Abstract
Gold nanoparticle (AuNP) bioconjugates are increasingly being utilized in biomedicine due to their low toxicity on biological tissues and unique electronic and chemical properties. They have been utilized in several biological applications, namely the manufacture of nanomaterials, biosensing, electron microscopy, and drug delivery systems. Mainly, immuno-assays often employ gold nanoparticles (AuNPs) to enhance the detection of a biological component. This paper presents a study on the bioconjugation of AuNPs with Horse Radish Peroxidase conjugated Human Papilloma Virus 16/18 Early 6 antibodies (CIP5) against Early 6 (E6) oncoprotein that is overexpressed in cervical carcinoma progression through physical adsorption. This bioconjugate can be employed in a diagnostic immunoassay for cervical cancer screening. The study also demonstrated that the antibody pI, gold colloidal solution pH, and amount of antibody determine the generation of stable Antibody–AuNPs bioconjugates
Keywords
Bioconjugates, Gold nanoparticles, Nanobiotechnology, Human papillomavirus virus, Early 6 (E6) protein, Immunoassay
Introduction
HPV 16/18 E6 oncoprotein has been evaluated as a useful biomarker with prognostic abilities as it can detect pre-cancer and cancerous states of cervical cancer progression [1-3]. A positive E6 assay indicates a high correlation to the cervical cancerous phenotype, not the potential for cervical cancer, thus high specificity in triaging patients during screening [4-6]. E6 levels of expression associate directly with the severity of cervical intraepithelial neoplasia (CIN) lesions and the risk of progression to invasive carcinoma [7].
Although the current screening interventions utilizing the E6 oncoprotein from high-risk HPV strains have significantly high specificity, the sensitivity is relatively low. OncoE6 test showed 69.6% sensitivity for High-Grade Squamous Intraepithelial Lesion (HSIL) (CIN3+) precancerous lesions [3]. HPV16/18/45 E6 test produced sensitivity and specificity of CIN2+ as 42.8% and 94.3%, respectively, and for CIN3+ as 54.2% and 93.8%, respectively [8,9]. More advanced methods of detecting E6 oncoprotein are therefore required to produce a sensitivity between 76.3%-97.2%, which is indicative of diagnostic test quality [10].
Nanobiotechnology is being used in cancer diagnostics to enhance detection. Nanotechnology refers to the utilization of materials at nanoscale ranging from 1 to 100 nm in scientific fields such as chemistry, biology, physics, and engineering [11-14]. At the nanoscale, these materials exhibit different properties from bulk material [15]. Nanobiotechnology is hence the application of nanosized tools and systems in the study of biological phenomena. It is applied in prevention (nano vaccines), diagnosis (in vivo diagnostics and in-vitro diagnostics), and treatment (drug delivery) of cancer nanooncology of human diseases [16,17]. The types of nanomaterials include metalbased (gold (Au) and silver (Ag) nanoparticles), oxides (superparamagnetic iron oxide (SPIO) nanoparticles, silver (Ag) oxide nanoparticles), liposomes, dendrimers, quantum dots, nanosphere, carbon nanotube, nanofibers, nanolayers like graphene sheets, biopolymers, among others [18].
Both in vivo diagnostics and in vitro diagnostics utilize biosensors for cancer diagnosis. Biosensor devices contain a biological recognition element capable of detecting the presence of a specified biological analyte such as proteins, isoenzymes, nucleic acids, metabolites or hormones, and a transducer that converts the biochemical signal into a quantifiable electrical signal [19]. The transducers depend on the signal from biological recognition element: optical transducer (luminescence, fluorescence, interferometry and colorimetric), magnetic (electrical, magnetic force), electrochemical (amperometry, potentiometry, and conductometry), calorimetric (heat thermistors), mechanical (force), piezoelectric (pressure or mass changes) [19]. In vivo sensors consist of quantum dots, nanoshells, among others, to detect biochemical changes in biological systems through imaging, while in vitro sensors utilize nanoparticles, nanowires, graphene sheets, among others [20].
