Abstract
Background: HIV/AIDS remains a major global public health challenge despite significant progress in treatment. New infections and HIV-related deaths persist, fueled by disparities in prevention and care access.
Purpose: This review synthesizes recent advances across key domains - from vaccine development to novel treatments to omics approaches – that collectively hold promise for ending the HIV/AIDS pandemic.
Main body: Multiple innovative HIV vaccine platforms are now in early-phase trials, including mRNA vaccines as well as conserved epitope and mosaic constructs for broader immunogenicity. Long-acting injectable antiretrovirals represent a major milestone in HIV treatment, while gene editing techniques offer future curative potential. Leveraging multi-omics data through genomics, transcriptomics, proteomics, and metabolomics provides systems-level insights into viral persistence and new therapeutic opportunities. The gut microbiome is increasingly recognized as a mediator of HIV progression, spurring research into probiotic/prebiotic supplementation and fecal transplantation. Across these domains, integration of artificial intelligence and machine learning will likely accelerate discovery.
Conclusion: Despite past setbacks, the HIV cure effort has renewed momentum. Translating emerging tools like long-acting antiretrovirals and omics profiling into broader clinical application could bend the pandemic’s trajectory. Innovation must be paired with ensuring equitable access to maximize global impact.
Keywords
HIV, AIDS, Vaccine, Antiretroviral, Gene editing, CRISPR, Multi-omics, Microbiome, Artificial intelligence
Background
HIV/AIDS originated from multiple cross-species transmissions of simian immunodeficiency viruses (SIVs) found in African primates to humans [1]. SIVs likely jumped to humans in the early 1900s when humans hunted chimpanzees and monkeys for meat [2]. The virus further adapted in humans until the current pandemic HIV strain emerged around the 1920s. The origins of the global pandemic were traced back to the Democratic Republic of Congo around the 1920s, from where the virus spread globally along transportation routes [3].
HIV is transmitted between humans primarily through sexual contact, sharing of contaminated needles, and vertical transmission from mother to child [4]. The virus progressively impairs the immune system by attacking and destroying CD4 T cells, resulting in life-threatening opportunistic infections [5]. Without treatment, most HIV-infected individuals will develop AIDS in an average of 8-10 years after initial infection [6].
Once inside host cells, HIV converts its RNA genome into DNA using reverse transcriptase [7]. This viral DNA integrates into the host cell genome and hijacks host cell machinery to produce new virus particles. HIV exhibits high genetic variability and rapid mutation, enabling escape from host immune responses and antiviral drugs. The latent HIV reservoir formed early in resting memory CD4 T cells is a major barrier to curing infection [8]. Since its emergence, HIV/AIDS has caused over 36 million deaths globally [9]. In 2020, nearly 38 million people were living with HIV infection. While no effective vaccine exists yet, antiretroviral therapy (ART) can effectively suppress viral replication, enabling HIV-infected individuals to achieve near-normal lifespans [10]. However, only 28.2 million people currently access ART, highlighting global disparities. Continued biomedical innovation and equitable access to prevention and treatment remain essential to end the HIV/AIDS pandemic [11].
There are complex bidirectional relationships between HIV/AIDS and many other diseases [12]. HIV infection itself as well as antiretroviral therapies used to treat it can increase the risk for a number of health conditions. Likewise, several diseases can impact the progression and management of HIV [13-14]. Liver diseases such as viral hepatitis and cirrhosis are common co-morbidities with HIV [15,16]. HIV infection can accelerate the progression of liver disease, while liver dysfunction can complicate HIV treatment [17]. Those with HIV have higher rates of chronic kidney disease and faster decline in renal function. Diabetes prevalence is also increased with HIV, partly due to certain antiretrovirals [18].
Cardiovascular disease risk is elevated with HIV infection, driven by chronic inflammation and metabolic changes [19-22]. Links have also been found between HIV and some cancers, including Hodgkin lymphoma, anal, breast, cervical, colorectal, and liver cancers. The cancer-causing virus HPV may contribute to this increased risk [23,24]. HIV appears to alter susceptibility to infectious diseases like tuberculosis and bacterial pneumonia [25]. Those living with HIV are much more prone to severe illness and death from these conditions [26]. The bacteria H. pylori can cause gastrointestinal ulcers and is more common among HIV-positive individuals [27].
The research fits into the existing literature. The existing literature shows that pre-exposure Prophylaxis has been a significant advancement, providing antiretroviral drugs to individuals at high risk of HIV to prevent infection. However, adherence has been a challenge. Long-acting formulations such as injectables or implants are being researched to address the issue of daily pill adherence. Research in HIV vaccine development has been ongoing for years. Developing an effective HIV vaccine remains a challenge due to the virus's ability to mutate rapidly. Innovative approaches, such as mosaic vaccines that target multiple strains, are being explored. Technologies like CRISPR-Cas9 have shown potential in gene therapy. Scientists are exploring the possibility of using gene editing to modify immune cells, e.g., T cells, to make them resistant to HIV or to enhance the body's natural ability to fight the virus. Topical microbicides, such as gels or rings, are being investigated as a method for preventing HIV transmission during sexual activity.
These products may provide an additional layer of protection, especially for individuals who cannot negotiate condom use. Telemedicine and mHealth technologies have the potential to improve access to HIV prevention and treatment services, particularly in remote or underserved areas. These technologies can be used for remote consultations, medication adherence support, and health education. Engaging communities in HIV prevention and treatment efforts is crucial. Innovations include community-led testing and counseling, peer support programs, and community health workers who are vital in reaching populations facing barriers to traditional healthcare services. Combining HIV prevention and treatment services with other essential healthcare services such as family planning, tuberculosis screening, and mental health services can improve health outcomes and reduce the stigma associated with HIV.
