Abstract
Idiopathic normal pressure hydrocephalus (iNPH) is a special type of hydrocephalus that is characterized by cognitive decline, gait disturbance, and urinary incontinence. It can lead to dementia and bedridden within 1-3 years. Without surgical treatment in time, the prognosis was bleak. We report an iNPH case misdiagnosed with Alzheimer’s disease, with a disease course of 3 years. The main manifestations of the patient were walking impairment, memory loss, urinary incontinence, repeated falls, and hallucinations, bedridden in the late stage, with a modified Rankin Scale (mRS):5. After undergoing the ventriculoperitoneal shunt, due to the low shunt pressure, the patient developed severe headache, repeated and severe subdural hemorrhage and effusion. After adjusting the shunt pressure in time, the patient recovered well with an mRS: 3, which was inconsistent with the previous belief that the operation was ineffective for patients with a disease course of more than 3 years. This case suggests that elderly patients with iNPH can still benefit from timely surgery even if the disease course of more than 3 years. Special attention should be paid after the operation, and the CSF shunt pressure should avoid setting too low, which may induce serious complications such as subdural hemorrhage or effusion.
Keywords
Hydrocephalus, Subdural hematoma, Ventriculoperitoneal shunt, Dementia, Subdural effusion
Introduction
Normal pressure hydrocephalus (NPH) is a type of hydrocephalus with dilated ventricles and normal cerebrospinal fluid (CSF) pressure that affects the elderly. The secondary NPH (sNPH) has clear etiology, but that of idiopathic NHP (iNPH) is unknown, which is mostly believed to be related to hypertension, diabetes, and cerebrovascular disease [1-3]. The prevalence of iNPH based on computerized tomography (CT)/magnetic resonance (MR) images is approximately 1.6% among people over 61 years of age, and 8.9% among people aged 80 years and more in Japan [4,5]. The iNPH is mainly manifested as a triad of cognitive decline, gait disturbance, and urinary incontinence [6], with progressive aggravation. The clinical symptoms and imaging manifestations of iNPH in the elderly are similar to those of Alzheimer’s and other diseases, making it prone to misdiagnosis and missed diagnosis [7]. Here, we summarized and analyzed the clinical data of an elderly patient with iNPH, including symptom evolution, surgical procedure, severe postoperative complications, and the corresponding treatment. The purpose was to attract clinical attention to avoid misdiagnosis and missed diagnosis, prevent delayed surgical treatment, and reduce the occurrence of serious postoperative complications.
Case Presentation
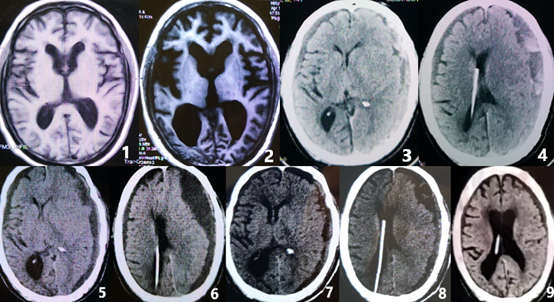
The patient, a 76-year-old Chinese man, was admitted to the hospital in a wheelchair with a complaint of “slow walking and memory loss for 3 years, difficulty in urinating for 2 years, and difficulty in walking for 1 month” on April 17, 2021. The patient was a junior high school graduate and a technical worker and a nonsmoker. He had a history of viral encephalitis and surgery for prostatic hyperplasia in May 2017. The brain magnetic resonance imaging (MRI) scan (August 3, 2017, Figure 1) showed slight atrophy of the cerebral cortex and slight dilation of the ventricular system.
