Abstract
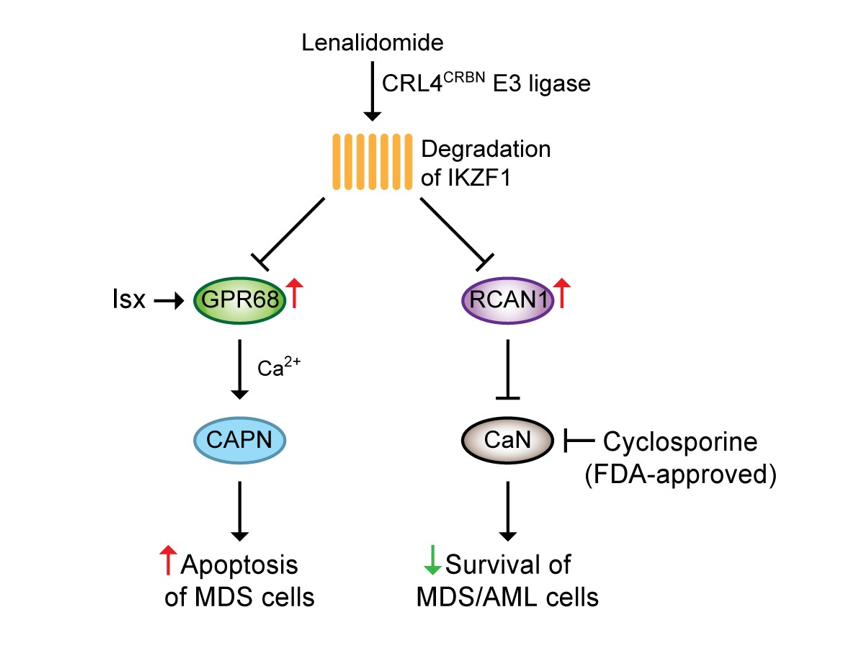
The immunomodulatory drug lenalidomide is used for the treatment of certain hematologic malignancies, including myelodysplastic syndromes (MDS). Lenalidomide interacts with cereblon (CRBN), a component of the CRL4CRBN E3 ubiquitin ligase complex, leading to ubiquitination and subsequent degradation of substrates, such as transcription factor Ikaros (Ikaros family zinc finger 1, IKZF1). With a genome loss of function screen, we recently identified two novel pathways mediated by lenalidomide in MDS. In this review, we summarized the major findings of these two pathways and their clinical implications. Depletion of G protein-coupled receptor 68 (GPR68) or an endogenous calcineurin (CaN) inhibitor, regulator of calcineurin 1 (RCAN1), reversed the inhibitory effect of lenalidomide on MDSL cells, an MDS cell line. Intriguingly, both GPR68 and RCAN1 expression levels were upregulated in MDSL cells after treatment with lenalidomide that was dependent on diminishment of IKZF1, indicating that IKZF1 functioned as a transcription repressor for GPR68 and RCAN1. Mechanistic studies revealed that upregulation or activation of GPR68 induced a Ca2+/calpain pro-apoptotic pathway, while upregulation of RCAN1 inhibited the CaN pro-survival pathway in MDSL cells. Notably, the pharmacological CaN inhibitor, cyclosporine, enhanced the sensitivity to lenalidomide in MDS as well as acute myeloid leukemia (AML). Surprisingly, pretreatment with lenalidomide reversed the immunosuppressive effects of cyclosporine on T lymphocytes. Our studies suggest that lenalidomide mediates degradation of IKZF1, leading to derepression of GPR68 and RCAN1 that activates the Ca2+/calpain pro- apoptotic pathway and inhibits the CaN pro-survival pathway, respectively. Our studies implicate that cyclosporine extends the therapeutic potential of lenalidomide to myeloid malignancies without compromising immune function.
