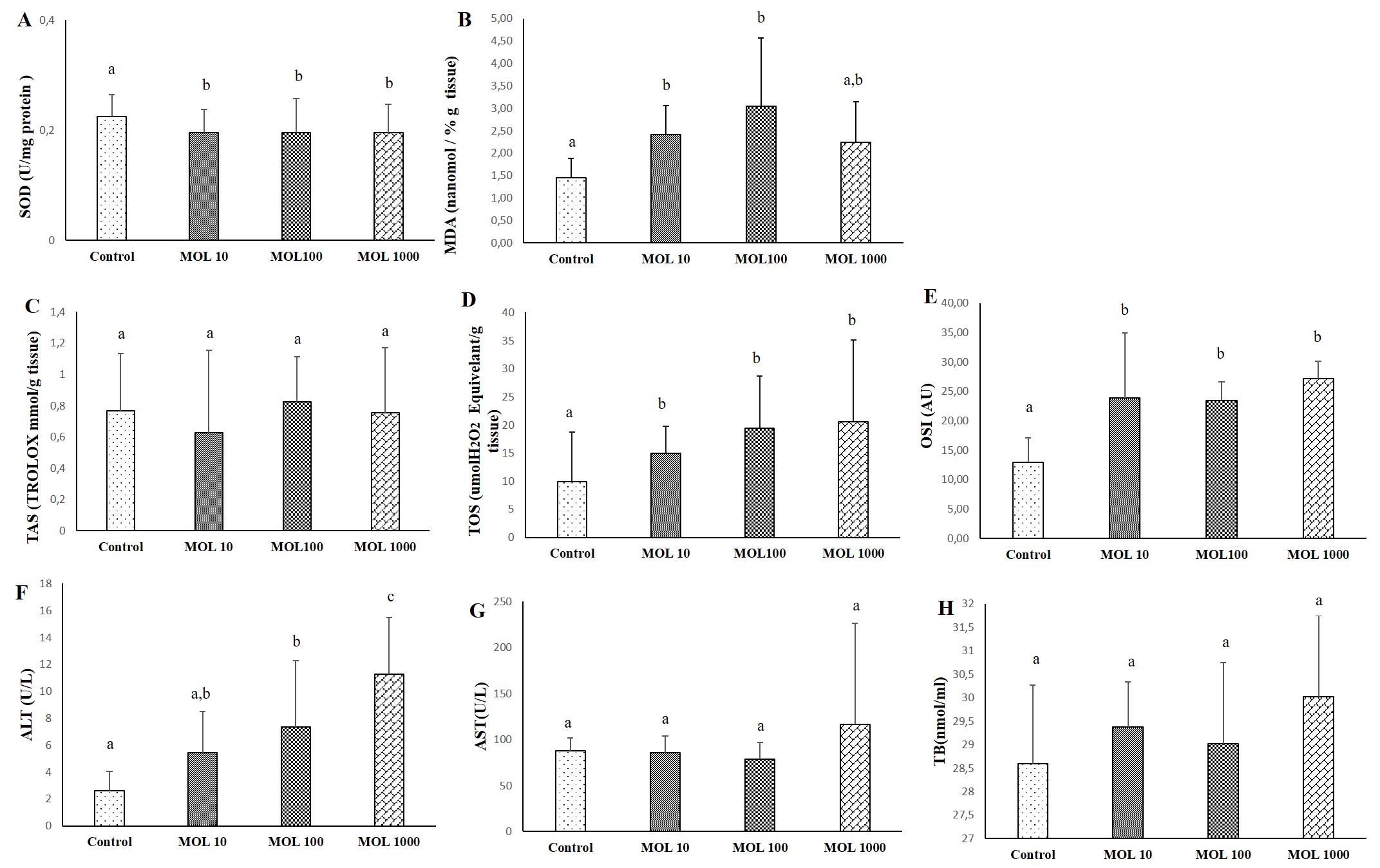
The results of SOD, MDA, TAS, TOS, OSI, ALT, AST, and TB evaluated liver tissue and serum are given in Figure1. The decrease in SOD values was statistically significant in liver tissue in the MOL groups compared to the control group (p<0.05) (Figure 1A). No significant difference was found between MOL10, MOL100, and MOL1000. MDA values are given in Figure 1B. The increase in MDA value was statistically significant in liver tisue in the MOL10 and MOL100 groups compared to the control group (p<0.05).