This review synthesizes recent advances that collectively hold promise for bending the trajectory of the HIV/AIDS epidemic. It offers a unique emphasis on emerging tools like gene editing, immunotherapy, and microbiome-based interventions that could overcome critical roadblocks to ending AIDS. Harnessing these innovations and ensuring their equitable global implementation will be essential to capitalize on the hard-won scientific progress achieved against this devastating pandemic.
HIV/AIDS Statistics and Studies
Recent research continues to demonstrate the profound global burden of HIV/AIDS and the inequities that persist in its distribution and disease outcomes. Though the annual number of new HIV infections declined by 52% from the peak in 1997 to 2020, an estimated 1.5 million people worldwide still acquired HIV in 2020 [28]. Sub-Saharan Africa remains disproportionately impacted, accounting for over two-thirds of new global HIV infections that year [29]. Women and key populations including men who have sex with men, people who inject drugs, sex workers, and prisoners carry a disproportionate share of new infections in many settings [30]. Encouragingly, the implementation and expansion of antiretroviral therapy (ART) in the past two decades has led to substantial declines in HIV/AIDS-related mortality globally and in countries like Ghana and Brazil [31]. However, treatment coverage and viral suppression rates remain suboptimal, and many patients still experience delayed diagnosis and ART initiation, highlighting the need for improved HIV testing and linkage to care [32]. Many countries suffer from HIV/AIDS. Sub-Saharan Africa is disproportionately impacted. Key populations face high infection rates. Access to prevention and treatment remains limited. Cultural stigma impedes HIV response efforts. Health system weaknesses exacerbate disparities. Global solidarity and investment are needed.
Recent Advances in HIV Vaccine Development
Messenger RNA vaccines
Messenger RNA (mRNA) vaccines represent a promising new approach in HIV vaccine development. mRNA vaccines work by delivering mRNA encoding viral antigenic proteins into host cells, which then translate the mRNA and express the antigenic proteins to stimulate an immune response. mRNA vaccines can be quickly adapted to different viral strains and offer logistical advantages over more conventional platforms. Early research shows mRNA HIV vaccines are capable of inducing both antibody and cellular immune responses against HIV proteins. Phase 1 clinical trials are underway examining mRNA vaccines encoding HIV envelope proteins. Though still early, mRNA technology provides a versatile new tool in the HIV vaccine development arsenal [33,34]. Figure 1 depicts the multiple steps involved in mRNA vaccine function and activation of the immune system. Upon vaccine administration, mRNAs enter host cells through endocytosis or direct diffusion across the cell membrane. Ribosomes then translate the mRNA sequences into encoded protein antigens. Proteasomes process these proteins into peptide fragments that are presented on the cell surface by major histocompatibility complexes. Antigen presentation activates CD4+ and CD8+ T cells through T cell receptor binding, stimulating humoral and cellular adaptive immunity. Additionally, innate immune sensors detect the exogenous mRNAs and trigger production of proinflammatory cytokines and interferons. Overall, the figure outlines key events in mRNA vaccine-induced immune activation [35].
Conserved Epitope/mosaic vaccine approaches
Most HIV vaccine strategies have focused on generating strain-specific responses effective against matching viruses. However, HIV's high mutation rate enables rapid immune escape through changes in key epitopes targeted by strain-specific antibodies. Conserved epitope vaccines aim to focus immune responses on sites of vulnerability on the HIV envelope conserved across viral strains. This may enable broader protection. Mosaic vaccines similarly incorporate gene fragments from diverse HIV strains to provide wider coverage. Preclinical studies demonstrated promising, broad neutralizing antibody responses using computationally optimized conserved epitope and mosaic immunogens. These approaches are now advancing into early stage human clinical testing [36].
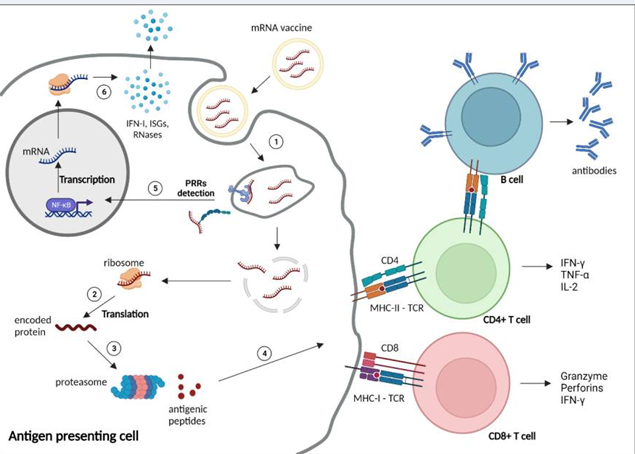
Figure 1. Mechanisms of mRNA vaccine function and immune system activation [35].