Introduction
Thalidomide, lenalidomide and pomalidomide are synthetic immunomodulatory drugs (IMiDs) that have recently drawn attention in both clinics and basic research [1]. Thalidomide was synthesized from glutamic acid and was banned due to its teratogenicity in pregnant women [1]. Lenalidomide is a 4-amino-glutamyl analogue of thalidomide and is approved for the treatment of certain hematologic malignancies. Lenalidomide is used for the treatment of lower-risk red blood cell (RBC) transfusiondependent myelodysplastic syndromes (MDS) with deletion of chromosome 5q (del(5q)) with or without additional cytogenetic abnormalities [2-4]. MDS patients with del(5q) exhibit much higher hematologic and cytogenetic responses than those without del(5q) [3-6]. In contrast to lower-risk MDS patients, the response to lenalidomide monotherapy is poor in patients with higherrisk del(5q) MDS and acute myeloid leukemia (AML), especially in those with TP53 mutations [7,8]. Therefore, lenalidomide in combination with other drugs are being evaluated. Indeed, better responses are observed in patients with higher-risk del(5q) MDS and AML who are treated with lenalidomide in combination with hypomethylating agent azacitidine than lenalidomide monotherapy [8-11]. Despite a high response to lenalidomide in lowerrisk del(5q) MDS, half of the patients relapse within 2-3 years, which may be associated with the malignant MDS stem cells [5,12,13]. Accumulating evidence implicate that lenalidomide selectively inhibits the del(5q) clone, which is associated with modulation of several haploinsufficient genes that are localized on the deleted 5q regions, such as cell division cycles 25C (CDC25C) and protein phosphatase 2 phosphatase activator (PTPA, also known as PP2A), secreted protein acidic and cysteine rich (SPARC), ribosomal protein S14 (RPS14) and miR-145 [14-21].
In addition to the direct effects on the pathological MDS clones, lenalidomide also exerts pleiotropic effects on immune cells [22,23]. In response to lipopolysaccharide (LPS), peripheral blood mononuclear cells (PBMC) secreted less tumor necrosis factor alpha (TNFα), interleukin 1 beta (IL-1β) and interleukin 6 (IL-6) but more interleukin 10 (IL-10) in the presence of lenalidomide [24]. T cells express T cell receptor (TCR) and coreceptors (i.e. CD4 and CD8) that recognize the antigen peptides presented by major histocompatibility complex (MHC) on antigen presenting cells (APC). Upon recognizing antigen/MHC complex, TCR and coreceptors, together with the co- stimulatory signal provided by CD28 on T cells and B7 on APC, activate signaling pathways that lead to proliferation, survival, differentiation, cytokine secretion, expression of cytokine receptors and cytotoxicity of T cells [25]. Lenalidomide is shown to directly activate CD28 and the subsequent signaling pathways, resulting in secretion of interleukin 2 (IL-2) and interferon gamma (IFNγ) [26,27]. In addition, lenalidomide, in combination with dexamethasone, is approved for the treatment of multiple myeloma (MM) that is associated with its immunomodulatory effects [1,24,26-33].
Recently, cereblon (CRBN) is identified as a primary target that directly binds IMiDs and mediates the teratogenic and anti-tumor activities [34-36]. CRBN, together with CUL4, DDB1 and ROC1, forms the CRL4CRBN E3 ubiquitin ligase complex with CRBN as the substrate adaptor [34,37,38]. In the presence of lenalidomide, CRBN binds several proteins, such as Ikaros (Ikaros family zinc finger 1, IKZF1), Aiolos (Ikaros family zinc finger 3, IKZF3) and casein kinase 1 isoform alpha (CSNK1A1, also known as CK1α), leading to ubiquitination and subsequent degradation of these substrates by proteasome[39-42]. In order to better understand the mechanism of action of lenalidomide in MDS cells, we performed a genome-wide RNA interference screen and identified novel signaling pathways that modulated the sensitivity to lenalidomide in MDS/AML [43,44]. Here we summarized the major discoveries about the newly discovered signaling pathways mediated by lenalidomide in MDS/AML. Our studies provide insights into rational combinatorial therapy of lenalidomide in myeloid malignancies.
