Abstract
Nosocomial infections in the form of bacterial biofilms on medical implants continue to pose a significant challenge for medical professionals in its treatment. This review addresses the basic biofilm formation process and illustrates in detail its manifestation in causing chronic infections. Bacterial biofilms are described as a microbial consortium attached to a substratum by exopolymeric substances. Biofilms trap soluble organic compounds and act as a sink for nutrients as well as inorganic compounds. They immobilize extracellular enzymes and DNA, to propagate the microbial colony on implant surfaces, and manifest the disease. Frequently, the persistent chronic infections arise due to biofilm formation on the implanted devices and cause severe inconvenience to the patients. These infections are difficult to eradicate and require a strong antibiotics course, which can penetrate the biofilm to control, however, in some cases it warrants the removal of the implanted device. Finally, this review highlights recent advances in antimicrobial compounds that aid in preventing implant infections.
Keywords
Bacteria, Biofilms, Nosocomial, Infections, Staphylococcus, Antimicrobials
Introduction
Human body implants are rapidly covered by a conditioning film (which contains the biochemical moieties) when they interact with human body fluids and its components. This surface-bound biochemical blend elicits a chemotactic response to attract bacteria that are transmitted through infection or by contamination. The attached bacteria subsequently form micro-colonies and result in the formation of bacterial biofilms. These microbial biofilms manifest a sequence of growth phases and become a complex biological unit that is difficult to treat with antibiotics [1].
Historically, microorganisms have been primarily categorized as free-living suspended cells. They are described based on their growth rate in nutrient rich culture media. Antony von Leeuwenhoek first described the microbiological phenomenon, in 1684, when he observed human dental plaque under the microscope, he described the miniscule organisms pictorially, and he named it as scurf [2]. From the old fundamental microbiological studies of Robert Koch and the proposed postulates [2], until the 1970s, microbes were studied as single cells dispersed, free-floating organisms i.e., planktonic mode of life. Subsequently, it was Zobell in 1942 [1,2], who reiterated that bacteria universally attach on submerged surfaces and grow as sessile communities. In 1978, Costerton [3] formally introduced the term ‘biofilm’. Later on, the biofilm research picked up the pace and from the 1980, there is a spurt in biofilm research [1]. Experimenting with the planktonic pure cultures of bacteria as models, microbiologists were able to investigate many fatal disease-causing bacteria and it resulted in development of new antimicrobial agents to kill the pathogenic bacteria [3]. Later on, the irregular use of antibiotics resulted in the rise of multi-drug-tolerant bacteria and the concern on how to kill the antibiotic resistant bacteria [2]. This has led to a re-assessment of the microbial life cycle and it is accepted, that bacteria grow within exopolymeric matrices called biofilms that provides bacteria with mechanisms to tolerate antibiotics. In the initial period of 20th century, several investigators stated that majority of bacteria are not free-living, however, adhere to surfaces and sediments particles [1,3-7].
Later on, microbiologists realized that microorganisms prefer sessile mode of life and were directly implicated in causation of diseases. This observation was proved when Pseudomonas aeruginosa cell aggregates were found in the sputum of the patients whose lungs are infected with cystic fibrosis. Today biofilm research is described as a unique science and attracting advanced research. Although the initial biofilm research was oriented towards aquatic systems and industrial fouling problems, subsequently, their role in biocorrosion and material deterioration was consolidated [1,2]. Of late, medical biofilms have drawn more attention after some chronic human infections have been listed due to biofilm mode of contamination. These microbial infections have created serious concerns among medical professional on deciding the mode of treatment [3,5,8].
In this mini review, the author addresses the readers on what are biofilms, how they colonize or infect the human body implants. Once the bacteria adhere to the implants and the nearby tissue, they manifest their dominance by producing metabolic products that disintegrate the surrounding tissue and create suitable condition for their growth. Thus, the biofilm bacteria have showed to elicit definite mechanisms to proliferate the infection deep into the tissue. First the bacterial adhesion to the tissue, spreading the community structure and modifying the surrounding milieu and finally detach from the biofilm and travel in the circulatory system for a new tissue to infect. Figure 1 illustrates the various factors that play a role in biofilm development. Therefore, the challenges in medical biofilms are twofold: 1) establishing an accurate diagnosis with speciation/sensitivity and eradicating the infection. 2) Multiple strategies to improve diagnostic accuracy, which prevents biofilm formation on body implants, to mobilize/detach or weaken the biofilm or to target specific bacteria embedded in the biofilm [9-11].