Differences in TAS level were not statistically significant between groups (Figure 1C). The increase in TOS levels in the MOL10, MOL100, and MOL1000 groups were statistically significant compared to the control group (p<0.05) (Figure 1D). The increase in OSI values in the MOL10, MOL100, and MOL1000 groups were statistically significant compared to the control group (p<0.05) (Figure 1E).
The increase in ALT values in the MOL100 and MOL1000 groups were statistically significant compared to the control group (p<0.05) The increase in ALT values in the MOL1000 groups were statistically significant compared to MOL10 and MOL100 groups (p<0.05) (Figure 1F). Differences in AST and TB levels were not statistically significant between groups (Figures 1G and 1H).
Figure 1. Descriptive statistical criteria for SOD (A), MDA (B), TAS (C), TOS (D), OSI (E) in liver tissues and ALT (F), AST (G), TB (H) in serum. Data are given as mean ± SD and comparison between groups was made with Kruskal Wallis test. a, b, c the difference between the group with different superscript letters on the same line is statistically significant (p<0.05) (n=10).
Lung tissue
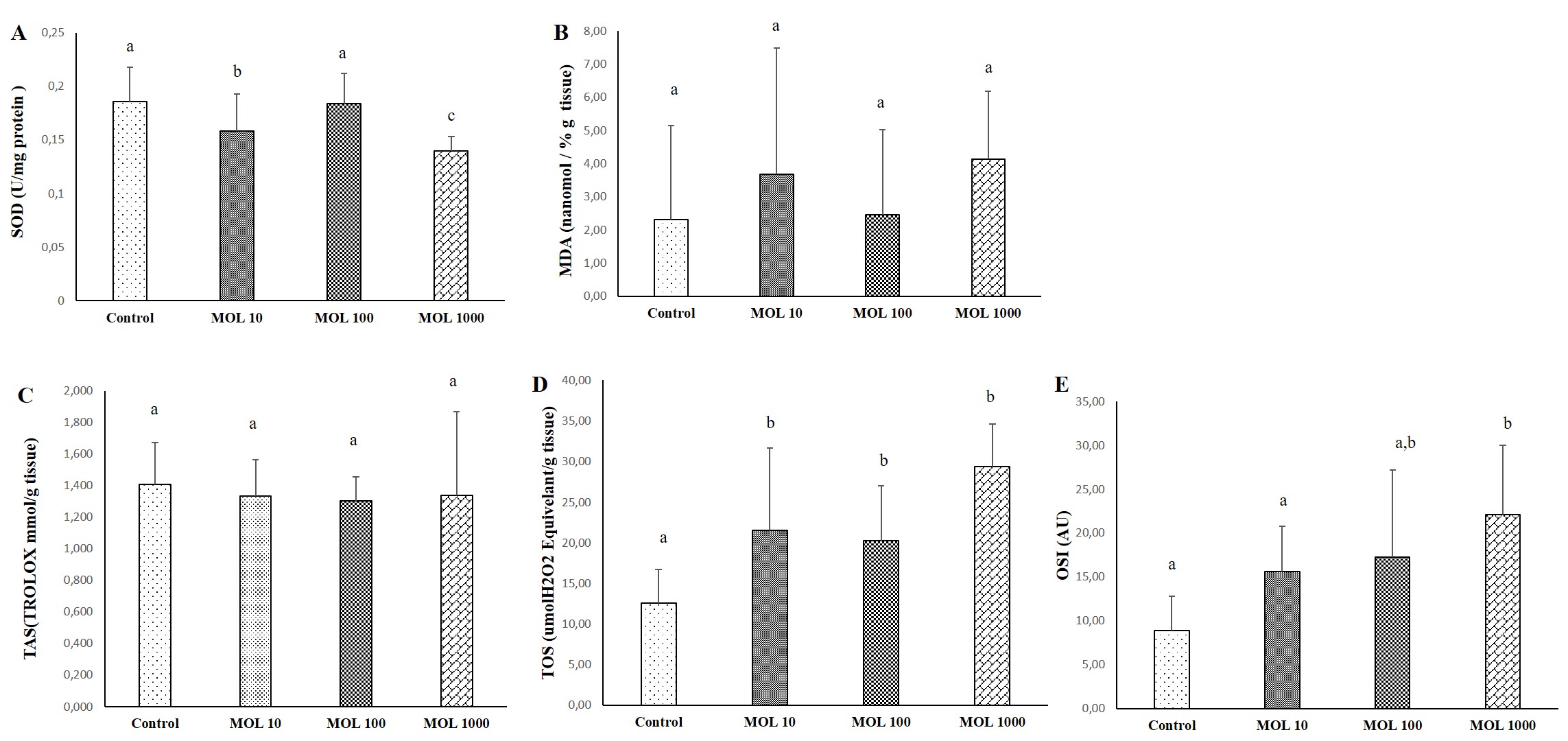