Lenalidomide regulates the GPR68/Ca2+/calpain pathway in MDS
In order to understand the mechanism of action of lenalidomide in MDS, we characterized the effects of lenalidomide on an MDS cell line, MDSL cells. The MDSL cells, initially derived from a low-risk MDS patients with del(5q) [45,46], contained a mixed populations of CD34+ and CD34- cells that behaved differentially in response to lenalidomide. In the presence of lenalidomide, the CD34- MDSL cells exhibited more Annexin V+ cells, while the CD34+ MDSL cells formed fewer colonies in semisolid methylcellulose. In liquid culture, both CD34+ and CD34- MDSL cells grew less efficiently in the presence of lenalidomide. Our findings suggested that lenalidomide exerted pleiotropic inhibitory effects on MDSL cells, including inhibition on growth, survival and clonogenicity. Intriguingly, depletion of CRBN reversed the inhibitory effects of lenalidomide on MDSL cells, indicating that lenalidomide acted through the CRL4CRBN E3 ubiquitin ligase complex.
In order to identify the genes that were critical for lenalidomide-mediated inhibitory effects on MDSL cells, we performed a genome-wide RNA interference screen in MDSL cells [43]. We found that depletion of a G proteincoupled receptor, GPR68, reversed the inhibitory effects of lenalidomide on MDSL cells. In addition, GPR68 mRNA and protein levels were upregulated in MDSL cells after treatment with lenalidomide. Among the identified targets of the CRL4CRBN E3 ubiquitin ligase complex (i.e. IKZF1, IKZF3 and CK1α), the promoter region of GPR68 gene locus contained binding peaks for IKZF1, indicating that IKZF1 may regulate GPR68 expression. As expected, depletion of IKZF1 increased GPR68 expression, while overexpression of wild type IKZF1, but not degradationresistant IKZF1, reduced GPR68 expression in MDSL cells, indicating that IKZF1 acted as a transcription repressor, repressing GPR68 expression. These results suggested that in the presence of lenalidomide, IKZF1 was degraded by the CRL4CRBN E3 ubiquitin ligase/proteasome system, leading to derepression of GPR68 in MDSL cells.
In response to extracellular protons or overexpression, GPR68 undergoes conformational changes, leading to association with G proteins (Gq/11) and subsequent inositol phosphate formation and cytosolic calcium (Ca2+) accumulation [47]. Further studies revealed that upregulation of GPR68 in MDSL cells upon treatment with lenalidomide led to accumulation of cytosolic Ca2+ ions that was required for lenalidomide-mediated inhibitory effect on MDSL cells. Screening of pharmacological inhibitors that targeted Ca2+ -related signaling pathways revealed that inhibition of calpain reversed apoptosis in MDSL cells after treatment with lenalidomide, indicating that lenalidomide activated a Ca2+/calpain pro-apoptotic pathway in MDSL cells through derepressing GPR68. Notably, 3,5-disubstituted isoxazoles (Isx), a GPR68 agonist [48], significantly increased cytosolic Ca2+ levels and apoptosis and reduced colony formation in MDSL cells in the presence of lenalidomide, indicating that both overexpression and activation of GPR68 could enhance lenalidomide-mediated inhibitory effects on MDSL cells.
Lenalidomide regulates the RCAN1/CaN pathway in MDS
Despite our observation in that GPR68 agonist Isx enhanced the inhibitory effects of lenalidomide on MDSL cells through inducing a Ca2+/calpain pro-apoptotic pathway, Isx has not been approved by the U.S. Food and Drug Administration (FDA) for any clinical applications. This prompted us to look for alternative candidates that could enhance the sensitivity to lenalidomide in MDS. From the genome-wide RNA interference screen in MDSL cells, we found that depletion of regulator of calcineurin 1 (RCAN1) also reversed the inhibitory effect of lenalidomide on MDSL cells. RCAN1 is the endogenous inhibitor of the serine/threonine phosphatase calcineurin (CaN) [49], a critical signaling molecule during T cell activation [50,51]. The pharmacological inhibitor of CaN, cyclosporine, is an FDA-approved drug that is used to prevent immune rejection after organ transplantation [52]. We therefore examined the effect of the RCAN1/CaN pathway on the sensitivity to lenalidomide in MDS.
Similar to GPR68, RCAN1 mRNA and protein levels were also upregulated in MDSL cells after treatment with lenalidomide. Intriguingly, depletion of IKZF1 also increased RCAN1 expression in MDSL cells, indicating that IKZF1 acted as a transcription repressor, repressing RCAN1 expression as well. These data suggested that through degrading IKZF1, lenalidomide derepressed the expression of both GPR68 and RCAN1 in MDSL cells. In contrast to GPR68, we failed to find any obvious binding peaks for IKZF1 in the promoter region of RCAN1 gene locus, indicating that IKZF1 may regulate RCAN1 expression through a different mechanism. Indeed, recent studies implicate IKZF1 functions as a tumor suppressor in T cell leukemia via global regulation of the enhancer/ super-enhancer landscape [53].