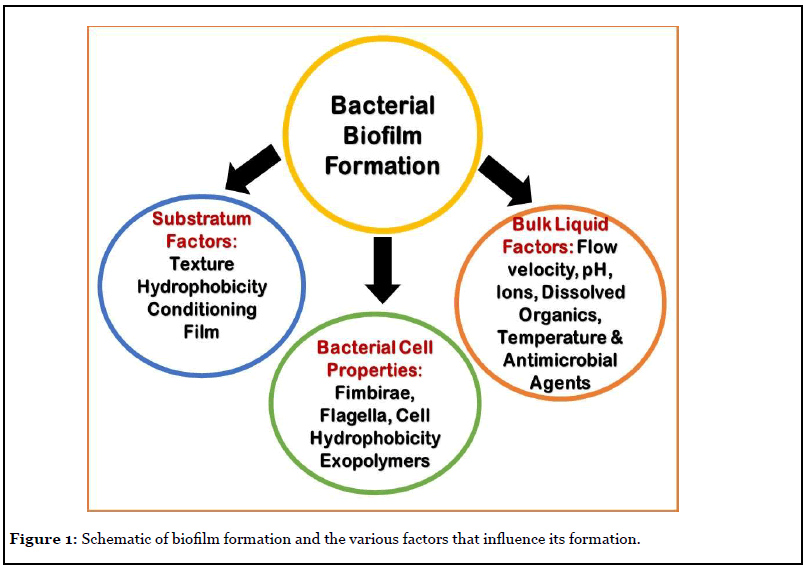
Any surface, organism, plants and materials are ideal surfaces for bacterial adhesion and biofilm development, which include; eye contact lenses and all types of medical implants as well as transcutaneous devices. In most conditions, the substratum can be a nutrition/carbon source for the organism, similarly, external coatings on the implant device retard bacterial adhesion. However, no one has developed an implant surface that prevents biofilm formation, likewise, no bacterial species happens to be in a completely free living in all growth forms [1,9,11].
Bacterial Exopolymers
Exopolymeric substances of bacteria form the core component of the biofilm glycocalyx. The glycocalyx is a biomolecular unit of protein (glycoproteins) and lipid-linked to the carbohydrates, which make up the exopolymeric matrix of the biofilm, this is also termed as the slime layer [1]. This carbohydrate-lipid-proteins complex is fully hydrated with water in the biofilm matrix. In most bacteria, exopolymeric substances or glycocalyx has an anionic property and functions as a go through system to trap necessary nutrients and minerals from the nearby milieu [12]. The glycocalyx provides a definite gradation in protection to the individual cells against antibiotics, antibodies, biosurfactants, phages, and grazers such as amoebae and macrophages. In principle, the exopolymeric matrix makes a 3-D biomolecular shield that protects biofilm bacteria from all attacks [12-14].
The exopolymeric substance of biofilms is generally composed of polysaccharides, proteins, and extracellular- DNA. Exopolymers are synthesized intra-cellularlly and are released outside of the cell and distributed in the immediate milieu. The exopolymeric matrix serve as a scaffold for carbohydrates, proteins, and nucleic acids to chemically bond and polymerize. The properties of the exopolymeric constituents and its structure differ from bacteria to bacteria. Commonly exopolymers are not explicit to biofilms, yet they are specific to bacteria. The synthesis of exopolymer is increased as a result of chemical stress; colanic acid (known as M antigen and is a heteropolysaccharide compound) synthesis in Escherichia coli and alginate production in Pseudomonas aeruginosa are relevant examples. Likewise, three exopolymers are linked with P. aeruginosa, Pel, Psl and alginate (D-mannuronate residues intermixed with L-guluronate). Alginate does not have a role in P. aeruginosa biofilm formation, it gives strength to the biofilm structure and protects the cells from antibiotic action and it also helps in overpowering host immune response as well as plays an important role in propagating the infection. Biomolecules, Psl and Pel are two main components in biofilm initiation, psl genes encode mannose-rich exopolymers containing pentamer units (D-mannose, L-rhamnose and D-glucose), that helps in primary bacterial adhesion, while Pel genes synthesize glucose-rich exopolymers. In Staphylococcus bacteria the exopolymers are termed as polysaccharide intercellular adhesin (PIA - a poly N-acetyl glucosamine molecule), it is a linear exopolymer composed of β-1,6- linked glucosamine residues. In Bacillus subtilis the biofilm exopolymer is termed as poly-δ-glutamate (3).