The patient developed slow walking 3 years prior, with a slightly flexed posture and forward leaning body when walking. Meanwhile, the patient also developed memory loss, manifesting as often losing things, forgetting the names of friends, and choosing more and more wrong cards when playing. The patient’s speech was reduced, with slower speech speed, and he became easily agitated. Afterwards, the patient’s walking gradually slowed down and occasionally fell. By then, he could stand up on his own, and occasionally needed assistance. The patient developed difficulty in urination 2 years prior, from standing for 10-20 minutes. He had frequent urination at night and increased to approximately 1 time per hour. The patient also developed constipation. His speech was reduced significantly, and he responded passively. He could no longer play cards for poor memory. The patient had undergone a brain MRI scan due to a fall, which showed slight atrophy of the cerebral cortex, thinning of the occipital cortex, dilatation of the lateral ventricles, blunt anterior horns of the lateral ventricles, obvious enlargement of the posterior horns, slight widening of the Sylvian fissure, a disproportionately enlarged subarachnoid space hydrocephalus (DESH) sign. He was then diagnosed with Alzheimer’s disease in a local hospital, but no treatment was provided. The patient experienced occasional hallucinations 1 year prior. He saw a deceased friend in the sky, which happened before falling asleep at night. The patient had self-awareness and often told the family about this, but nobody paid attention. The falls of the patient gradually increased. A repeat brain MRI scan showed atrophy of the cerebral cortex, significant dilation of the lateral ventricles, DESH sign. One month before admission, the patient displayed aggravated difficulty in walking, with needs for assistance, and became bedridden and wheelchair bound. He fell repeatedly while urinating at night, had aggravated difficulty in urinating, and the urinary incontinence at night became severe. The patient’s speech was significantly reduced, and he needed to be reminded or called repeatedly to give a response. The patient’s memory also declined significantly. He could not recall the names of his family members, and he forgot to eat and questioned his family members for not giving him food.
On admission, the patient was conscious, indifferent, and unresponsive, with masked face, and he could respond to part of the questions. His bilateral pupils were round and equal in size, with sensitive light reflexes. The bilateral nasolabial folds were symmetrical, and the tongue was not deviated when put out. The muscle strength of the four extremities was at level 5, and the tendon reflexes of the four extremities were active, with increased muscle tone (cogwheel rigidity) in the four extremities. The sensations were symmetrical, and the pathological reflexes were negative.
He had a Mini-Mental State Examination (MMSE) score of 10, an mRS score of 5, and an iNPH grading scale (iNPHGS) score of 10. The time to walk in a straight line of 10 m was approximately 40 s with assistance, and the number of steps was 45. The patient then underwent lumbar puncture, and the CSF pressure was 165 mmH2O. The results of CSF routine and biochemistry tests were normal. At 8 h, 24 h, and 48 h after CSF drainage, the reassessed MMSE scores of the patient were 12, 16, and 12, respectively. The results of 10 Meter Walk Test (10MWT) were 37 s, 34 s, and 39 s, and the results of 10 Meter Walk Steps (10MWS) were 43 steps, 39 steps, and 44 steps, respectively.
After admission, the patient underwent the complete blood count, liver function, kidney function, plasma electrolytes, coagulation function, thyroid function, and tumor markers tests, and the results were all normal. The electrocardiogram showed sinus rhythm. Abdominal color Doppler ultrasonography showed normal liver, gallbladder, pancreas, and spleen; small kidney stones in both kidneys; mild prostatic hypertrophy; and urinary retention in the bladder, with a volume of about 100 ml.
A chest CT scan showed chronic bronchitis and emphysema in both lungs. A brain MRI scan (April 12,2021, Figure 2) showed significant atrophy of the cerebral cortex, significant dilation of the ventricular system, significant widening, and enlargement of the posterior horns of the lateral ventricles, significant widening of the Sylvian fissure, DESH sign, and significant irregular depression of the top of the lateral ventricles.