Consistent with the function of RCAN1 in T cells, depletion of RCAN1 in MDSL cells resulted in increased activity of CaN, indicating that RCAN1 also functioned as an inhibitor of CaN in MDSL cells. To understand the function of the RCAN1/CaN pathway in MDSL cells, we used cyclosporine to inhibit CaN activity. As expected, treatment of cyclosporine resulted in increased activity of CaN. Consistent with the function of CaN in T cells [50,51], treatment of cyclosporine resulted in increased Annexin V+ cells in MDSL cells, indicating that CaN was constitutively activated and provided a pro-survival signal in MDSL cells. Our results suggested that in addition to inducing the GPR68/Ca2+/calpain pro-apoptotic pathway, lenalidomide also inhibited the CaN pro-survival pathway via derepressing RCAN1 expression in MDSL cells. Lenalidomide crosslinked the GPR68/Ca2+/calpain and the RCAN1/CaN pathways through degrading IKZF1.
Cyclosporine enhances the sensitivity to lenalidomide in MDS
Given that CaN provided a pro-survival signal in MDSL cells, we examined the effect of cyclosporine on the sensitivity to lenalidomide in MDS. We pretreated MDSL cells with control or lenalidomide, followed by cotreatment with control or cyclosporine. Co-treatment with lenalidomide and cyclosporine induced more Annexin V+ cells than single treatment with lenalidomide or cyclosporine in MDSL cells. In addition, MDSL cells grew far fewer colonies in the presence of lenalidomide and cyclosporine than in the presence of lenalidomide only in methylcellulose. These results suggested that cyclosporine enhanced the sensitivity to lenalidomide in MDSL cells. In addition, we examined the effect of cyclosporine on the sensitivity to lenalidomide in primary bone marrow samples from MDS patients. We found more Annexin V+ cells in two MDS specimens after co-treatment with lenalidomide and cyclosporine than single treatment with lenalidomide or cyclosporine. Notably, one of the MDS patients was diagnosed with RAEBII, higher-risk MDS, indicating that cyclosporine enhanced the sensitivity to lenalidomide in both lower- and higher-risk MDS.
Cyclosporine enhances the sensitivity to lenalidomide in AML
We next examined the effect of cyclosporine on the sensitivity to lenalidomide in AML. TF-1 cells are a del(5q) AML cell line that is sensitive to lenalidomide. Similar to MDSL, co-treatment of lenalidomide and cyclosporine significantly increased Annexin V+ cells in TF-1 cells compared to single treatment with lenalidomide or cyclosporine. In addition, we examined the effect of cyclosporine on the sensitivity to lenalidomide in AML patient-derived xenograft (PDX) models. All three PDX models were derived from pediatric AML after relapse who failed to respond to chemotherapy [54]. Among the three PDX models, one was sensitive to lenalidomide as evidenced by increased Annexin V+ after treatment with lenalidomide. Co-treatment with lenalidomide and cyclosporine induced more Annexin V+ cells in the lenalidomide-sensitive PDX model than single treatment with lenalidomide or cyclosporine. Surprisingly, cotreatment with lenalidomide and cyclosporine induced apoptosis in the two PDX models that were resistant to lenalidomide. Intriguingly, the lenalidomidesensitive PDX model contained wild type p53, while the lenalidomide-resistant PDX models contained mutant p53, which was consistent with clinical observations in that p53 mutation was associated with resistance to lenalidomide [2,8]. In addition, the three PDX models contained MLL arrangements and complex karyotypes, indicating that cyclosporine enhanced the sensitivity to lenalidomide in AML irrespective of the cytogenetic aberrations.