Novel adjuvant formulations
Adjuvants enhance vaccine immunogenicity and are critical components of subunit vaccines. Alum has been the most widely used adjuvant but is suboptimal for vaccines needing potent cellular immunity, like HIV vaccines. Newer adjuvants aim to drive stronger, more durable T and B cell responses. Formulations using TLR agonists show excellent safety and immunogenicity in non-human primates and humans. Lipid-based nanoparticles delivering vaccine antigens and adjuvants also offer promise through sustained antigen release and lymph node targeting. Their biodegradability and potential for scalable manufacturing make novel adjuvants appealing HIV vaccine components [37]. Table 1 summarizes emerging HIV vaccine platforms in clinical development.
|
Vaccine Platform |
Key Features |
Stage of Testing |
|
mRNA vaccines [33-35] |
Rapid production, versatile, robust cellular and humoral immunity |
Phase 1 trials ongoing |
|
Conserved epitope/mosaic vaccines [36] |
Optimized bioinformatically for broad coverage, focus immune responses on vulnerable sites |
Early human trials |
|
Novel adjuvant formulations [37] |
Stimulate potent, durable immune responses |
Safety and immunogenicity demonstrated in humans |
Emerging HIV Treatment Strategies
Long-acting antiretroviral therapies
Current antiretroviral therapy (ART) for HIV requires daily oral administration, presenting adherence challenges. Long-acting injectable and implantable ART formulations that only require monthly or less frequent administration are in development. Cabotegravir and rilpivirine long-acting injections have demonstrated virologic suppression comparable to daily oral ART in clinical trials. Other long-acting ART agents like lenacapavir and islatravir are also showing promise. By reducing dosing frequency, long-acting ART aims to improve adherence, decrease reservoir seeding, and bolster immune recovery [38,39].
Gene editing approaches (e.g. CRISPR)
Gene editing with CRISPR/Cas9 allows precise modification of host DNA to confer HIV resistance in CD4 T cells and hematopoietic stem cells. Early stage human trials of ex vivo CRISPR approaches demonstrated feasibility and safety, with treated cells persisting for months. However, challenges remain around scalability, delivery, off-target effects, and adequate efficiency. Combining CRISPR with other emerging technologies like mRNA or gene therapy vectors may help overcome current limitations to durability [40].
Therapeutic vaccines
Rather than prevent initial infection, therapeutic vaccines aim to enhance immune control of HIV post-infection to reduce viral reservoirs and enable ART-free remission. Vaccine strategies in development include vectored vaccines, mRNA vaccines, dendritic cell-based approaches, and combination immunotherapy. The BCN 02 therapeutic vaccine showed sustained control of viral load after ART interruption in early human testing. While no therapeutic vaccine is approved yet, the concept continues to be pursued, including in combination with other cure strategies [41-43].
Immune-based therapies
Immune modulators and monoclonal antibodies (mAbs) also show therapeutic promise by enhancing antiviral immunity or directly targeting HIV. mAbs like ibalizumab bind HIV's CD4 binding site, blocking viral entry. The TLR7 agonist vesatolimod induces antiviral cytokines and activated HIV transcription. interleukin-7 increases CD4 counts and reinvigorates exhausted T cells. While not curative alone, immunotherapies may play a supplementary role in achieving post-treatment control of HIV [44,45].
Nanotechnology for targeted drug delivery
Nanoscale drug delivery systems, such as polymeric nanoparticles, lipid nanoparticles and nanosuspensions, allow manipulated delivery and improved biodistribution of antiretroviral drugs. Nanoformulations of rilpivirine, dolutegravir, and efavirenz have exhibited sustained release, cell/tissue targeting, and favorable pharmacokinetics in preclinical studies. While still early in development, nanotechnology offers the potential to overcome current ART limitations related to systemic toxicities, adverse effects, and suboptimal pharmacokinetics [46,47]. Table 2 summarizes key points of each of the five HIV treatment approaches described.
|
Category |
Specific Strategies |
Key Features |
|
Long-acting ART [38,39] |
- Cabotegravir/rilpivirine injections - Lenacapavir injection - Islatravir implant |
- Monthly or less frequent dosing - Improves adherence - More constant drug levels |
|
Gene editing [40] |
- CRISPR/Cas9 |
- Confers HIV resistance - Ex vivo modification of CD4 T cells |
|
Therapeutic vaccines [41-43] |
- mRNA vaccines - Dendritic cell vaccines |
- Reduces viral reservoir - Enhances immune control |
|
Immunotherapies [44,45] |
- Broadly neutralizing mAbs - Immune modulators |
- Inhibits viral entry/replication - Restores immune function |
|
Nanotechnology [46,47] |
- Polymeric nanoparticles - Lipid nanoparticles |
- Manipulated drug delivery - Improved pharmacokinetics |
The Microbiome in HIV Pathogenesis and Treatment
Gut microbiota changes with HIV infection
Advances in DNA sequencing have enabled detailed characterization of the gut microbiota in HIV infection. Studies consistently demonstrate that HIV infection dramatically alters the gut microbial composition, leading to reduced microbial diversity, abundance, and functionality. Specific taxa depleted in the HIV gut microbiota have immunomodulatory and barrier protective properties. Microbiome disruption likely contributes to the chronic immune activation and inflammation underlying HIV progression. Antiretroviral therapy only partially normalizes the microbiota, suggesting persistent dysbiosis [53].
Probiotics/prebiotics as adjuvant therapy
Supplementation with probiotics or prebiotics aims to restore beneficial microbes depleted in HIV infection. Small clinical trials found certain probiotic strains reduced immune activation, inflammation, and microbial translocation markers among HIV patients on ART. Vaginal probiotics also decreased vaginal dysbiosis and inflammation in HIV-positive women. However, results vary across different probiotic formulations. Further research on optimal species, strains, dosing, and delivery methods is warranted to translate microbiome-targeted interventions into clinical care [54,55].
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) from healthy donors more comprehensively restores microbial communities. Case studies demonstrated FMT from HIV-negative donors into HIV patients temporarily enriched microbial diversity and anti-inflammatory taxa. Larger clinical trials are underway to formally evaluate immunologic and virologic effects of FMT in HIV infection. Critical questions remain around donor selection, recipient preparation, delivery routes, and long-term safety. Addressing these knowledge gaps could position FMT as a novel adjuvant to augment ART [56].