The results of SOD, MDA, TAS, TOS, and OSI evaluated lung tissue is given in Figure 2. The decrease in SOD values was statistically significant in lung tissue in the MOL10 and MOL1000 groups compared to the control group (p<0.05) (Figure 2A). No significant difference was found between MOL100 and control. MDA values are given in Figure 2B. No significant difference was found between MOL groups and control.
Differences in TAS level were not statistically significant between groups (Figure 2C). The increase in TOS levels in the MOL10, MOL100, and MOL1000 groups were statistically significant compared to the control group (p<0.05) (Figure 1D). The increase in OSI values in the MOL1000 groups were statistically significant compared to the control and MOL10 groups (p<0.05).
Figure 2. Descriptive statistical criteria for SOD (A), MDA (B), TAS (C), TOS (D), OSI (E) parameters in lung tissues. Data are given as mean ± SD and comparison between groups was made with Kruskal Wallis test. a, b, c the difference between the group with different superscript letters on the same line is statistically significant (p<0.05) (n=10).
Heart tissue
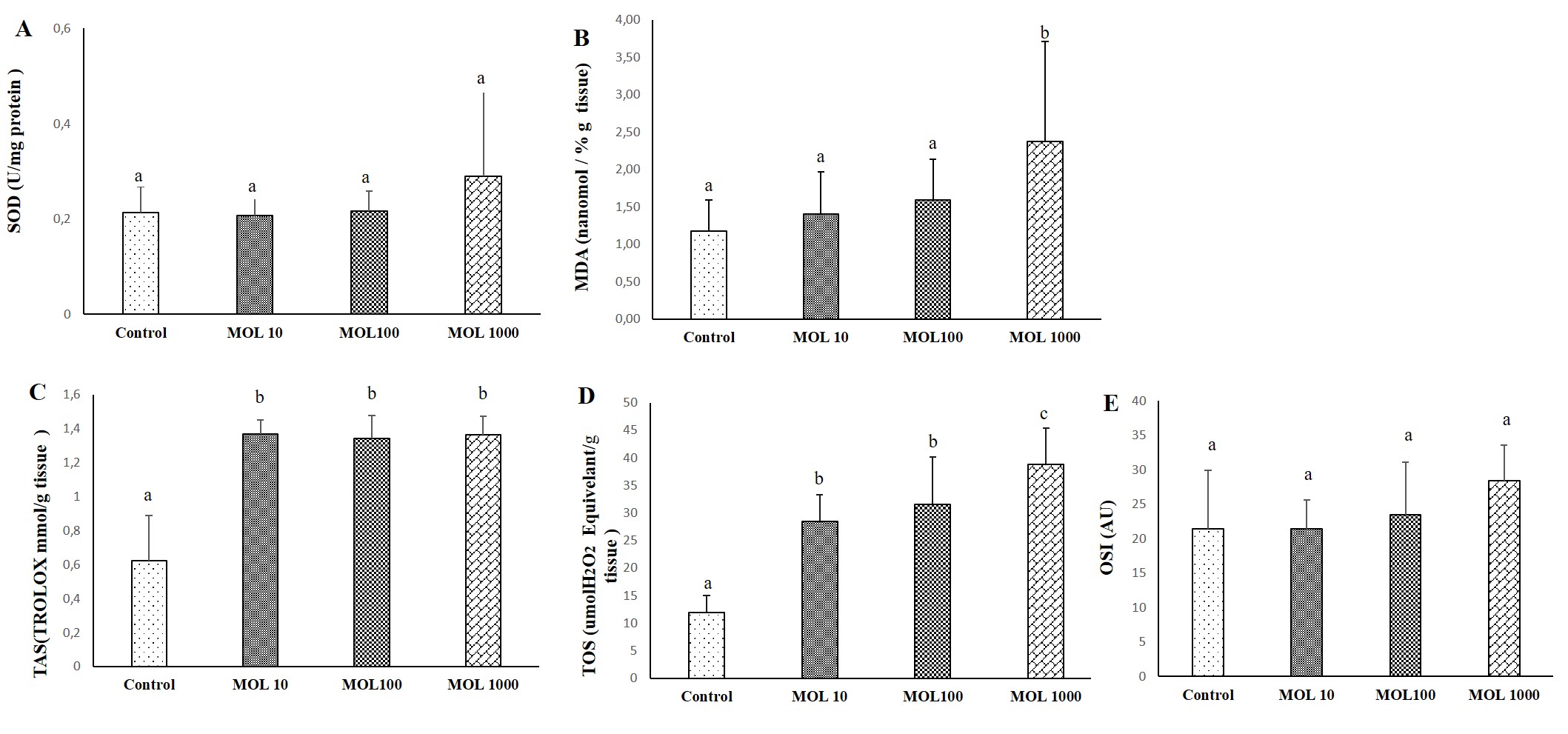
The results of SOD, MDA, TAS, TOS, and OSI evaluated heart tissue is given in Figure 3. The chance of SOD values not statistically significant in heart tissue in the MOL groups compared to the control group (Figure 3A). No significant difference was found between MOL10, MOL100, and MOL1000. MDA values are given in Figure 3B. The increase in MDA value was statistically significant in liver tisue in the MOL1000 groups compared to the control group (p<0.05).
Differences in TAS level were statistically significant in the MOL groups compared to the control group (Figure 3C). The increase in TOS levels in the MOL10, MOL100, and MOL1000 groups were statistically significant compared to the control group (p<0.05). TOS value was statistically significant in the MOL1000 groups compared to the MOL10 and MOL100 groups (Figure 3D). The increase in OSI values were not statistically significant between groups (Figure 3E).
Figure 3. Descriptive statistical criteria for SOD (A), MDA (B), TAS (C), TOS (D), OSI (E) parameters in heart tissues. Data are given as mean ± SD and comparison between groups was made with Kruskal Wallis test. a, b, c the difference between the group with different superscript letters on the same line is statistically significant (p<0.05) (n=10).