Biosensors are increasingly being used in cancer diagnostics, primarily because in cancer development, cancer-specific biomarkers are elevated or reduced; hence their detection and identification enhance diagnosis [19]. Biosensors are also applicable at the point of care testing to enable screening. They exhibit high sensitivity, specificity, reproducibility, and cheap instrumentation [19]. Early cervical cancer diagnostics have been improved by the application of nanotechnologies [21]. For example, the ultra-bright fluorescent mesoporous silica nanoparticle has been utilized to detect folate (Fa) receptors on cervical cells surface and fluoresce. The brighter the fluorescence, the more, the more advanced cervical carcinoma [22]. Secondly, adding monodispersed inorganic silica nanoparticles into organic dyes used to detect HR-HPV DNA using DNA microarrays has amplified the DNA microarray technology signal [23]. Thirdly, nanotechnology has been used to detect proteins; for example, the piezoelectric (contains quartz crystals) immuno-sensor that detects protein p16INK4a for early cervical cancer detection [24].
Gold nanoparticle (AuNP) bioconjugates in particular, are increasingly being utilized in several biological applications. AuNPs have low toxicity on biological tissues. Additionally, they possess characteristic optical and electronic properties, such as enhanced absorption and/or scattering at certain resonance wavelengths. When subjected to irradiation, resonant oscillations of the metal free electrons across the nanoparticles are induced, a phenomenon called Surface plasmon resonance (SPR) [25-29]. These oscillations lie within visible frequencies and result in the strong characteristic optical absorbance and scattering properties of the AuNPs [29]. These resonance wavelengths are highly dependent on the morphology, distance between the particles and dielectric medium of the AuNPs [28,29]. A colloidal gold solution of 20 nm particles size has an SPR band with a lambda maximum of about 520 nm that gives the solution a red color. As the core/shell ratio increases, the SPR band exhibits a red-shift due to increase in wavelength by a few nanometers [26,29,30]. When there is a shift from red to blue color, it’s an indication of plasmonplasmon interactions due to possible gold nanoparticle aggregation. This property is utilized when AuNP-labeled conjugates bind to their target leading to aggregation of the nanoparticles and a detectable shift in the optical signal hence a colorimetric assay [31,32].
This study developed a bioconjugate of gold nanoparticles with HPV 16 E6/18 E6-HRP (C1P5) antibody that can be utilized to sensitively detect E6 oncoprotein biomarker in cervical cancer screening in a colorimetric assay.
Materials and Methods
Bioconjugation preparation
The pH of 500 μl mouse IgG1 HPV 16 E6/18 E6- HRP (CP15) monoclonal antibodies (Santa Cruz Biotechnologies, USA Cat# sc-460) was determined using a pH Meter. The pH of 1000 μl 20nm colloidal spherical monodispersed citrate stabilized gold nanoparticles (Sigma Aldrich) was adjusted to a pH unit of 0.5 higher than the iso-electric point (IEP) of the antibody molecule to be adsorbed using 20 mM borate buffer pH 8.7. Using Jenway UV- VIS Spectrophotometer Model 6800, the lambda maximum of the colloidal gold was measured before bioconjugation.
Bioconjugation optimization
A bioconjugation optimization protocol was carried out to determine the amount of antibody needed for maximum bioconjugation. This was accomplished by diluting 200 μg/ml HPV 16 E6/18 E6-HRP (CP15) antibodies in 1X PBS buffer pH 7.4 (Beijing Solarbio Science & Technology Co. Ltd) a range of 0 mg/ml to 0.012 mg/ml. To each dilution, 100 μl of the gold solution was added. This mixture was incubated for two hours to allow for passive adsorption [33]. The absorption spectra of each mixture were obtained to confirm the concentration at which bioconjugation took place using Jenway Model 6800 UVVIS Spectrophotometer [27].