Integration of Traditional Medicine
Herbal medicines from various cultures
Traditional herbal medicines have long been used to treat symptoms related to HIV/AIDS across cultures worldwide. African medicinal plants like Hypoxis hemerocallidea (African potato) and Sutherlandia frutescens demonstrate immunomodulatory properties in vitro. Chinese herbs, including formulas like yi ai ping, also have putative mechanisms against HIV through effects on viral entry, replication, and immune function. The Indian Ayurvedic system utilizes herbs like Tinospora cordifolia as immune boosters for HIV patients. While promising, most traditional remedies require more rigorous study to confirm safety and efficacy [57-62].
Acupuncture, meditation, and other practices
Beyond herbs, traditional healing systems have incorporated practices like acupuncture, yoga, meditation, and massage into HIV care models. Small studies suggest acupuncture may reduce pain, fatigue, and gastrointestinal symptoms in people with HIV on ART. Yoga and mindfulness meditation appear to improve psychological health, coping, and quality of life. Therapeutic massage may also benefit psychological wellbeing, though robust clinical data is lacking. More research is needed on optimal protocols and integration with conventional treatment [63].
Safety, efficacy, and drug interactions
The inclusion of traditional medicine therapies into HIV care requires careful evaluation of safety, efficacy, and interactions with ART. Many herbal products remain poorly characterized and can have adverse effects or interfere with ART metabolism through effects on drug metabolizing enzymes like CYP450. However, traditional medicine can play an important role in patient-centered care models if practitioners skillfully screen for harmful herb-drug interactions and only recommend evidence-based therapies that safely complement ART. Ongoing research, open provider-patient communication, and appropriate regulation are critical [64,65].
Leveraging Multi-Omics Approaches
Genomics for host factors and viral resistance
Genomic analyses have furthered understanding of host factors influencing HIV acquisition, disease progression, and treatment outcomes. Genome-wide association studies identified specific polymorphisms associated with viral control and elite controller status. Comparative genomics of HIV-resistant animal species is uncovering novel restriction factors. Sequencing also detects minor viral variants harboring drug resistance mutations missed by standard genotyping. As sequencing costs decrease, integrating host and viral genomics into routine care may guide therapy [48].
Transcriptomics for biomarkers and drug targets
Analyzing global gene expression by RNA sequencing provides insights into mechanisms of HIV replication, latency, reactivation, and pathogenesis. Transcriptomic profiling identified host biomarkers of inflammation, immune activation, and reduced immune function that are associated with and predict disease progression. Analyses also illuminate how HIV modulates host pathways, uncovering dependencies that may inform new therapeutic targets. Single-cell transcriptomics offers additional depth by deconvoluting responses within complex cell mixtures [49].
Proteomics for functional insights
Mass spectrometry-based proteomics defines functional profiles by quantifying thousands of host and viral proteins simultaneously. Comparative studies revealed global protein-level dysregulation reflecting increased inflammation, coagulation, and apoptosis in HIV infection. Proteomics also elucidated virion composition and assembly, interactions of viral proteins with host machinery, and how viral protein abundance changes with treatment. As proteomics workflows and databases mature, proteome-wide scanning may uncover new prognostic markers and therapeutic opportunities [50].
Metabolomics for monitoring disease progression
Metabolomic analyses detect metabolic perturbations associated with HIV infection and treatment effects. Marked changes were observed in amino acid, lipid, and energy metabolism. Machine learning models applied to metabolomic data could stratify patients by disease severity. Integration with other omics layers (multi-omics) provides a systems view of HIV pathogenesis not discernible from individual datasets alone. While still an emerging technology, metabolomics offers substantial potential for monitoring disease progression and treatment response [51,52]. Table 3 illustrates multi-omics approaches to analyze HIV.
|
Omics Layer |
Technology |
Insights Generated |
|
Genomics [48] |
DNA sequencing |
Host factors, viral resistance |
|
Transcriptomics [49] |
RNA sequencing |
Disease biomarkers, viral effects on host genes |
|
Proteomics [50] |
Mass spectrometry |
Functional profiles, virion composition |
|
Metabolomics [51,52] |
Mass spectrometry |
Monitoring disease progression and treatment response |
Artificial Intelligence for HIV Cure
AI for novel drug design
Artificial intelligence methods such as deep learning and neural networks have shown promise for accelerating and improving the HIV drug discovery process. AI can help screen millions of compounds to identify promising drug candidates by predicting activity and toxicity [66-69]. It can also model protein dynamics and interactions to design drugs that can better target the virus. Startups and major pharmaceutical companies have begun using AI for HIV drug design with some early successes. However, more research is still needed to fully realize the potential of these technologies [70-73].
Machine learning to analyze diverse omics data
Machine learning is being applied to analyze complex HIV omics data sets including genomics, transcriptomics, and proteomics data. By detecting patterns in these high-dimensional datasets, machine learning can uncover new insights into HIV persistence and pathogenesis [74,75]. Researchers have used these techniques to identify biomarkers of reservoir size and immune dysfunction. Machine learning has also shown promise for discovering novel cellular pathways and host-virus interactions. Integrating diverse omics data with advanced algorithms offers a new avenue for understanding and overcoming viral reservoirs [76,77].
AI to optimize treatment regimens
AI and reinforcement learning methods are now being leveraged to optimize HIV treatment strategies. Researchers have developed AI models to predict treatment outcomes and make dynamic treatment decisions for HIV patients. These personalized models can recommend optimal timing for switching drug regimens based on a patient's specific viral genetics and history. They can also suggest additional interventions such as immunotherapy to minimize the viral reservoir. Applying AI to HIV treatment data continues to be an active area of research for achieving sustained virologic response [74].