Pretreatment of lenalidomide reverses the immunosuppressive effect of cyclosporine
Upon recognizing the antigen/MHC complex, TCR, coreceptors and co-stimulation activate a series of signaling pathways, among which CaN and the subsequent nuclear factor of activated T cells (NFAT) play a critical role during T cell activation. The CaN/NFAT pathway promotes the production of IL-2, resulting in proliferation and survival of T cells [55,56]. After organ transplantation, T cells play a major role mediating immune rejection. In clinics, high-dose cyclosporine is used to prevent immune rejection through inhibiting the CaN/NFAT pathway and T cell response [57,58]. In contrast, low-dose cyclosporine is used in miscellaneous pathological disorders [59-61]. Abnormal immune function is also implicated in the pathogenesis of MDS [62-64]. In lower-risk MDS, autologous T cells mediate apoptosis in both MDS cells and normal hematopoietic cells [65]. In higher-risk MDS, T cells fail to recognize antigen/MHC complex on APC due to inhibitory signals, such as programmed death-1 (PD-1) and its ligand programed death ligand 1 (PD-L1), leading to defective tumor surveillance [66]. Therefore, immunosuppressive therapy, such as cyclosporine, is used for patients with lower-risk MDS, while immune checkpoint inhibitors are used for patients with higher risk MDS [64,67-69]. We examined the combined effects of lenalidomide and cyclosporine on T cell response. T cells were harvested from spleens of C57Bl6 mice and activated in the presence of anti-CD3 and anti-CD28 antibodies. T cell activation was inhibited by co-treatment with lenalidomide and cyclosporine but not single treatment with lenalidomide, indicating that cyclosporine inhibited T cell activation. Intriguingly, when we pretreated T cells with lenalidomide, co-treatment with lenalidomide and cyclosporine didn’t inhibit T cell activation, indicating that pretreatment of lenalidomide reversed the inhibitory effect of cyclosporine on T cell activation. Lenalidomide directly binds human CRBN but not mouse Crbn due to a mutation within the binding domain [41]. Our results indicated that lenalidomide reversed the immunosuppressive effect of cyclosporine through a CRBN-independent manner. Lenalidomide-mediated direct activation of CD28 may explain the reversion of cyclosporine’s immunosuppressive effect on T cells, which needs further clarification.
Conclusion
Our recent studies identify that lenalidomide mediates degradation of IKZF1, leading to derepression of GPR68 and RCAN1 (Figure 1). Upregulation of GPR68 activates a Ca2+/calpain pro-apoptotic pathway in MDS cells. In addition, GPR68 agonist Isx also activates the Ca2+/calpain pro-apoptotic pathway, thus enhancing the cytotoxicity of lenalidomide in MDS. However, the fact that Isx is not an FDA-approved drug limits its clinical application. Upregulation of RCAN1 inhibits the CaN pro-survival pathway in MDS cells. The pharmacological inhibitor of CaN, cyclosporine, induces apoptosis in MDS/AML cells, thus enhancing the cytotoxicity of lenalidomide in MDS/ AML. Surprisingly, pretreatment of lenalidomide reverses the immunosuppressive effect of cyclosporine on T cells. Our studies provide the rational therapeutic combination of lenalidomide and cyclosporine in myeloid malignancies.
Declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors’ Contributions
A.D. and J.F. wrote and approved the manuscript.
Funding
This work was supported by NIH (R01CA218076), St. Baldrick’s Foundation, Aplastic Anemia & MDS International Foundation and NSF (1736150).
References
2. Komrokji RS, List AF. Short-and long-term benefits of lenalidomide treatment in patients with lower-risk del (5q) myelodysplastic syndromes. Annals of Oncology. 2016 Jan 1;27(1):62-8.
3. Lian XY, Zhang ZH, Deng ZQ, He PF, Yao DM, Xu ZJ, et al. Efficacy and safety of lenalidomide for treatment of low-/intermediate-1-risk myelodysplastic syndromes with or without 5q deletion: a systematic review and metaanalysis. PloS One. 2016 Nov 8;11(11):e0165948.
4. Montoro J, Yerlikaya A, Ali A, Raza A. Improving treatment for myelodysplastic syndromes patients. Current Treatment Options in Oncology. 2018 Dec 1;19(12):66.
5. List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. New England Journal of Medicine. 2006 Oct 5;355(14):1456-65.
6. Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusiondependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011 Oct 6;118(14):3765-76.