Bioconjugation confirmation using Spectrophotometer
The absorbance spectrum was obtained for all dilutions through a scan within the standard range of wavelengths 400-900 nm. From the collected absorbance spectrum, the absorbance maximum (λmax) was determined.
Preparation of HPV 16 E6/18 E6-HRP (CP15)- AuNPS conjugate for downstream processes
About 100 μl of AuNPs was added into an Eppendorf tube, and the pH was adjusted to pH 7 using 1X PBS at pH 7.4. 200 μg/ml of the antibody was diluted to 4 μg/ ml by adding 20 μl of stock to 980 μl 1X PBS buffer to make four μg/ml concentration of the antibody. 900 μl of the antibody concentration obtained above, was added to the 100 μl AuNPs to make a final solution of 1000 μl. The antibody-gold nanoparticle solution was incubated for two hours at 4°C to allow passive adsorption. The solution was centrifuged at 2500 rpm for 1 minute to remove unbound antibody and supernatant discarded. The pelleted HPV 16 E6/18 E6-HRP (CP15) - AuNPS conjugate was resuspended in 1X PBS buffer pH 7.4. The centrifugation was repeated, and the pelleted conjugate stored undiluted at 4°C.
Results
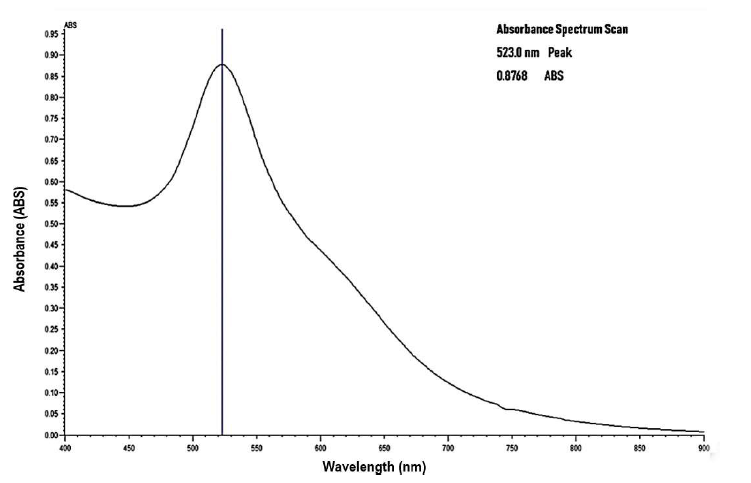
The pH of 500 μl HPV 16 E6/18 E6-HRP (CP15) antibody was obtained as pH 7.05. The pH of 1000 μl of the colloidal gold solution was adjusted to pH 7.83, a pH unit of about 0.5 higher than the iso-electric point (IEP) of the antibody molecule using 20 mM borate buffer pH 8.7 [33]. The maximum absorption for the 20 nm, OD 1 gold nanoparticles with a concentration of 6.54 x 1011 particles/mL, a peak SPR wavelength of 518-524 nm and extinction coefficient, of 9.21 x 108 (M-1 cm-1 before conjugation was at 523 nm as shown on Figure 1 [25].
Figure 1: Absorption Spectra of Gold Nanoparticles before conjugation using Jenway Model 6800 UV- VIS Spectrophotometer.