Conclusions
The HIV/AIDS pandemic persists as a global public health crisis, disproportionately impacting resource-limited regions. However, the review highlights recent biomedical advances holding promise to bend the trajectory of the epidemic. Innovative vaccine platforms like mRNA and mosaic antigens could enhance breadth and durability of protection. Long-acting antiretroviral formulations represent a breakthrough for adherence challenges. Approaches such as gene editing and immune therapies offer potential curative strategies, though obstacles around delivery and off-target effects remain. Multi-omics profiling illuminates new prognostic biomarkers and therapeutic targets. The microbiome emerges as a modifiable factor influencing HIV pathogenesis. While no single solution ensures epidemic control, synergistically translating these tools through rigorous clinical testing and equitable global access could transform the HIV/AIDS landscape. However, major barriers persist around financing, infrastructure, and political commitment required for widespread implementation, especially in heavily affected regions. Strengthening health systems and tackling social drivers like stigma remain crucial priorities alongside continued biomedical innovation.
Recommendations
To fully leverage these scientific advances and accelerate efforts to control and eventually end the HIV/AIDS pandemic, the following steps are recommended:
- Rapidly advance the most promising biomedical prevention strategies like mRNA vaccines through late-phase clinical testing and delivery optimization to determine field effectiveness.
- Expand access to new potent ART regimens like long-acting injections globally, with a focus on hardest-hit regions and populations.
- Support further research into curative approaches like CRISPR gene editing and immunotherapy, but also implement them cautiously and ethically.
- Incorporate emerging omics profiling and AI-based treatment optimization into routine HIV care to individualize prevention and treatment.
- Elucidate optimal probiotic/prebiotic formulations and fecal transplant protocols through rigorous clinical trials to translate microbiome findings.
- Promote evidence-based integration of traditional medicine where safe and effective; screen for herb-drug interactions.
- Foster collaborative, multidisciplinary research across public and private sectors to accelerate the most impactful ideas from lab to clinic.
List of Abbreviations
HIV: Human Immunodeficiency Virus; AIDS: Acquired Immunodeficiency Syndrome; SIV: Simian Immunodeficiency Virus; ART: Antiretroviral Therapy; mRNA: Messenger RNA; TLR: Toll-Like Receptor; MHC: Major Histocompatibility Complex; mAbs: Monoclonal Antibodies; CRISPR - Clustered Regularly Interspaced Short Palindromic Repeats; Cas9: CRISPR Associated Protein 9; FMT: Fecal Microbiota Transplantation; HPV: Human Papillomavirus; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; CKD: Chronic Kidney Disease; CVD: Cardiovascular Disease; GI: Gastrointestinal; Mtb: Mycobacterium tuberculosis; H. pylori: Helicobacter pylori; AI: Artificial Intelligence; PLHIV: People Living with HIV
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Availability of data and materials
All data is available, and sharing is available as well as publication.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript.
Acknowledgements
The authors thank all the researchers who have made great efforts in their studies. The authors would like to thank the Deanships of all the participating Universities for supporting this work. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Hokello J, Tyagi K, Owor RO, Sharma AL, Bhushan A, Daniel R, et al. New Insights into HIV Life Cycle, Th1/Th2 Shift during HIV Infection and Preferential Virus Infection of Th2 Cells: Implications of Early HIV Treatment Initiation and Care. Life. 2024 Jan 9;14(1):104.
3. Kirchner JT. Origin, Evolution, and Spread of HIV1 & HIV2. In: Hardy WD. Fundamentals of HIV Medicine 2023. New York: Oxford University Press; 2023.
4. Tumwebaze M, Rubaihayo J, Harold M. Appraisal of existing HIV/AIDS prevention and control measures and presentation of innovative strategies to end HIV/AIDs epidemic by 2030. Open Journal of Epidemiology. 2023 Jul 3;13(3):178-94.
5. Attaullah, Zeb K, Khan I, Ahmad R, Eldin SM. Transmission dynamics of a novel HIV/AIDS model through a higher-order Galerkin time discretization scheme. Scientific Reports. 2023 May 8;13(1):7421.
6. Abiodun OE, Adebimpe O, Ndako J, Oludoun O, Aladeitan B, Adeniyi M. Qualitative analysis of HIV and AIDS disease transmission: impact of awareness, testing and effective follow up. F1000research. 2022 Oct 7;11:1145.
7. Broyles LN, Luo R, Boeras D, Vojnov L. The risk of sexual transmission of HIV in individuals with low-level HIV viraemia: a systematic review. The Lancet. 2023 Aug 5;402(10400):464-71.
8. Mansouri M, Rumrill S, Dawson S, Johnson A, Pinson JA, Gunzburg MJ, et al. Targeting HIV-1 Reverse transcriptase using a fragment-based approach. Molecules. 2023 Mar 30;28(7):3103.
9. Milward de Azevedo Meiners MM, Araújo Cruz I, De Toledo MI. Adherence to antiretroviral therapy and viral suppression: Analysis of three periods between 2011 and 2017 at an HIV-AIDS center, Brazil. Frontiers In Pharmacology. 2023 Mar 31;14:1122018.
10. Addissouky TA, Wang Y, Megahed FA, El Agroudy AE, El Sayed IE, El-Torgoman AM. Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egyptian Liver Journal. 2021 Dec;11:86.
11. Addissouky TA, Ayman E. El-Agroudy, Abdel Moneim AK El-Torgoman and 1Ibrahim E. El-Sayed, Efficacy of biomarkers in detecting fibrosis levels of Liver Diseases. World Journal of Medical Sciences. 2019 Mar;16(1):11-8.