7. Ades L, Boehrer S, Prebet T, Beyne-Rauzy O, Legros L, Ravoet C, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood, The Journal of the American Society of Hematology. 2009 Apr 23;113(17):3947-52.
8. Möllgård L, Saft L, Treppendahl MB, Dybedal I, Nørgaard JM, Astermark J, et al. Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Haematologica. 2011 Jul 1;96(7):963-71.
9. Sekeres MA, Tiu RV, Komrokji R, Lancet J, Advani AS, Afable M, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higherrisk myelodysplastic syndromes. Blood, The Journal of the American Society of Hematology. 2012 Dec 13;120(25):4945-51.
10. Platzbecker U, Braulke F, Kündgen A, Götze K, Bug G, Schönefeldt C, et al. Sequential combination of azacitidine and lenalidomide in del (5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: a phase I study. Leukemia. 2013 Jun;27(6):1403-7.
11. Scherman E, Malak S, Perot C, Gorin NC, Rubio MT, Isnard F. Interest of the association azacitidine– lenalidomide as frontline therapy in high-risk myelodysplasia or acute myeloid leukemia with complex karyotype. Leukemia. 2012 Apr;26(4):822-4.
12. List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB. Efficacy of lenalidomide in myelodysplastic syndromes. New England Journal of Medicine. 2005 Feb 10;352(6):549-57.
13. Tehranchi R, Woll PS, Anderson K, Buza-Vidas N, Mizukami T, Mead AJ, et al. Persistent malignant stem cells in del (5q) myelodysplasia in remission. New England Journal of Medicine. 2010 Sep 9;363(11):1025-37.
14. Wei S, Chen X, McGraw K, Zhang L, Komrokji R, Clark J, et al. Lenalidomide promotes p53 degradation by inhibiting MDM2 auto-ubiquitination in myelodysplastic syndrome with chromosome 5q deletion. Oncogene. 2013 Feb;32(9):1110-20.
15. Wei S, Chen X, Rocha K, Epling-Burnette PK, Djeu JY, Liu Q, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proceedings of the National Academy of Sciences. 2009 Aug 4;106(31):12974-9.
16. Giagounidis A, Mufti GJ, Fenaux P, Germing U, List A, MacBeth KJ. Lenalidomide as a disease-modifying agent in patients with del (5q) myelodysplastic syndromes: linking mechanism of action to clinical outcomes. Annals of Hematology. 2014 Jan 1;93(1):1-1.
17. Pellagatti A, Jädersten M, Forsblom AM, Cattan H, Christensson B, Emanuelsson EK, et al. Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proceedings of the National Academy of Sciences. 2007 Jul 3;104(27):11406-11.
18. Venner CP, Woltosz JW, Nevill TJ, Deeg HJ, Caceres G, Platzbecker U, et al. Correlation of clinical response and response duration with miR-145 induction by lenalidomide in CD34+ cells from patients with del (5q) myelodysplastic syndrome. Haematologica. 2013 Mar 1;98(3):409-13.
19. Oliva EN, Cuzzola M, Nobile F, Ronco F, D’Errigo MG, Laganà C, et al. Changes in RPS14 expression levels during lenalidomide treatment in Low-and Intermediate-1-risk myelodysplastic syndromes with chromosome 5q deletion. European Journal of Haematology. 2010 Sep;85(3):231-5.
20. Stahl M, Zeidan AM. Lenalidomide use in myelodysplastic syndromes: insights into the biologic mechanisms and clinical applications. Cancer. 2017 May 15;123(10):1703-13.
21. Gaballa MR, Besa EC. Myelodysplastic syndromes with 5q deletion: pathophysiology and role of lenalidomide. Annals of Hematology. 2014 May 1;93(5):723-33.
22. Heise C, Carter T, Schafer P, Chopra R. Pleiotropic mechanisms of action of lenalidomide efficacy in del (5q) myelodysplastic syndromes. Expert Review of Anticancer Therapy. 2010 Oct 1;10(10):1663-72.
23. Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, et al. Mechanism of action of lenalidomide in hematological malignancies. Journal of Hematology & Oncology. 2009 Dec;2(1):1-0.
24. Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-a. The Journal of Immunology. 1999 Jul 1;163(1):380-6.