Kidney tissue
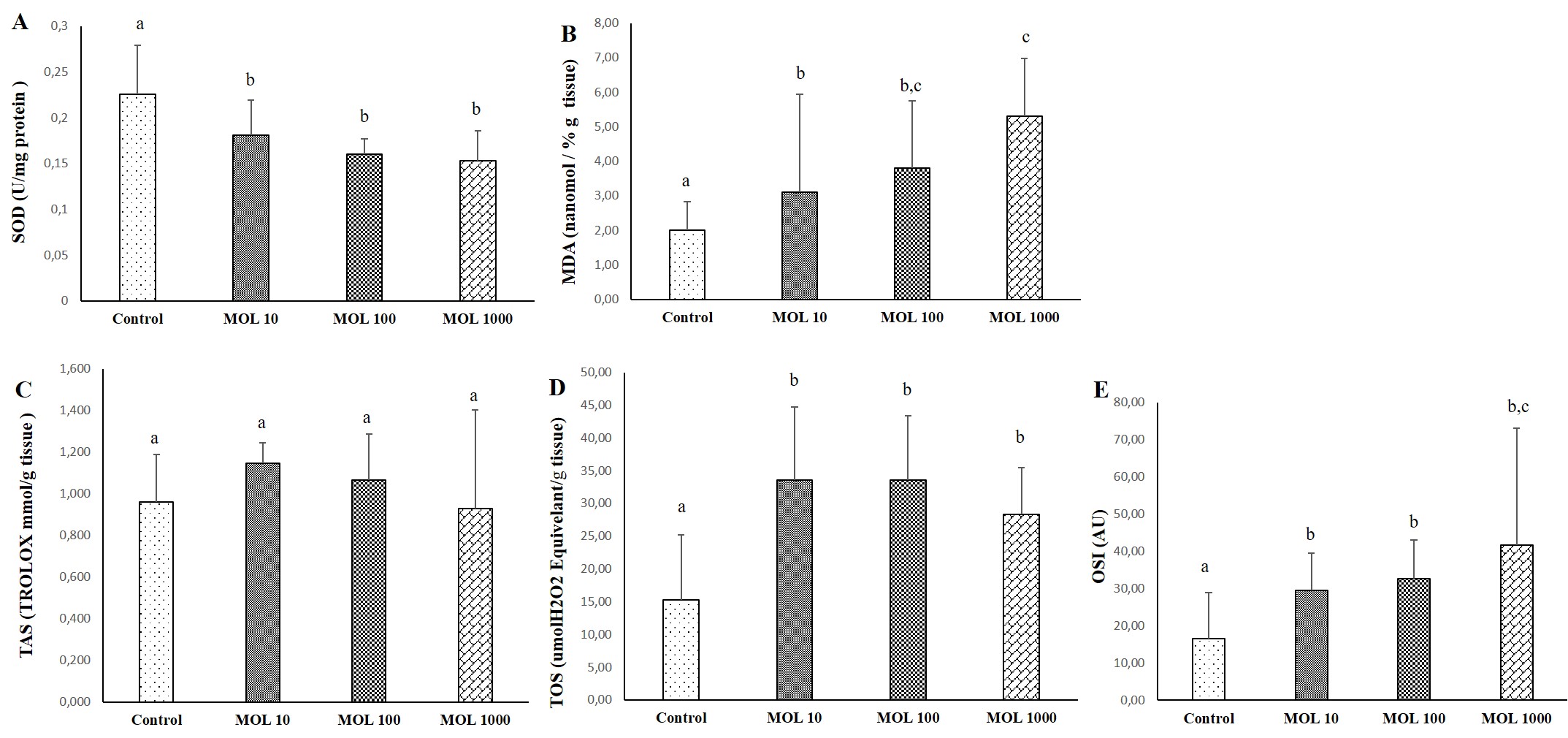
The results of SOD, MDA, TAS, TOS, and OSI evaluated kidney tissue is given in Figure 4. The decrease in SOD values was statistically significant in kidney tissue in the MOL groups compared to the control group (p<0.05) (Figure 4A). No significant difference was found between MOL10, MOL100, and MOL1000. MDA values are given in Figure 4B. The increase in MDA value was statistically significant in kidney tisue in the MOL10, MOL100, and MOL1000 groups compared to the control group (p<0.05). MDA value was statistically significant in the MOL1000 groups compared to the MOL10 group (p<0.05).
Differences in TAS level were not statistically significant between groups (Figure 4C). The increase in TOS levels in the MOL10, MOL100, and MOL1000 groups were statistically significant compared to the control group (p<0.05) (Figure 4D).
The increase in OSI values in the MOL10, MOL100, and MOL1000 groups were statistically significant compared to the control group (p<0.05). OSI value was statistically significant in the MOL1000 groups compared to the MOL10 and MOL100 groups (p<0.05) (Figure 4E).