12. Addissouky TA, El Agroudy AE, El-Torgoman AM, El Sayed IE, Ibrahim EM. Efficiency of alternative markers to assess liver fibrosis levels in viral hepatitis B patients. Biomedical Research. 2019 Jan 15;30(2):351-6.
13. Addissouky T. Detecting liver fibrosis by recent reliable biomarkers in viral hepatitis patients. American Journal of Clinical Pathology. 2019 Oct 1;152:S85.
14. El Agroudy AE, Elghareb MS, Addissouky TA, Elshahat EH, Hafez EH. Serum hyaluronic acid as non invasive biomarker to predict liver fibrosis in viral hepatitis patients. Journal of Bioscience and Applied Research. 2016 May 24;2(5):326-33.
15. El Agroudy AE, Elghareb MS, Addissouky TA, Elshahat EH, Hafez EH. Biochemical study of some non invasive markers in liver fibrosis patients. Journal of Bioscience and Applied Research. 2016 May 23;2(5):319-25.
16. Addissouky TA, Khalil AA, El Agroudy AE. Assessment of potential biomarkers for early detection and management of Glomerulonephritis Patients with Diabetic Diseases. American Journal of Clinical Pathology. 2023 Nov;160(Supplement_1):S18-S19.
17. Addissouky T, Ali M, El Sayed IE, Wang Y. Revolutionary innovations in diabetes research: from biomarkers to genomic medicine. Iranian Journal of Diabetes and Obesity. 2023 Dec 28;15(4):228-42.
18. Matodzi HJ, Lowton K, Miseer P. Assessing HIV transmission knowledge in psychiatric patients in Johannesburg, South Africa. South African Journal of Psychiatry. 2023;29(1):2040.
19. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Elarabany N, et al. Shaping the future of cardiac wellness: exploring revolutionary approaches in disease management and prevention. Journal of Clinical Cardiology. 2024 Jan 5;5(1):6-29.
20. Liu Y, Deng S, Zhang Q, Guo Y, Wang Y, Li T, et al. MLIF modulates microglia polarization in ischemic stroke by targeting eEF1A1. Frontiers in Pharmacology. 2021 Sep 7;12:725268.
21. Addissouky TA, El Agroudy AE, Khalil AA. Developing a novel non-invasive serum-based diagnostic test for early detection of colorectal cancer. American Journal of Clinical Pathology. 2023 Nov 1;160(Supplement_1):S17.
22. Addissouky TA, Khalil AA. Detecting lung cancer stages earlier by appropriate markers rather than biopsy and other techniques. American Journal of Clinical Pathology. 2020 Oct;154(Supplement_1):S146-7.
23. Mremi A, Mswima J, Mlay MG, Bartholomew H, Alloyce JP, Mmbaga BT, et al. Cancer spectrum in HIV-infected patients: A zonal hospital experience in Tanzania. Cancer Treatment and Research Communications. 2020 Jan 1;25:100213.
24. Wong IK, Grulich AE, Poynten IM, Polizzotto MN, van Leeuwen MT, Amin J, et al. Time trends in cancer incidence in Australian people living with HIV between 1982 and 2012. HIV Medicine. 2022 Feb;23(2):134-45.
25. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Elarabany N, et al. Oxidative stress and inflammation: elucidating mechanisms of smoking-attributable pathology for therapeutic targeting. Bulletin of the National Research Centre. 2024 Jan 22;48(1):16.
26. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Molecular Pathways in Sepsis Pathogenesis: Recent Advances and Therapeutic Avenues. Journal of Cellular Immunology. 2024 Jan 20;5(6):174-83.
27. Azevedo-Pereira JM, Pires D, Calado M, Mandal M, Santos-Costa Q, Anes E. HIV/Mtb co-infection: from the amplification of disease pathogenesis to an “emerging syndemic”. Microorganisms. 2023 Mar 27;11(4):853.
28. Tian X, Chen J, Wang X, Xie Y, Han D, Fu H, et al. Global, regional, and national HIV/AIDS disease burden levels and trends in 1990-2019: a systematic analysis for the global burden of disease 2019 study. Frontiers in Public Health. 2023 Feb 15;11:1068664.
29. Boah M, Yeboah D, Kpordoxah MR, Issah AN, Adokiya MN. Temporal trend analysis of the HIV/AIDS burden before and after the implementation of antiretroviral therapy at the population level from 1990 to 2020 in Ghana. BMC Public Health. 2023 Jul 20;23(1):1399.
30. Sameen S, Lakhdir MP, Azam SI, Asad N. Evaluating knowledge about HIV and discriminatory attitudes among Pakistani women of reproductive age using 2017-18 Demographic Health Survey data. Scientific Reports. 2023 Oct 19;13(1):17849.
31. Lua I, Silva AF, Guimarães NS, Magno L, Pescarini J, Anderle RV, et al. The effects of social determinants of health on acquired immune deficiency syndrome in a low-income population of Brazil: a retrospective cohort study of 28.3 million individuals. The Lancet Regional Health–Americas. 2023 Aug 1;24:100554.
32. Ijaiya MA, Olowu A, Oguntade HA, Anjorin S, Uthman OA. HIV research output in African Countries between 1986–2020. PLOS Global Public Health. 2023 Jun 22;3(6):e0000544.
33. Chapman R, van Diepen M, Douglass N, Hermanus T, Moore PL, Williamson AL. Needle-Free Devices and CpG-Adjuvanted DNA Improve Anti-HIV Antibody Responses of Both DNA and Modified Vaccinia Ankara-Vectored Candidate Vaccines. Vaccines. 2023 Feb 7;11(2):376.
34. Akbari E, Seyedinkhorasani M, Bolhassani A. Conserved multiepitope vaccine constructs: A potent HIV-1 therapeutic vaccine in clinical trials. Brazilian Journal of Infectious Diseases. 2023 Jul 10;27:102774.