In case of Staphylococcus epidermidis, the production of exopolymeric substance is a critical factor, in biofilm formation. The composition of the exopolymeric matrix, gives stability to the biofilm, this makes the infected implants more difficult to treat. Nevertheless, the progression of biofilm development by S. epidermidis is multi-phasic and thus warrants a comprehensive study on the metabolomics of the biofilm that provides numerous target sites to control implant infections [15,16].
Exo-protein molecules are the other key biofilm matrix components, they are involved in biofilm formation by promoting cell surface interactions with polysaccharides. Amyloids are tough protein molecules that have a key role in the development of biofilm topology. The Fap amyloid gene expression by Pseudomonas bacteria result in cell aggregation and this boosts bacterial adhesion and subsequent biofilm development. Similarly, amyloid biomolecule of B. subtilis, TasA release fibrous material that help cells to aggregate and also nurture them to grow in harsh conditions. Biofilm-associated proteins from S. aureus (bap) and E. faecalis (esp) are involved in the progression of the infection and support biofilm formation. Similarly, exo-enzymes cleave the biomolecular matrix of the mature biofilm and increases the invasive property of the bacterial infection. These exo-enzymes make fragments of exopolymeric substance and provide organic carbon and energy molecules to cells, particularly during the period of nutrient stringent conditions. The biofilm dispersal requires enzymatic action to cleave the complex polymers to release the cells, which will initiate a new biofilm lifecycle [10]. Figure 2 explains the different bacterial types that are involved in implant infections.
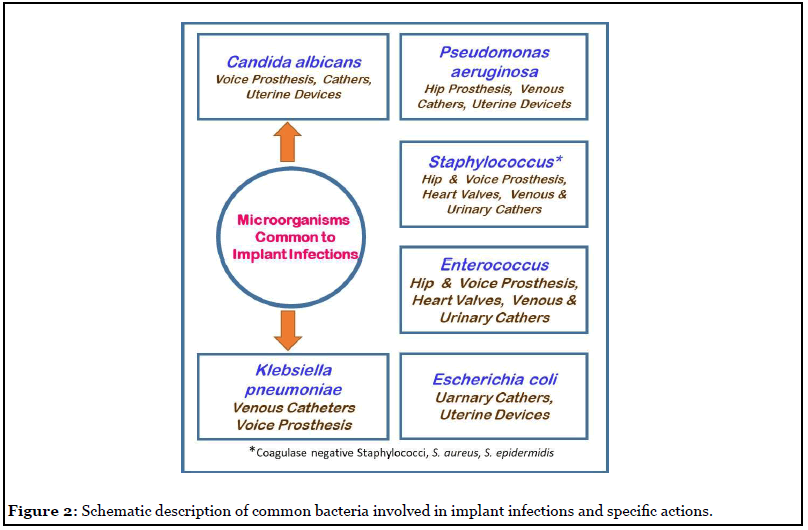
Extracellular nucleic acids (eDNA) are considered as fragments of damaged cells genome, until it was reported that DNA polymerase I prevents P. aeruginosa biofilm formation. The extracellular DNA is also an actively secreted product of a living cell, indicating its importance in biofilm development. There are also reports [17,18] that it is an important factor for bacterial adhesion and in primary biofilm formation. However, when the distance between a bacterial cell and surface becomes primary minimum (in nm scale range), the overall negative charge of eDNA interacts with chemical components of the implant surface through van der Waals force, aiding in initial bacterial adhesion. In addition, eDNA is able to chelate metal ions and some antibiotics and neutralize their toxicity to the cell. There are literature reports [17] that eDNA complexes Mg2+ ions and stimulate the cell metabolic machinery resulting in resistance of P. aeruginosa biofilm cells. Although, there are speculative reports [1,17] that eDNA plays a significant role in biofilm development and persistence, still more innovative studies are warranted to understand the mechanism [2,5].