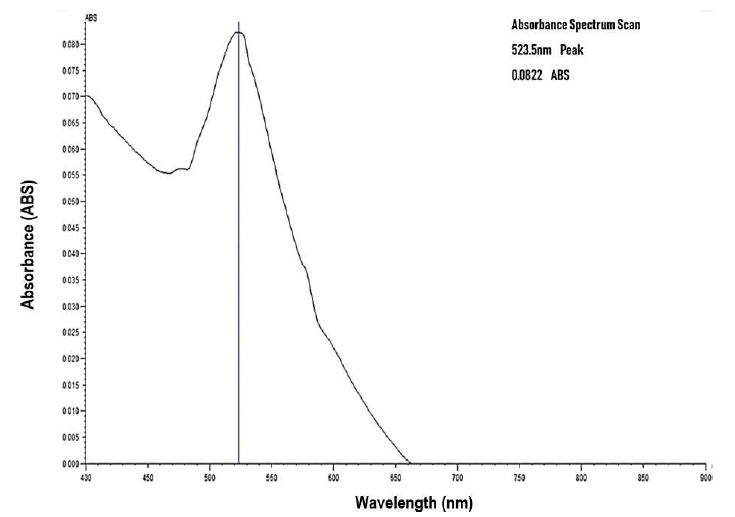
Figure 2: Absorption Spectra of Gold Nanoparticles after conjugation with 4.0 μg/ml concentration of antibody using Jenway Model 6800 UV- VIS Spectrophotometer.
After two hours of incubation, a redshift of lambda maximum by 0.5nm was observed at antibody concentration 4.0 μg/ml from the initial 523.0 nm as illustrated in Figure 2.
The Lambda maximum obtained for the range of antibody concentrations 0 mg/ml to 0.012 mg/ml is outlined in Table 1. Only one concentration exhibited a red shift.
| HPV 16 E6/18 E6-HRP antibody concentration (μg/ml) |
Absorption Maximum after 2 h incubation (nm) |
|---|---|
| 0 | 523.0 |
| 0.3 | 519.5 |
| 0.6 | 520.0 |
| 0.9 | 520.5 |
| 1.2 | 521.0 |
| 2.0 | 522.0 |
| 4.0 | 523.5 |
| 6.0 | 522.0 |
| 8.0 | 522.0 |
| 10.0 | 521.0 |
| 12.0 | 521.5 |
Discussion
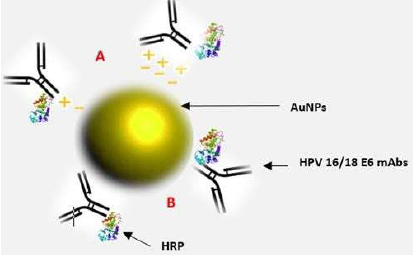
Bioconjugation of AuNPs with antibody against E6 oncoprotein occurs upon the successful binding of the monoclonal antibodies to the AuNPs surface, causing the surface plasmon resonance (SPR) spectrum to redshift, that is, increase in wavelength by a few nanometers with a color shift from red to blue [26,29]. The redshift indicated in this study was 0.5 nm. The SPR absorption of AuNPs is dependent on size. Normally, the SPR absorption maxima ranging from 517 nm to about 575 nm are exhibited by AuNPs with diameter range from 9 nm to 99 nm [26,29,31]. The SPR in the study is 523 nm which corresponds to about 520 nm within the range of 517 nm and 575 nm [25]. The antibody replaces the negative charge citrate capping on the surface of citrate stabilized colloidal gold nanoparticles. Therefore, both electrostatic and hydrophobic interactions involved. This is called passive adsorption a non-covalent method of bioconjugation (Figure 3) [27,33,34].
Figure 3: Showing bioconjugate: HPV 16 E6/18 E6-HRP (CP15)-AuNPs. It comprises 20 nm citrate stabilized monodispersed spherical AuNPs passively adsorbed to horseradish peroxidase (HRP) enzymelabeled HPV 16/18 E6 (C1P5) mouse monoclonal antibodies through (A) electrostatic and (B) hydrophobic interactions.
For optimal bioconjugation to occur, the following critical steps were involved. Firstly, the adjustment of the pH of the colloidal gold solution to pH 7.83, a pH unit of about 0.5 higher than the iso-electric point (IEP) of the antibody molecule to be adsorbed whose isoelectric point is about pH 7.00 [35]. This ensured maximum hydrophobic interactions that are exhibited at the IEP of antibody; hence maximal bioconjugation was achieved [34,36].