35. Matarazzo L, Bettencourt PJ. mRNA vaccines: a new opportunity for malaria, tuberculosis and HIV. Frontiers in Immunology. 2023 Apr 24;14:1172691.
36. Cohen KW, Fiore-Gartland A, Walsh SR, Yusim K, Frahm N, Elizaga ML, et al. Trivalent mosaic or consensus HIV immunogens prime humoral and broader cellular immune responses in adults. The Journal of Clinical Investigation. 2023 Feb 15;133(4):e163338.
37. Singh A, Boggiano C, Eller MA, Maciel Jr M, Marovich MA, Mehra VL, et al. Optimizing the Immunogenicity of HIV Vaccines by Adjuvants–NIAID Workshop Report. Vaccine. 2023 Jul 12;41(31):4439-46.
38. Sension MG, Brunet L, Hsu RK, Fusco JS, Cochran Q, Uranaka C, et al. Cabotegravir+ rilpivirine long-acting injections for HIV treatment in the US: real world data from the OPERA cohort. Infectious Diseases and Therapy. 2023 Dec;12(12):2807-17.
39. Overton ET, Richmond G, Rizzardini G, Thalme A, Girard PM, Wong A, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with human immunodeficiency virus 1 type 1 infection: 152-week results from ATLAS-2M, a randomized, open-label, phase 3b, noninferiority study. Clinical Infectious Diseases. 2023 May 1;76(9):1646-54.
40. Gutiérrez-Rodríguez A, Cruz-Fuentes CS, Genis-Mendoza AD, Nicolini H. CRISPR/Cas9 genome editing approaches for psychiatric research. Brazilian Journal of Psychiatry. 2023 May 12;45:137-45.
41. Addissouky TA, El Sayed IE, Ali MM, Wang Y, El Baz A, Khalil AA, et al. Can vaccines stop cancer before it starts? Assessing the promise of prophylactic immunization against high-risk preneoplastic lesions. Journal of Cellular Immunology. 2023 Nov 29;5(4):127-40.
42. Kopycinski J, Yang H, Hancock G, Pace M, Kim E, Frater J, et al. Therapeutic vaccination following early antiretroviral therapy elicits highly functional T cell responses against conserved HIV-1 regions. Scientific Reports. 2023 Oct 11;13(1):17155.
43. Vieira V, Lim N, Singh A, Leitman E, Dsouza R, Adland E, et al. Slow progression of pediatric HIV Associates with early Cd8+ T cell PD-1 expression and a stem-like phenotype. JCI insight. 2023 Feb 2;8(3):e156049.
44. Struble EB, Rawson JM, Stantchev T, Scott D, Shapiro MA. Uses and challenges of antiviral polyclonal and monoclonal antibody therapies. Pharmaceutics. 2023 May 19;15(5):1538.
45. Matsui Y, Miura Y. Advancements in Cell-Based Therapies for HIV Cure. Cells. 2023 Dec 28;13(1):64.
46. Joseph TM, Kar Mahapatra D, Esmaeili A, Piszczyk Ł, Hasanin MS, Kattali M, et al. Nanoparticles: Taking a unique position in medicine. Nanomaterials. 2023 Jan 31;13(3):574.
47. Onugwu AL, Nwagwu CS, Onugwu OS, Echezona AC, Agbo CP, Ihim SA, et al. Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases. Journal of Controlled Release. 2023 Feb 1;354:465-88.
48. Thami PK, Choga WT, Dandara C, O'Brien SJ, Essex M, Gaseitsiwe S, et al. Whole genome sequencing reveals population diversity and variation in HIV-1 specific host genes. Frontiers in Genetics. 2023 Dec 20;14:1290624.
49. Zhou Z, Zhong Y, Zhang Z, Ren X. Spatial transcriptomics deconvolution at single-cell resolution using Redeconve. Nature Communications. 2023 Dec 1;14(1):7930.
50. Birhanu AG. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clinical Proteomics. 2023 Dec;20(1):32.
51. Wendt CH, Samorodnitsky S, Lock EF, Kruk M, Morris A, Leung JM, et al. Lung and plasma metabolome in HIV-associated obstructive lung disease. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2022 Nov 1;91(3):312-8.
52. Lu L, Yang Y, Liu X, Cao W, Li T. Altered plasma metabolites and inflammatory networks in HIV-1 infected patients with different immunological responses after long-term antiretroviral therapy. Frontiers in Immunology. 2023 Sep 27;14:1254155.
53. Martínez-Sanz J, Talavera-Rodríguez A, Díaz-Álvarez J, Rodríguez JM, Alba C, Montes ML, et al. A gut microbiome signature for HIV and metabolic dysfunction-associated steatotic liver disease. Frontiers in Immunology. 2023 Dec 14;14:1297378.
54. Roy S, Dhaneshwar S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World Journal of Gastroenterology. 2023 Apr 4;29(14):2078.
55. Ji J, Jin W, Liu SJ, Jiao Z, Li X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm. 2023 Dec;4(6):e420.
56. Malik A, Malik MI. Fecal Microbiota Transplantation in Human Immunodeficiency Virus-Infected Patient Population: A Systematic Review and Meta-Analysis. Gastroenterology Research. 2023 Aug;16(4):209-16.
57. Addissouky TA, Ali MM, El Sayed IE, Wang Y. Recent advances in diagnosing and treating helicobacter pylori through botanical extracts and advanced technologies. Archives of Pharmacology and Therapeutics. 2023 Nov 3;5(1):53-66.