Figure 4. Descriptive statistical criteria for SOD (A), MDA (B), TAS (C), TOS (D), OSI (E) parameters in kidney tissues. Data are given as mean ± SD and comparison between groups was made with Kruskal Wallis test. a, b, c the difference between the group with different superscript letters on the same line is statistically significant (p<0.05) (n=10).
Discussion
The use of MOL, which is patient friendly, safe, tolerable, and orally effective, is common for outpatients for the treatment of COVID-19. MOL, the first oral drug to receive emergency use authorization during the pandemic process, completed Phase 1 and Phase 2 clinical trials in approximately 15 months. It is currently in Phase 3 of clinical trials. Its effectiveness in the treatment of COVID-19 has accelerated the development of this drug. To date, there is no toxicity information for MOL in the literature [30]. When the ease of use and efficacy in treatment are evaluated, the side effects seen in the patients can be ignored. However, a detailed study on its long-term use is not yet available in the literature. In this study, the effects of using different doses of MOL in many tissues were evaluated. Our study findings indicate that molnupiravir, whose toxicity is not mentioned in the literature, may cause tissue damage by disrupting the oxidant/antioxidant balance in different organs in a dose-dependent manner [5].
It is recommended that the use of MOL causes an increase in ALT in patients and caution is recommended when using it in patients with liver dysfunction [31]. Although AST and ALT are considered as indicators of liver tissue damage, AST may be more helpful in diagnosing cardiac and pulmonary infarctions. In liver cells, 40% of AST is localized in mitochondria, while all of ALT is in the cytoplasm. Therefore, the increase in ALT is higher than the increase in AST, since acute liver injuries are mostly in the cytoplasm [32]. In our study findings, while the use of MOL caused an increase in ALT in direct proportion to the dose, the increase in AST was not found to be statistically significant. While the use of MOL decreases the antioxidant enzyme SOD activity in the liver tissue, while increasing the amount of MDA, which is a marker of oxidative damage, may cause hepatotoxicity. While using MOL, lower doses and long-term use can be preferred by evaluating liver functions. The use of antioxidants can be preferred against toxicity that may occur in the liver [33].
Once absorbed, MOL is converted in the plasma to EIDD-1931, the active nucleoside analogue [34]. EIDD-1931 was found to be widely bioavailable in the lungs and successfully converted into its triphosphate form in the central nervous system in pharmacokinetic and distribution studies in mice, rats, ferrets, and dogs [35]. In experimental animal model studies of the use of MOL in the literature, lung pathogenesis and respiratory viral spread evaluations were made. Since the MOL effect was investigated on animals infected with the virus in these studies, it was reported that it was effective in preventing the pathology that developed due to the virus, but no information was given about the side effects [36]. However, since animals in our study used MOL without any viral infection, it is not correct to compare them with these data. Our study findings are that it increases the oxidative damage in the lung tissue and causes the deterioration of the antioxidant/oxidant balance. It shows that it may be beneficial to use it together with antioxidants mentioned in the literature to reduce the damage that may occur due to the use of MOL while preventing viral growth in the lung tissue [37].
In a study investigating the effects of antiviral drugs used in the treatment of COVID-19 in individuals with metabolic diseases with other drugs used, it was reported that the use of MOL is safe for heart patients [38]. No interaction has been observed when used with antidiabetic, cardiovascular, and hypertensive drugs. It is reported that it does not cause any change in ECG findings, does not cause arrhythmia or hypertensive effects. However, there are many studies in the literature that the COVID-19 virus causes damage to vascular and endothelial tissues as well as heart tissue [39].
In a recent study evaluating the effects of antiviral drugs on renal function, all antiviral drugs used in the treatment of COVID-19 were evaluated. It has been stated that the use of MOL is 2-10 times more effective in slowing down virus replication compared to other antiviral drugs, but no negative effects on kidney functions have been reported. Our findings showed that it increased oxidative stress in kidney tissue. Increasing lipid peroxidation and decreasing SOD activity may create risky conditions for kidney tissue [40]. Patients with chronic kidney disease should be followed in the use of MOL.