Secondly, the concentration of antibody needed was optimized through titrations of the antibody as too little antibody adsorbed to the gold surface, will cause aggregation upon addition of electrolytes present in standard buffers. Bioconjugation occurred at 4.0μg/ml HPV 16 E6/18 E6- HRP (CP15) antibody concentration. The bioconjugate formed was prepared and pelleted conjugate stored undiluted at 4°C for use in E6 oncoprotein biomarker detection in cervical cancer screening. It can be stored for 12-18 months.
The absorption spectrum obtained after bioconjugation was fairly smooth (Figure 2) due to possible antibody contamination with small contaminants such as sodium azide. Dialyzing the antibody with a buffer solution is highly recommended in subsequent analysis. It could also arise from contamination of gold colloidal solution during pipetting hence aliquoting the gold solution during use is to be maintained. Despite the challenges, passive adsorption of gold nanoparticles to antibody was achieved, and testing the antibody sensitivity on E6 protein will further verify the findings.
Early cervical cancer diagnostics have been improved by the application of nanotechnologies [21,31]. Mainly, immuno-assays often employ gold nanoparticles (AuNPs) to enhance the detection of a biological component. Gold nanoparticle (AuNP) bioconjugates are increasingly being utilized in biomedicine due to their low toxicity on biological tissues and unique electronic and chemical properties such as unique surface characteristics, optical properties, and consistency [31,32,37]. The Bioconjugate herein uses an antibody conjugated to horseradish peroxidase (HRP) enzyme; thus, conjugation to AuNPs will further enhance visual enzyme-linked immunosorbent assay (ELISA) based colorimetric detection of the E6 oncoprotein analyte [38].
Conclusion
It was possible to conjugate mAbs against HPV 16/18 E6 oncoprotein to 20 nm citrate stabilized gold nanoparticles through passive adsorption. The bioconjugate formed was HPV 16 E6/18 E6- HRP (CP15)-AuNPs. Currently, there is no report on the development of a gold nanoparticle bioconjugate with mAbs against the E6 protein of HPV 16/18 to enhance the signal of the immunoassay involving the E6 protein and the antibody thereof. This HPV 16 E6/18 E6-HRP (CP15)-AuNPs conjugate can be utilized in immunoassays for further verification of the bioconjugate’s bio-functionality.
Acknowledgments
We would like to express our gratitude to the staff at Molecular Biology and biotechnology PAUSTI Laboratory and University of Nairobi Histopathology laboratory for their support and creating a conducive environment in which the lab investigations were carried out, Dr. Dickson Andala for the support in the preliminary stages of the research study and James G. Maina for valuable feedback during the research period.
References
2. Zhang JJ, Cao XC, Zheng XY, Wang HY, Li YW. Feasibility study of a human papillomavirus E6 and E7 oncoprotein test for the diagnosis of cervical precancer and cancer. Journal of International Medical Research. 2018 Mar;46(3):1033-42.
3. Schweizer J, Berard-bergery M, Bisht A, Ho M, Ho T, Labiad Y, et al. E6 Based Rapid Diagnostic Test for Cervical Pre-Cancer and Cancer. 2010;58. Available from: http://arborvita.com/news_press/media/ AVantageHPVE6Dx-1.pdf
4. Justin J, Burns M. Large Study Evaluating Screening Tests Including HPV E6 Test for Cervical Cancer Screening. Interim Findings Presented at 27th International Papillomavirus Conference in Berlin, Germany [Internet]. 2011 [cited 2018 Oct 9]. 2011. Available from: http://arborvita.com/news_press/pr_ avdxberlininterim_09192011.php
5. Mantovani F, Banks L. The Human Papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001 Nov;20(54):7874–87.
6. Jiang P, Yue Y. Human papillomavirus oncoproteins and apoptosis. Experimental and Therapeutic Medicine. 2014 Jan 1;7(1):3-7.