Biofilm Growth
Normally, biofilms have a distinctive growth mode for microbes that are dissimilar to their planktonic counterparts. In bacterial adhesion process, the biofilm cells go through phenotypic changes to the chemotactic reaction elicited by the conditioned substratum. In all the biofilm development phases, the cells undergo fission reproduction and form micro-colonies that are specific to bacteria of the same genus. The micro-environments or niches in the biofilm are explicit for each bacterial genus, having varied growth phases/rates, and with time, these microbial communities synchronize and result in the development of a structurally complex biofilm. Flagella a locomotory unit of a bacterial cell plays a critical role in the initial transport of a cell to an implant surface. A representative structure of biofilm is defined in four stages: adhesion, micro-colony formation, mature phase, and dispersion. The initial bacterial adhesion phase is further categorized into two phases, first the reversible adhesion of bacterial cells and within short time, secondly, it transforms into an irreversible mode with the secretion of exopolymers. Bacterial cell surface constituents respond to the exopolymeric molecules and covalently bond to the cell membrane components. For example, Staphylococcus aureus has >20 surface recognizing biomolecular receptors that respond to the exopolymer synthetic genes. Generally, autolysins mediate the initial attachment of biofilm cells by non-covalent bacterial adhesion [1,2,5].
In a biofilm, bacterial growth is heterogeneous, and it initially forms a monolayer, which transforms into a colony of mushroom shape. A 100 μm thick biofilm has different types of bacteria that are organized to render exclusive metabolic rate and oxygen-tolerance phases. For instance, anoxic bacteria reside at the implant/biofilm interface to avoid oxygen exposure. Bacterial types that are enmeshed in the biofilm matrix chemically connect to each other and act in synergy performing various metabolic functions. As the biofilm matures, the biofilm matrix trap more bacteria and replenish nutrients from the surrounding milieu. More biomolecular scaffolds of proteins, eDNA, polysaccharides are added to strengthen the biofilm. As the biofilm commences the dispersal phase, bacteria synthesize desorbing enzymes, which disperse the biofilm bacterial cells. The dispersal phase is initiated by multitude of factors, such as nutrition deficiency, extreme competition among bacterial cells for growth, over-grown population, etc. Dispersal generally occurs in a portion of biofilm and subsequently spreads. The ejection of planktonic bacteria from the biofilm promotes the commencement of a new biofilm at new sites [17].
Several factors stimulate planktonic microbes to shift to biofilm mode of growth. Comparatively, free living bacterial cells have higher cell division and growth rate. Still, the biofilm mode of growth looks natural and is a major form of microbial life. There are numerous reasons that transform a bacterial cell to biofilm mode. Firstly, the adhesion process of bacteria elicit the genetic response in the cell to form biofilm. This metabolic transformation will be able to augment the bacteria to survive in severe ecological conditions or to overcome the high antibiotic doses. The biofilm property of bacteria can help in anchoring to the implant or body tissue. In has been reported that biofilm bacteria are 1000 times more tolerant to antibiotics/antimicrobials when compared to their planktonic counterparts. Secondly, the exopolymeric matrix of the biofilm protects bacteria cells, from antibacterial compounds by controlling their distribution into the biofilm. The biofilm mode limits bacterial movement and surges cell density and biomass. This process provides an ideal condition for exchange of antibiotic tolerant genes by conjugation process. Nevertheless, gene transfer rate through horizontal mode is ominously high in biofilms than in the free-living bacteria. Signaling molecules like cyclic-di-GMP, quorum-sensing chemicals, efflux pumps and persister cells assist in the progression of biofilm and promote antibiotic tolerance and resistance to biofilm cells. Thoughtful studies on biofilm formation, how the cells respond to chemical cues in surrounding milieu and contribute to disease is a compounded challenge that needs concerted research efforts [18-20].
Staphylococcus aureus and Biofilm Infections
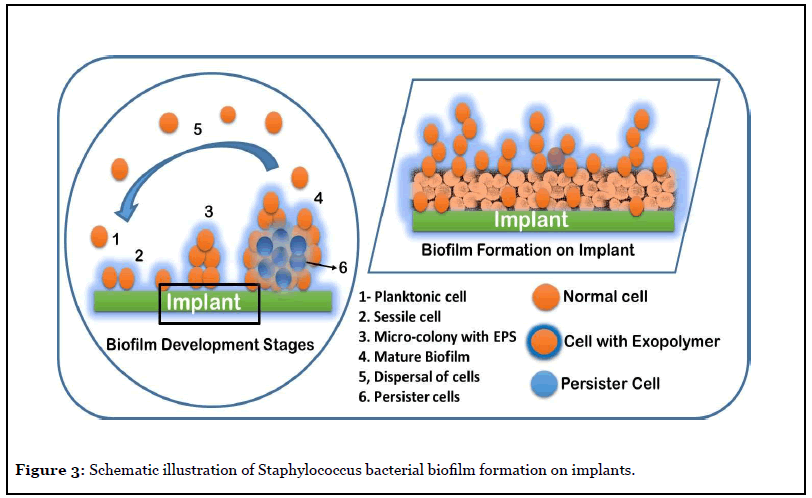
Figure 3 illustrates the biofilm process typical of Staphylococcus species, which are a major cause for hospital acquired infections and represents a serious liability for the healthcare professionals. The mode of S. aureus invasion into human body, its adhesion and biofilm formation on medical implants and infecting the surrounding host tissue play a major role in the establishment of chronic infection. The S. aureus biofilm formation, and the exopolymer matrix, protects the cells from anti-microbial agents such as antibiotics and oxidizing agents, immune defense cells. Thus, the biofilms formed on body implants are resilient and difficult to eradicate infections. The dispersal of biofilm cells can result in the spread of the infection to other tissues or organs thereby spreading the infection in the whole body [10,21].