58. Ikinyom N, Lamwaka AV, Malagala AT, Ndyomugyenyi EK. Ethnobotanical study of nutraceutical plants used to manage opportunistic infections associated with HIV/AIDS in Acholi sub-region, Northern Uganda. Tropical Medicine and Health. 2023 Sep 1;51(1):50.
59. Addissouky TA, Megahed FA, Elagroudy AE, El Sayed IE. Efficiency of mixture of olives oil and figs as an antiviral agent: a review and perspective. International Journal of Medical Science and Health Research. 2020 Aug;4(4):107-11.
60. Malik U, Pal D. HIV and the Role of Various Medicinal Plants Against Infection in Humans, with Possible Mechanism of Action and Functions of Secondary Metabolites. In: Anti-Viral Metabolites from Medicinal Plants. Cham: Springer International Publishing; 2022 Dec 14. pp. 1-22.
61. Addissouky TA, Khalil AA, El Agroudy AE. Assessing the efficacy of a modified triple drug regimen supplemented with mastic gum in the eradication of Helicobacter Pylori Infection. American Journal of Clinical Pathology. 2023;160(Supplement_1):S19.
62. Mosavat SH, Pasalar M. Complementary and alternative medicine use among people living with HIV in Shiraz, Southern Iran. Frontiers in Public Health. 2023 Oct 5;11:1206665.
63. Uebelacker LA, Cherenack EM, Busch A, Baker JV, Pinkston M, Gleason N, et al. Pharmacologic and non-pharmacologic treatments for chronic pain used by patients with pain, HIV, and depression. AIDS and Behavior. 2022 Mar;26(3):864-73.
64. Kong L, Xie X, Fu Y, Gan L, Yang X, Ma S, et al. Clinical efficacy, safety, and subjective experience based on ePRO in HIV‐infected individuals administered Bictegravir/Emtricitabine/Tenofovir Alafenamide in southwest China. Immunity, Inflammation and Disease. 2023 Aug;11(8):e974.
65. Addissouky TA, Ali M, Sayed IE, Wang Y. Emerging advanced approaches for diagnosis and inhibition of liver fibrogenesis. The Egyptian Journal of Internal Medicine. 2024 Dec;36(1):19.
66. Peng Y, Chen J, Chen ZS, Peng F, Liu Z. Current drugs for HIV-1: from challenges to potential in HIV/AIDS. Frontiers in Pharmacology. 2023 Oct 26;14:1294966.
67. Addissouky TA, Ali MMA, El Sayed IET, Wang Y, Khalil AA. Translational insights into molecular mechanisms of chemical hepatocarcinogenesis for improved human risk assessment. Advances in Clinical Toxicology. 2024;9(1):294.
68. Addissouky TA, Wang Y, El Tantawy El Sayed I, Majeed MAA, Khalil AA. Emerging technologies and advanced biomarkers for enhanced toxicity prediction and safety pharmacology. Advances in Clinical Toxicology. 2024;9(1):293.
69. Addissouky TA, Wang Y, El Tantawy El Sayed I, Majeed MAA, Khalil AA. Transforming toxicity assessment through microphysiology, bioprinting, and computational modeling. Advances in Clinical Toxicology. 2024;9(1):295.
70. Han R, Yoon H, Kim G, Lee H, Lee Y. Revolutionizing medicinal chemistry: the application of artificial intelligence (AI) in early drug discovery. Pharmaceuticals. 2023 Sep 6;16(9):1259.
71. Addissouky TA, Sayed IE, Ali MM, Wang Y, Baz AE, Khalil AA, et al. Latest advances in hepatocellular carcinoma management and prevention through advanced technologies. Egyptian Liver Journal. 2024 Jan 2;14(1):2.
72. Vora LK, Gholap AD, Jetha K, Thakur RR, Solanki HK, Chavda VP. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics. 2023 Jul 10;15(7):1916.
73. Addissouky TA, Ali MM, El Sayed IE, Wang Y, El Baz A, Elarabany N, et al. Preclinical promise and clinical challenges for innovative therapies targeting liver fibrogenesis. Archives of Gastroenterology Research. 2023 Nov 14;4(1):14-23.
74. Addissouky TA, El Sayed IE, Ali MM. Regenerating Damaged Joints: The Promise of Tissue Engineering and Nanomedicine in Lupus Arthritis. J Clinical Orthopaedics and Trauma Care. 2024;6(2):2694-0248.
75. Addissouky TA, El Sayed IE, Ali MM. Conservative and Emerging Rehabilitative Approaches for Knee Osteoarthritis Management. J Clinical Orthopaedics and Trauma Care. 2024;6(2):2694-0248.
76. Mikaeloff F, Gelpi M, Benfeitas R, Knudsen AD, Vestad B, Høgh J, et al. Network-based multi-omics integration reveals metabolic at-risk profile within treated HIV-infection. Elife. 2023 Feb 16;12:e82785.
77. Addissouky TA, Wang Y, El Sayed IE, Baz AE, Ali MM, Khalil AA. Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches. Beni-Suef University Journal of Basic and Applied Sciences. 2023 Sep 2;12(1):80.
78. Espineira S, Flores-Piñas M, Chafino S, Viladés C, Negredo E, Fernández-Arroyo S, et al. Multi-omics in HIV: searching insights to understand immunological non-response in PLHIV. Frontiers in Immunology. 2023 Aug 15;14:1228795.
79. Seboka BT, Yehualashet DE, Tesfa GA. Artificial intelligence and machine learning based prediction of viral load and CD4 status of people living with HIV (PLWH) on anti-retroviral treatment in Gedeo Zone Public Hospitals. International Journal of General Medicine. 2023 Dec 31:435-51.