The patient underwent ventriculoperitoneal shunt (VPs) with a pressure-adjustable drainage tube, and the drainage pressure was set as 110 mmH2O. The postoperative head CT scan (April 15, 2021) showed that the hydrocephalus was alleviated, the ventricles were narrowed, and the widening of the Sylvian fissure was reduced. After recovering from the anesthesia, the patient’s speech gradually became clear and fluent, and his memory was improved. He could recognize his family members and answer questions with accuracy. Moreover, he could go to the toilet with assistance, and the incontinence was recovered. Subsequently, the patient developed severe and persistent headache after the operation, mainly on the forehead and the top of the head, which was aggravated by sitting up and alleviated by lying down. He resisted getting out of bed due to the headache, which affected his diet, and the amount and frequency of food intake decreased significantly. The patient woke up several times at night and found that his limbs were weak, which was apparent on the right side. An emergency brain MRI scan with diffusion-weighted imaging (DWI) was performed to rule out cerebral infarction. The symptoms were relieved after increasing fluid infusion, and hypoperfusion was considered due to insufficient intake. After 3 weeks of bed rest, the patient’s headache was relieved, and he could walk slowly without assistance. He could eat, get dressed, wash his face, brush his teeth, and go to the toilet by himself, but still needed help in bathing. However, the patient developed dizziness after standing for a long time or going to the toilet, which could be relieved after resting in bed. He occasionally fell when urinating at night, without unconsciousness, convulsion, or incontinence. At the 10th week after the operation, the patient fell while walking and developed drowsiness. He could not recognize his family members nor eat or walk. Besides, he also had incontinence. A head CT scan (June 29, 2021, Figure 3 and 4) showed subdural hemorrhage and significantly narrowed ventricles with midline shift. The patient underwent burr-hole craniotomy and drainage twice for subdural hematoma in 3 days. After the operation, the symptoms were improved, and the patient became conscious and could recognize his family members. He was able to eat, and the incontinence was recovered. One week after the operation, the patient developed drowsiness and incontinence again. A repeat head CT scan (July 16, 2021. Figure 5 and 6) showed significant subdural effusion, and the ventricles were compressed and deformed, with significant midline shift. Burr-hole craniotomy and drainage were repeated. In addition, the ventriculoperitoneal drainage pressure increased to 150 mmH2O. The patient’s condition was improved, and his consciousness was restored. He resumed walking and could take care of himself in daily life, but still needed help in bathing. A repeat head CT scan (July 25, 2021, Figure 7 and 8) showed that the subdural effusion, ventricular deformation, and midline shift were significantly reduced.
Postoperatively, the patient recovered well and was discharged 2 weeks after the last operation. The patient did not require medication. During follow-up at 3 months, 1 year, the patient’s mRS score was 3, and the iNPHGS score was 5. The head CT scan (January 21, 2022, Figure 9) showed that the subdural effusion was significantly alleviated. The follow-up on January 1, 2024, revealed that the patient adequately took care of himself, with an mRS score of 3.
Figure 1. T1MRI (August 3, 2017) scan showed slight atrophy. Figure 2. T1MRI (April 12,2021) scan showed significant atrophy of the cerebral cortex, significant dilation of the ventricular system, significant widening, and enlargement of the posterior horns of the lateral ventricles, significant widening of the Sylvian fissure, DESH sign. Figure 3-4. CT scan (June 29, 2021) showed subdural hemorrhage and significantly narrowed ventricles with midline shift. Figure 5-6. CT (July 16, 2021) scan showed significant subdural effusion, and the ventricles were compressed and deformed, with significant midline shift. Figure 7-8. CT (July 25, 2021) scan showed the subdural effusion, ventricular deformation, and midline shift were significantly reduced. Figure 9. CT scan (January 21, 2022) showed the subdural effusion was significantly alleviated after 6 months again.
Discussion
The main clinical manifestations of iNPH in the elderly are the triad of gait disturbance, cognitive decline, and urinary incontinence, of which gait disturbance is the most common and the earliest to appear, and it is also the earliest to improve after CSF shunt [8]. The early symptoms of patients are mainly gait disturbance, decreased stride length, and slowed walking, accompanied by varying degrees of memory loss. With the prolongation of the disease course, the symptoms are gradually aggravated, and the gait disturbance becomes severe. Standing and turning need support, especially difficulty in turning round and getting up [6]. Memory loss is gradually aggravated, and hallucination and behavioral abnormalities may occur. In severe cases, the patients cannot recognize their family members, accompanied by incontinence, and they cannot take care of themselves.
Brain MRI and CT scans are important means for the diagnosis of iNPH. The imaging evaluation of iNPH mainly takes Evans index (EI)>0.3 as the diagnostic criterion of ventriculomegaly. iNPH mainly manifests as dilatation of the ventricular system to varying degrees, supratentorial hydrocephalus, and significant enlargement of the temporal horns and posterior horns of lateral ventricles; the brain atrophy is not evident in the early stages, accompanied by DESH sign and periventricular white matter lesions [9]. Different from iNPH, patients with Alzheimer’s disease mainly show manifestations of cortical atrophy in the frontal, temporal, and parietal association areas, and ventriculomegaly is not evident in the early stages [10].