Pre-clinical studies showed that MOL yielded no significant chromosomal damage or genotoxicity. Studies and findings on tissue damage caused by the use of MOL in experimental animals are not sufficient. Most of the studies are directed towards the antiviral efficacy of MOL. Considering the improvements observed due to the decrease in the effect of the virus, the damages that may occur in the tissue were not evaluated by ignoring them [41]. The result of our study shows that while providing new information to the literature with this aspect, attention should be paid to the use of the drug. If the use of MOL is an inevitable necessity, long-term use at lower doses may be recommended. Studies have shown that the use of antioxidants may be beneficial in reducing the side effects of antiviral drugs, as well as in reducing the rate of spread of the virus by strengthening the body's defense system. [35,39]. Taking antioxidants together with MOL can reduce tissue damage due to MOL. In future studies, examining the effects of MOL use at different doses and treatment times together with antioxidant supplementation will provide more reliable and applicable results.
Consequently, our study findings and previous studies have shown that MOL used in virus treatment is dose-dependently effective on the oxidant/antioxidant system in different tissues. It is known that changes in the oxidant/antioxidant system cause structural damage that causes loss of function in tissues. For this reason, the use of antioxidants together with MOL may be beneficial to reduce tissue damage that may occur in the use of MOL.
Confict of Interest
The authors declare no competing interests
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. This work was not supported.
Ethics Approval
This study was carried out with approval of Ethical Committee of Experimental Animals of the Faculty of Medicine in Inonu University (2022/7-3). The authors have no ethical conflicts to disclose.
Author Contributions
Conceptualization, K.T.; methodology, K.T. software, K.T.; validation, K.T., S.S..; formal analysis, K.T. and S.S.; investigation, K.T.; data curation K.T., S.S..; writing—original draft preparation, K.T.; writing—review and editing, K.T. and S.S.; All authors have read and agreed to the published version of the manuscript.
References
2. Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Ann Med. 2022;54(1):516-23.
3. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386(6):509-20.
4. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
5. Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szewczyk LJ, et al. Molnupiravir, an Oral Antiviral Treatment for COVID-19. medRxiv. 2021.
6. Schou TM, Joca S, Wegener G, Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19 - A systematic review. Brain Behav Immun. 2021;97:328-48.
7. Khoo SH, Fitzgerald R, Fletcher T, Ewings S, Jaki T, Lyon R, et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother. 2021;76(12):3286-95.
8. Gordon CJ, Tchesnokov EP, Schinazi RF, Gotte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021;297(1):100770.
9. Law MF, Ho R, Law KWT, Cheung CKM. Gastrointestinal and hepatic side effects of potential treatment for COVID-19 and vaccination in patients with chronic liver diseases. World J Hepatol. 2021;13(12):1850-74.
10. Yoshikawa T, Naito YJJmaj. What is oxidative stress? 2002;45(7):271-6.
11. Delgado-Roche L, Mesta FJAomr. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. 2020;51(5):384-7.
12. Adams L, Franco MC, Estevez AGJEb, medicine. Reactive nitrogen species in cellular signaling. 2015;240(6):711-7.
13. Schieber M, Chandel NSJCb. ROS function in redox signaling and oxidative stress. 2014;24(10):R453-R62.
14. Antelmann H, Helmann JDJA, signaling r. Thiol-based redox switches and gene regulation. 2011;14(6):1049-63.
15. Ntyonga-Pono M-PJTPAMJ. COVID-19 infection and oxidative stress: an under-explored approach for prevention and treatment? 2020;35(Suppl 2).
16. Cecchini R, Cecchini ALJMh. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. 2020;143:110102.
17. Lavillette D, Barbouche R, Yao Y, Boson B, Cosset F-L, Jones IM, et al. Significant redox insensitivity of the functions of the SARS-CoV spike glycoprotein: comparison with HIV envelope. 2006;281(14):9200-4.
18. Mihalopoulos M, Dogra N, Mohamed N, Badani K, Kyprianou NJEuf. COVID-19 and kidney disease: molecular determinants and clinical implications in renal cancer. 2020;6(5):1086-96.