7. Shi WJ, Liu H, Wu D, Tang ZH, Shen YC, Guo L. E6/ E7 proteins are potential markers for the screening and diagnosis of cervical pre cancerous lesions and cervical cancer in a Chinese population. Oncology letters. 2017 Nov 1;14(5):6251-8
8. Andreas M. Kaufmann P. Screening Methods for HPV and Dysplasia Detection - ppt download [Internet]. Clinic for Gynecology,Charité-Universitätsmedizin Berlin, Germany © AMK; 2017. p. 1–38. Available from: https://slideplayer.com/slide/12137628/
9. Mariano VS, Lorenzi AT, Scapulatempo-Neto C, Stein MD, Resende JC, Antoniazzi M, Villa LL, Levi JE, Longatto-Filho A, Fregnani JH. A Low-cost HPV immunochromatographic assay to detect high-grade cervical intraepithelial neoplasia. PloS one. 2016;11(10): e0164892.
10. Qiao YL, Jeronimo J, Zhao FH, Schweizer J, Chen W, Valdez M, Lu P, Zhang X, Kang LN, Bansil P, Paul P. Lower cost strategies for triage of human papillomavirus DNA-positive women. International journal of cancer. 2014 Jun 15;134(12):2891-901.
11. Goksuluk D, Korkmaz S, Zararsiz G, Karaagaoglu AE. easyROC: an interactive web-tool for ROC curve analysis using R language environment. RJ. 2016 Dec 1;8(2):213-30.
12. Mohamed WS, Alzaid M, SM Abdelbaky M, Amghouz Z, García-Granda S, M Abu-Dief A. Impact of Co2+ Substitution on Microstructure and Magnetic Properties of CoxZn1-xFe2O4 Nanoparticles. Nanomaterials. 2019 Nov;9(11):1602.
13. Mohamed WS, Abu-Dief AM. Synthesis, characterization and photocatalysis enhancement of Eu2O3-ZnO mixed oxide nanoparticles. Journal of Physics and Chemistry of Solids. 2018 May 1;116:375-85.
14. Abu-Dief AM, Hamdan SK. Functionalization of magnetic nano particles: synthesis, characterization and their application in water purification. American Journal of Nanosciences. 2016 Nov 9;2(3):26-40.
15. Abu-Dief AM, Abdelbaky MS, Martínez-Blanco D, Amghouz Z, García-Granda S. Effect of chromium substitution on the structural and magnetic properties of nanocrystalline zinc ferrite. Materials Chemistry and Physics. 2016 May 1;174:164-71.
16. Islam MT, Uddin MA. Biosensors, the emerging tools in the identification and detection of cancer markers. Journal of Gynecology and Women’s Health. 2017;5(4):555667.
17. Mansoori GA, Mohazzabi P, McCormack P, Jabbari S. Nanotechnology in cancer prevention, detection and treatment: bright future lies ahead. World Review of Science, Technology and Sustainable Development. 2007 Jan 1;4(2):226.
18. AAH A-DA and A-M. SF Journal of Nanochemistry and Nanotechnology Functionalization of Magnetic Nanoparticles for Drug. 2018;1(May):1–6.
19. Patel SP, Patel PB, Parekh BB. Application of nanotechnology in cancers prevention, early detection and treatment. Journal of cancer research and therapeutics. 2014 Jul 1;10(3):479.
20. Jainish P, Prittesh P. Biosensors and biomarkers: promising tools for cancer diagnosis. Int J Biosen Bioelectron. 2017;3(4):00072.
21. Jaishree V, Gupta PD. Nanotechnology: a revolution in cancer diagnosis. Indian Journal of Clinical Biochemistry. 2012 Jul 1;27(3):214-20.
22. Chen J, Gu W, Yang L, Chen C, Shao R, Xu K, Xu ZP. Nanotechnology in the management of cervical cancer. Reviews in medical virology. 2015 Mar;25:72-83.