25. Sharpe AH, Abbas AK. T-cell costimulation–biology, therapeutic potential, and challenges. The New England Journal of Medicine. 2006 Sep 7;355(10):973-5.
26. LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004 Mar 1;103(5):1787-90.
27. Dredge K, Marriott JB, Todryk SM, Muller GW, Chen R, Stirling DI, et al. Protective antitumor immunity induced by a costimulatory thalidomide analog in conjunction with whole tumor cell vaccination is mediated by increased Th1-type immunity. The Journal of Immunology. 2002 May 15;168(10):4914-9.
28. Henry JY, Labarthe MC, Meyer B, Dasgupta P, Dalgleish AG, Galustian C. Enhanced cross-priming of naive CD8+ T cells by dendritic cells treated by the IM i D s® immunomodulatory compounds lenalidomide and pomalidomide. Immunology. 2013 Jul;139(3):377-85.
29. Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006 Jul 15;108(2):618-21.
30. Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid®(lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunology, Immunotherapy. 2008 Dec 1;57(12):1849-59.
31. Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunology, Immunotherapy. 2009 Jul 1;58(7):1033-45.
32. Görgün G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood, The Journal of the American Society of Hematology. 2010 Oct 28;116(17):3227-37.
33. Holstein SA, McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. 2017 Apr 1;77(5):505-20.
34. Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010 Mar 12;327(5971):1345-50.
35. Lopez-Girona AE, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012 Nov;26(11):2326-35.
36. Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood, The Journal of the American Society of Hematology. 2011 Nov 3;118(18):4771-9.
37. Xin W, Xiaohua N, Peilin C, Xin C, Yaqiong S, Qihan W. Primary function analysis of human mental retardation related gene CRBN. Molecular Biology Reports. 2008 Jun 1;35(2):251-6.
38. Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Molecular Cell. 2007 Jun 22;26(6):775-80.
39. Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014 Jan 17;343(6168):301-5.
40. Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014 Jan 17;343(6168):305-9.
41. Krönke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, et al. Lenalidomide induces ubiquitination and degradation of CK1a in del (5q) MDS. Nature. 2015 Jul;523(7559):183-8.
42. Guirguis AA, Ebert BL. Lenalidomide: deciphering mechanisms of action in myeloma, myelodysplastic syndrome and beyond. Current Opinion in Cell Biology. 2015 Dec 1;37:61-7.
43. Fang J, Liu X, Bolanos L, Barker B, Rigolino C, Cortelezzi A, et al. A calcium-and calpain-dependent pathway determines the response to lenalidomide in myelodysplastic syndromes. Nature Medicine. 2016 Jul;22(7):727-34.
44. He X, Dou A, Feng S, Roman-Rivera A, Hawkins C, Lawley L, Zhang J, Wunderlich M, Mizukawa B, Halene S, Patel A. Cyclosporine enhances the sensitivity to lenalidomide in MDS/AML in vitro. Experimental Hematology. 2020 May;86:21-27 e2.
45. Matsuoka A, Tochigi A, Kishimoto M, Nakahara T, Kondo T, Tsujioka T, et al. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia. 2010 Apr;24(4):748-55.
46. Tohyama K, Tohyama Y, Nakayama T, Ueda T, Nakamura T, Yoshida Y. A novel factor-dependent human myelodysplastic cell line, MDS92, contains haemopoietic cells of several lineages. British Journal of Haematology. 1995 Dec;91(4):795-9.
47. Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Protonsensing G-protein-coupled receptors. Nature. 2003 Sep;425(6953):93-8.
48. Russell JL, Goetsch SC, Aguilar HR, Coe H, Luo X, Liu N, et al. Regulated expression of pH sensing G Proteincoupled receptor-68 identified through chemical biology defines a new drug target for ischemic heart disease. ACS Chemical Biology. 2012 Jun 15;7(6):1077-83.
49. Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. Journal of Biological Chemistry. 2000 Mar 24;275(12):8719-25.
50. Rusnak F, Mertz P. Calcineurin: form and function. Physiological reviews. 2000 Jan 10;80(4):1483-521.
51. Hayden-Martinez K, Kane LP, Hedrick SM. Effects of a constitutively active form of calcineurin on T cell activation and thymic selection. The Journal of Immunology. 2000 Oct 1;165(7):3713-21.