However, the accuracy of the Evans index is also controversial due to its influence on ventricular variability [11]. MRI measurement of the corpus callosum angle is also commonly used for the diagnosis of NPH. In INPH patients, the corpus callosum angle often fluctuates between 52-80°, which is usually greater than 90° compared to Alzheimer's disease and normal control groups. Its accuracy, sensitivity, and specificity are 93%, 97%, and 88%, respectively [12]. Phase contrast MRI (PC-MRI) technology can be non-invasive, accurate, and sensitive to evaluate the velocity and direction of CSF flow. In NPH patients, the peak flow velocity and stroke volume of CSF in the midbrain aqueduct were significantly higher than those in the communicating hydrocephalus, normal control group, and small vessel disease group [13].
Surgery is the main treatment for iNPH in the elderly, including VPs, ventriculoarterial shunt, and lumbar-peritoneal shunt, of which VPs is the first choice. The most common complications of VPs are postoperative headache, subdural effusion, spontaneous epidural hemorrhage, and infection. In this case, the CSF shunt pressure was lowered by about 50 mmH2O after VPs. The patient developed persistent and severe headache after the operation, mainly in the frontal and parietal parts of the head, which was aggravated by standing and alleviated by bed rest, suggesting an over drainage of CSF. Subsequently, subdural hematoma and subdural effusion occurred, which required repeated drilling and drainage. After increasing the pressure of the shunt valve, the headache and subdural effusion were improved, which confirmed the over drainage. Over drainage of CSF can lead to unilateral or bilateral subdural hematoma. The symptoms of the patient are often aggravated again, and coma may occur in severe cases. If detected in time, drainage of hematoma can be performed early; combined with increasing the ventriculoperitoneal shunt pressure, most patients can generally recover well [14]. Therefore, some scholars suggest that after VPs, the initial shunt pressure should be set at 20 mmH2O lower than the initial CSF pressure. The patient should be reexamined 1-3 months after the operation, and the shunt pressure should be gradually lowered according to the patient’s clinical symptoms and the degree of ventricular dilatation, with an appropriate pressure adjustment range of 10-20 mmH2O each time. Frequent adjustment and excessive reduction should be avoided, which may lead to over drainage and induce subdural or epidural hemorrhage or effusion.
The patient’s symptoms may be improved immediately after VPs. Walking and incontinence are the earliest and fastest to recover, and memory and speech functions also recover gradually, continuing to improve 3 months after the operation; some patients continue to improve 1 year after VPs, and more than half of the patients can maintain clinical improvement for more than 6 years [15]. Some scholars believe that the disease course has a relationship with postoperative recovery. Patients with a disease course of 6-11 months have better recovery after surgery. The longer the disease course, the more serious the damage to the brain, and the worse the recovery. Surgery may be ineffective for those with a disease course of more than 3 years, but there are also some researchers who believe that the course of the disease has no correlation with the postoperative recovery [16-18]. In this case, the disease course of the patient was 3 years, and the symptoms gradually worsened till the patient could not recognize his family members and became bedridden. In the late stage, he could not take care of himself at all, with an mRS score of 5. He recovered well after VPs, with an mRS score of 3. The patient benefited significantly after VPs, indicating that elderly patients with iNPH may still benefit from timely surgery even if the disease course exceeds 3 years.
Conclusions
The incidence of iNPH in elderly patients is high. Combined with the typical triad of iNPH, MRI and other imaging features, and CSF drainage test, the diagnosis can be confirmed. VPs is the main treatment for iNPH in the elderly. Special attention should be paid after the operation, and the CSF shunt pressure should be gradually decreased to avoid adjusting it too frequently or setting it too low, which may induce headache secondary to intracranial hypotension, and even serious complications such as subdural hemorrhage or effusion.
Statement of Ethics
This study was conducted in line with the principles of the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Ethical approval is not required for this study in accordance with local guidelines.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Funding Sources
Shenzhen Guangming District Special Fund for Economic Development, China, No. 2020R01006. University of Chinese Academy of Sciences-Shenzhen Hospital discipline construction capacity improvement project, No. HRF-2020023. Suzhou Science and Technology Plan Project, No.sys2018012.
Author Contributions
Wang QY contributed to manuscript writing and editing, data collection and analysis; Li QJ contributed to manuscript editing, clinical data collection and analysis; all authors have read and approved the final manuscript.
Data Availability Statement
All data generated or analyzed in this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author, Dr. Qingjun Li.