19. Abdi A, Jalilian M, Sarbarzeh PA, Vlaisavljevic ZJDr, practice c. Diabetes and COVID-19: A systematic review on the current evidences. 2020;166:108347.
20. Prasad SN, Muralidhara. Protective effects of geraniol (a monoterpene) in a diabetic neuropathy rat model: attenuation of behavioral impairments and biochemical perturbations. J Neurosci Res. 2014;92(9):1205-16.
21. Tanbek K, Ozerol E, Yilmaz U, Yilmaz N, Gul M, Colak C. Alpha lipoic acid decreases neuronal damage on brain tissue of STZ-induced diabetic rats. Physiol Behav. 2022;248:113727.
22. Arslan AK, Yaşar Ş, Çolak C, Yoloğlu SJTKB. WSSPAS: an interactive web application for sample size and power analysis with R using shiny. 2018;10(3):224-46.
23. Çolak C, PARLAKPINAR HJJoTOMC. Hayvan deneyleri: in vivo denemelerin bildirimi: ARRIVE Kılavuzu-Derleme. 2012;19(2):128-31.
24. Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407-21.
25. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497-500.
26. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clinical biochemistry. 2004;37(4):277-85.
27. Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clinical biochemistry. 2004;37(2):112-9.
28. Erel O. A new automated colorimetric method for measuring total oxidant status. Clinical biochemistry. 2005;38(12):1103-11.
29. Demirbag R, Yilmaz R, Erel O, Gultekin U, Asci D, Elbasan Z. The relationship between potency of oxidative stress and severity of dilated cardiomyopathy. Can J Cardiol. 2005;21(10):851-5.
30. Singh AK, Singh A, Singh R, Misra A. Molnupiravir in COVID-19: A systematic review of literature. Diabetes Metab Syndr. 2021;15(6):102329.
31. Fischer W, Eron JJ, Holman W, Cohen MS, Fang L, Szewczyk LJ, et al. Molnupiravir, an oral antiviral treatment for COVID-19. 2021.
32. Reedy DW, Loo AT, Levine RA. AST/ALT ratio > or = 1 is not diagnostic of cirrhosis in patients with chronic hepatitis C. Dig Dis Sci. 1998;43(9):2156-9.
33. Tanbek K, Ozerol E, Bilgic S, Iraz M, Sahin N, Colak CJM. Protective effect of Nigella sativa oil against thioacetamide-induced liver injury in rats. 2017;6(1):96-103.
34. Pourkarim F, Pourtaghi-Anvarian S, Rezaee H. Molnupiravir: A new candidate for COVID-19 treatment. Pharmacol Res Perspect. 2022;10(1):e00909.
35. Painter GR, Natchus MG, Cohen O, Holman W, Painter WP. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Curr Opin Virol. 2021;50:17-22.
36. Schafer A, Martinez DR, Won JJ, Moreira FR, Brown AJ, Gully KL, et al. Therapeutic efficacy of an oral nucleoside analog of remdesivir against SARS-CoV-2 pathogenesis in mice. bioRxiv. 2021.
37. Ozdemir R, Gokce IK, Taslidere AC, Tanbek K, Gul CC, Sandal S, et al. Does Chrysin prevent severe lung damage in Hyperoxia-Induced lung injury Model? International immunopharmacology. 2021;99:108033.
38. S KS, P AA, B S, Kalala KP, Pm A, Sabarathinam S. Drug interaction risk between cardioprotective drugs and drugs used in treatment of COVID-19: A evidence-based review from six databases. Diabetes Metab Syndr. 2022;16(3):102451.
39. Tarnawski AS, Ahluwalia A. Endothelial cells and blood vessels are major targets for COVID-19-induced tissue injury and spreading to various organs. World J Gastroenterol. 2022;28(3):275-89.
40. Ekici K, Temelli O, Parlakpinar H, Samdanci E, Polat A, Beytur A, et al. Beneficial effects of aminoguanidine on radiotherapy-induced kidney and testis injury. Andrologia. 2016;48(6):683-92.
41. Focosi D. Molnupiravir: From Hope to Epic Fail? Viruses. 2022;14(11):2560.