52. Hamawy MM. Molecular actions of calcineurin inhibitors. Drug news & perspectives. 2003 Jun;16(5):277.
53. Ding Y, Zhang B, Payne JL, Song C, Ge Z, Gowda C, et al. Ikaros tumor suppressor function includes induction of active enhancers and super-enhancers along with pioneering activity. Leukemia. 2019 Nov;33(11):2720-31.
54. Wunderlich M, Brooks RA, Panchal R, Rhyasen GW, Danet-Desnoyers G, Mulloy JC. OKT3 prevents xenogeneic GVHD and allows reliable xenograft initiation from unfractionated human hematopoietic tissues. Blood. 2014 Jun 12;123(24):e134-44.
55. Shaw JP, Utz PJ, Durand DB, Toole JJ, Emmel EA, Crabtree GR. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202- 5.
56. Chen L, Rao A, Harrison SC. Signal integration by transcription-factor assemblies: interactions of NF-AT1 and AP-1 on the IL-2 promoter. InCold Spring Harbor Symposia on Quantitative Biology 1999 Jan 1;64:527-532.
57. Henderson DJ, Naya I, Bundick RV, Smith GM, Schmidt JA. Comparison of the effects of FK-506, cyclosporin A and rapamycin on IL-2 production. Immunology. 1991 Jul;73(3):316.
58. Medeiros M, Lumini J, Stern N, Castañeda- Hernández G, Filler G. Generic immunosuppressants. Pediatric Nephrology. 2018 Jul 1;33(7):1123-31.
59. Lowe NJ, Wieder JM, Rosenbach A, Johnson K, Kunkel R, Bainbridge C, et al. Long-term low-dose cyclosporine therapy for severe psoriasis: effects on renal function and structure. Journal of the American Academy of Dermatology. 1996 Nov 1;35(5):710-9.
60. Bagnis CI, du Montcel ST, Beaufils H, Jouanneau C, Jaudon MC, Maksud P, et al. Long-term renal effects of low-dose cyclosporine in uveitis-treated patients: followup study. Journal of the American Society of Nephrology. 2002 Dec 1;13(12):2962-8.
61. Kessel A, Toubi E. Low-dose cyclosporine is a good option for severe chronic urticaria. Journal of Allergy and Clinical Immunology. 2009 Apr 1;123(4):970.
62. Montoro J, Gallur L, Merchán B, Molero A, Roldán E, Martínez-Valle F, et al. Autoimmune disorders are common in myelodysplastic syndrome patients and confer an adverse impact on outcomes. Annals of Hematology. 2018 Aug 1;97(8):1349-56.
63. Komrokji RS, Kulasekararaj A, Al Ali NH, Kordasti S, Bart-Smith E, Craig BM, et al. Autoimmune diseases and myelodysplastic syndromes. American Journal of Hematology. 2016 May;91(5):E280-3.
64. Glenthøj A, Ørskov AD, Hansen JW, Hadrup SR, O’Connell C, Grønbæk K. Immune mechanisms in myelodysplastic syndrome. International Journal of Molecular Sciences. 2016 Jun;17(6):944.
65. Baumann I, Scheid C, Koref MS, Swindell R, Stern P, Testa NG. Autologous lymphocytes inhibit hemopoiesis in long-term culture in patients with myelodysplastic syndrome. Experimental Hematology. 2002 Dec 1;30(12):1405-11.
66. Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PDL2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014 Jun;28(6):1280-8.
67. Stahl M, Bewersdorf JP, Giri S, Wang R, Zeidan AM. Use of immunosuppressive therapy for management of myelodysplastic syndromes: a systematic review and meta-analysis. Haematologica. 2020 Jan 1;105(1):102-11.
68. Platzbecker U. Treatment of MDS. Blood, The Journal of the American Society of Hematology. 2019 Mar 7;133(10):1096-107.
69. Stahl M, DeVeaux M, De Witte T, Neukirchen J, Sekeres MA, Brunner AM, et al. The use of immunosuppressive therapy in MDS: clinical outcomes and their predictors in a large international patient cohort. Blood Advances. 2018 Jul 24;2(14):1765-72.