23. Palantavida S, Guz NV, Woodworth CD, Sokolov I. Ultrabright fluorescent mesoporous silica nanoparticles for prescreening of cervical cancer. Nanomedicine: Nanotechnology, Biology and Medicine. 2013 Nov 1;9(8):1255-62.
24. Yang L, Huang X, Sun L. A piezoelectric immunosensor for early cervical cancer detection. Asian Pacific Journal of Cancer Prevention. 2014 Jan 1;15(21):9375-8.
25. Yang L, Huang X, Sun L. A piezoelectric immunosensor for early cervical cancer detection. Asian Pacific Journal of Cancer Prevention. 2014 Jan 1;15(21):9375-8.
26. Sigma-Aldrich. Gold Nanoparticles: Properties and Applications | Sigma-Aldrich [Internet]. Sigma-Aldrich Co. 2016 [cited 2020 May 7]. Available from: https:// www.sigmaaldrich.com/technical-documents/articles/ materials-science/nanomaterials/gold-nanoparticles. html
27. Introduction to Gold Nanoparticle Characterization – Cytodiagnostics [Internet]. [cited 2019 May 15]. Available from: http://www.cytodiagnostics.com/store/ pc/viewcontent.aspidpage=3
28. Jazayeri MH, Amani H, Pourfatollah AA, Pazoki- Toroudi H, Sedighimoghaddam B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sensing and bio-sensing research. 2016 Jul 1;9:17-22.
29. Ogarev VA, Rudoi VM, Dement’eva OV. Gold nanoparticles: synthesis, optical properties, and application. Inorganic Materials: Applied Research. 2018 Jan 1;9(1):134-40.
30. Azzazy HM, Mansour MM, Samir TM, Franco R. Gold nanoparticles in the clinical laboratory: principles of preparation and applications. Clinical Chemistry and Laboratory Medicine (CCLM). 2012 Feb 1;50(2):193-209.
31. Jain PK, El-Sayed IH, El-Sayed MA. Au nanoparticles target cancer. nano today. 2007 Feb 1;2(1):18-29.
32. Radwan SH, Azzazy HM. Gold nanoparticles for molecular diagnostics. Expert review of molecular diagnostics. 2009 Jul 1;9(5):511-24.
33. Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. Gold nanoparticles for the development of clinical diagnosis methods. Analytical and bioanalytical chemistry. 2008 Jun 1;391(3):943-50.
34. Ciaurriz P, Fernández F, Tellechea E, Moran JF, Asensio AC. Comparison of four functionalization methods of gold nanoparticles for enhancing the enzymelinked immunosorbent assay (ELISA). Beilstein journal of nanotechnology. 2017 Jan 25;8(1):244-53.
35. van de Plas P, Willems S, Leunissen J. GOLD NANOPARTICLE CONJUGATION Adsorption or Covalent Binding[Internet]. 2019 [cited 2019 May 15]. Available from: www.ScienceServices.eu
36. Agrisera. Molecular weight and isoelectric point of various immunoglobulins [Internet]. 2019 [cited 2019 Aug 29]. Available from: https://www.agrisera.com/en/ info/molecular-weight-and-isoelectric-point-of-variousimmunoglobulins. html
37. Ruiz G, Tripathi K, Okyem S, Driskell JD. PH Impacts the Orientation of Antibody Adsorbed onto Gold Nanoparticles. Bioconjugate Chemistry. 2019 Apr 17;30(4):1182–91.
38. Busch RT, Karim F, Weis J, Sun Y, Zhao C, Vasquez ES. Optimization and Structural Stability of Gold Nanoparticle-Antibody Bioconjugates. ACS Omega. 2019 Sep 17;4(12):15269–79.
39. Billingsley MM, Riley RS, Day ES. Antibodynanoparticle conjugates to enhance the sensitivity of ELISA-based detection methods. PloS one. 2017;12(5).:e0177592.