Biofilm Control and Research
The research on microbial biofilms is progressing in many directions, such as on biofilm-associated proteins and their proteomics as well the genetic response. Becker et al. [22] reported that biofilm associated genes of Staphylococcus aureus stimulate the enzymes of tricarboxylic acid cycle such as phosphoglycerate mutase, triose-phosphate isomerase, and alcohol dehydrogenase and inferred that oxygen limitation resulted in the upregulation of genes responsible for biofilm development. The major studies [19,21,23] are on evaluation of various control strategies for either preventing biofilm formation on medical devices or disrupting the already formed biofilms. In addition, other research activities [21,23] were oriented towards advancement of new methods for assessing and monitoring the antimicrobial activity and the role of biofilm associated genes in gaining antimicrobial resistance. With the universal acceptance of the biofilm phenotype microbiologists are targeting the role of biofilm phenotype initiating chronic diseases [23].
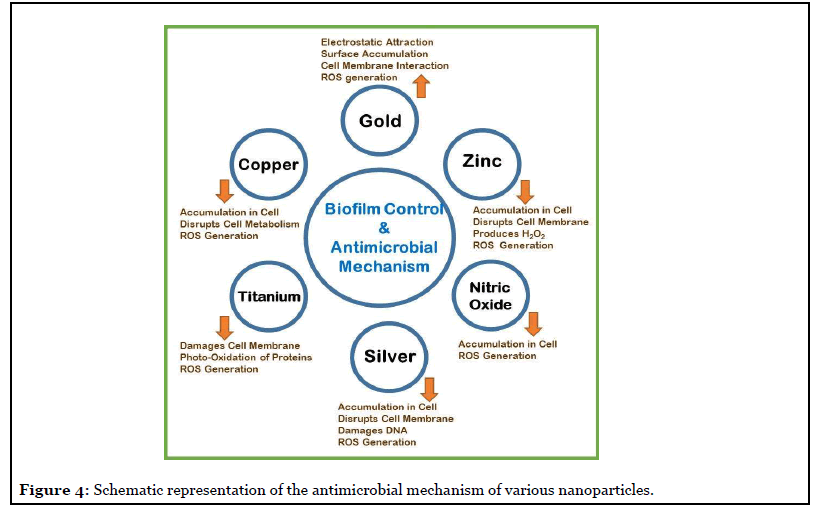
Some details of antimicrobial nanoparticles (NPs) and their probable antibacterial mechanisms are detailed in Figure 4. One promising biofilm control strategy is with NPs, the majority of them are coated on to materials. The NP impregnated drug delivery systems show potential application in the eradication of implant-associated biofilms. The majority of NPs have strong antibacterial activity, which can be attributed to at least one of the mechanisms illustrated in the schematic Figure 4. The main antimicrobial actions include; inhibition of cell wall/ membrane synthesis, disruption of metabolic processes, production of reactive oxygen species (ROS), photocatalysis, enzyme inhibition, and impairing DNA synthesis [24].
There is significant progress in the last two decades in distinguishing the role of biofilms in causing chronic infections. In addition, there were several studies to understand the role of biochemical and cellular processes in biofilm formation [17,18,23]. Nevertheless, there are only a few studies in the area of pathogenesis by biofilm infection and their interaction with host’s tissues in vivo [21,23]. There is lot of scope for both in vitro and in vivo experiments to understand the mechanisms involved in antibiotic resistance as well as in untying the immune response to chronic biofilm infections in the human system [25].