References
2. Jaraj D, Agerskov S, Rabiei K, Marlow T, Jensen C, Guo X, et al. Vascular factors in suspected normal pressure hydrocephalus: A population-based study. Neurology. 2016 Feb 16;86(7):592-9.
3. Bonney PA, Briggs RG, Wu K, Choi W, Khahera A, Ojogho B, et al. Pathophysiological Mechanisms Underlying Idiopathic Normal Pressure Hydrocephalus: A Review of Recent Insights. Front Aging Neurosci. 2022 Apr 28;14:866313.
4. Andersson J, Rosell M, Kockum K, Lilja-Lund O, Söderström L, Laurell K. Prevalence of idiopathic normal pressure hydrocephalus: A prospective, population-based study. PLoS One. 2019 May 29;14(5):e0217705.
5. Nakajima M, Yamada S, Miyajima M, Ishii K, Kuriyama N, Kazui H, et al. Guidelines for Management of Idiopathic Normal Pressure Hydrocephalus (Third Edition): Endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol Med Chir (Tokyo). 2021 Feb 15;61(2):63-97.
6. Hallqvist C, Grönstedt H, Arvidsson L. Gait, falls, cognitive function, and health-related quality of life after shunt-treated idiopathic normal pressure hydrocephalus-a single-center study. Acta Neurochir (Wien). 2022 Sep;164(9):2367-73.
7. Jeppsson A, Bjerke M, Hellström P, Blennow K, Zetterberg H, Kettunen P, et al. Shared CSF Biomarker Profile in Idiopathic Normal Pressure Hydrocephalus and Subcortical Small Vessel Disease. Front Neurol. 2022 Mar 3;13:839307.
8. Graff-Radford NR, Godersky JC. Normal-pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1986 Sep;43(9):940-2.
9. Mouton Paradot G, Baledent O, Sallioux G, Lehmann P, Gondry-Jouet C, Le Gars D. Apport de l'IRM de flux dans les hydrocéphalies à pression normale de l'adulte : intérêt prédictif dans les indications chirurgicales [Contribution of phase-contrast MRI to the management of patients with normal pressure hydrocephalus: Can it predict response to shunting?]. Neurochirurgie. 2010 Feb;56(1):50-4.
10. Kitagaki H, Mori E, Yamaji S, Ishii K, Hirono N, Kobashi S, et al. Frontotemporal dementia and Alzheimer disease: evaluation of cortical atrophy with automated hemispheric surface display generated with MR images. Radiology. 1998 Aug;208(2):431-9.
11. Takagi K, Watahiki R, Machida T, Onouchi K, Kato K, Oshima M. Reliability and Interobserver Variability of Evans' Index and Disproportionately Enlarged Subarachnoid Space Hydrocephalus as Diagnostic Criteria for Idiopathic Normal Pressure Hydrocephalus. Asian J Neurosurg. 2020;15(1):107-12.
12. Ishii K, Kanda T, Harada A, Miyamoto N, Kawaguchi T, Shimada K, et al. Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol. 2008;18:2678-83.
13. Eide PK, Pripp AH, Ringstad G. Magnetic resonance imaging biomarkers of cerebrospinal fluid tracer dynamics in idiopathic normal pressure hydrocephalus. Brain Commun. 2020;2(2):fcaa187.
14. Berger A, Constantini S, Ram Z, Roth J. Acute subdural hematomas in shunted normal-pressure hydrocephalus patients - Management options and literature review: A case-based series. Surg Neurol Int. 2018 Nov 28;9:238.
15. Farahmand D, Sæhle T, Eide PK, Tisell M, Hellström P, Wikkelsö C. A double-blind randomized trial on the clinical effect of different shunt valve settings in idiopathic normal pressure hydrocephalus. J Neurosurg. 2016 Feb;124(2):359-67.
16. Caruso R, Cervoni L, Vitale AM, Salvati M. Idiopathic normal-pressure hydrocephalus in adults: result of shunting correlated with clinical findings in 18 patients and review of the literature. Neurosurg Rev. 1997;20(2):104-7.
17. McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005 Oct;57(4):699-705; discussion 699-705.
18. Kim HS, Lee SU, Cha JH, Heo W, Song JS, Kim SJ. Clinical Analysis of Results of Shunt Operation for Hydrocephalus Following Traumatic Brain Injury. Korean J Neurotrauma. 2015 Oct;11(2):58-62.