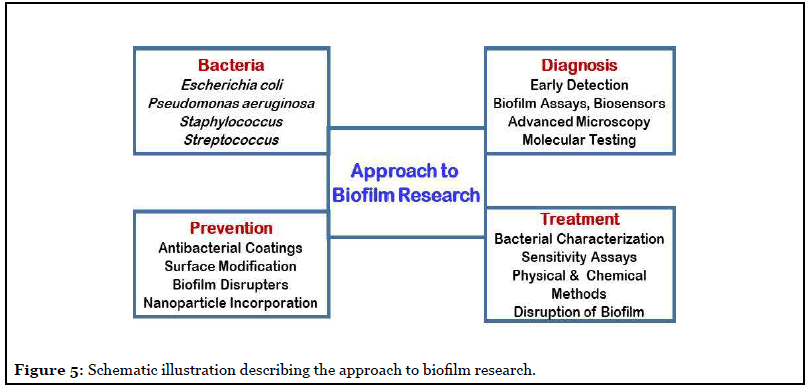
The often detected hospital acquired infections generally involve biofilms, they are the main cause of mortality in patients and one of the major concern for health care professionals. Therefore, advances in understanding both fronts; biofilm formation and biofilm resistance mechanism are necessary for stopping and handling longdrawn- out infections. Such studies demand advancement in technologies for assaying biomolecular composition of the biofilms and cell biochemistry and metabolic dynamics of biofilm-forming microorganisms. In addition, to animal models simulating chronic infections [26,27], in vivo, innovative methods for disrupting biofilms without the use of antibiotics are solicited [28]. The on-going advancements in interdisciplinary research to cognizant the biology and pathogenesis of biofilms, will provide the confidence towards development of new vistas in biofilm therapeutics. The outcome of the various research programs is aimed at development of novel approaches for biofilm control. The basic trait to successful control of biofilms lies in complete understanding of the biofilm phenotype and how different it is from the planktonic phenotype. Figure 5 illustrates the approach to biofilm research to decipher a curable determinant for controlling biofilm infections [8-11,17-19,24].
Acknowledgement
The author is grateful to the Director, Bhabha Atomic Research Centre, Mumbai, for providing all the facilities for biofilm research.
Conflict of Interest
The author has no conflict of interest as far the review article is concerned. There are no financial implications.
Author Contributions Statement
The corresponding author has prepared the concept for the review article. After a through literature survey, the contents of the mini review were drafted and completed.
References
2. Pelczar, M.J. Chan, E.C.S., Krieg, N.R. 1998. Microbiology: Application Based Approach. pp. 900. ISBN: 1259081753, 9781259081750, Tata McGraw-Hill Education, New Delhi.
3. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annual review of microbiology. 1995 Oct;49(1):711-45.
4. Hori K, Matsumoto S. Bacterial adhesion: from mechanism to control. Biochemical Engineering Journal. 2010 Feb 15;48(3):424-34.
5. Wimpenny J, Manz W, Szewzyk U. Heterogeneity in biofilms. FEMS microbiology reviews. 2000 Dec 1;24(5):661-71.
6. Tuson HH, Weibel DB. Bacteria–surface interactions. Soft matter. 2013;9(17):4368-80.
7. Bassler BL, Losick R. Bacterially speaking. Cell. 2006 Apr 21;125(2):237-46.
8. Arciola CR, Montanaro L, Costerton JW. New trends in diagnosis and control strategies for implant infections.
9. Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends in microbiology. 2001 Jan 1;9(1):34-9.
10. Shukla SK, Rao TS. Calcium-mediated modulation of Staphylococcal bacterial biofilms.
11. Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends in microbiology. 2005 Jan 1;13(1):34-40.
12. Rao TS, Kesavamoorthy R, Rao CB, Nair KV. Influence of flow on ordering characteristics of a bacterial biofilm. Current Science. 1997 Jul 10:69-74.
13. Moscovici M. Present and future medical applications of microbial exopolysaccharides. Frontiers in microbiology. 2015 Sep 29;6:1012.
14. Hanna A, Berg M, Stout V, Razatos A. Role of capsular colanic acid in adhesion of uropathogenic Escherichia coli. Applied and environmental microbiology. 2003 Aug 1;69(8):4474-81.
15. Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilmforming Staphylococcus epidermidis. Antimicrobial agents and chemotherapy. 2000 Dec 1;44(12):3357-63.
16. Neut D, C Van Der Mei H, K Bulstra S, J Busscher H. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta orthopaedica. 2007 Jan 1;78(3):299-308.
17. Shukla SK, Rao TS. Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. The Journal of antibiotics. 2013 Feb;66(2):55-60.
18. Shukla, S.K. Dugeshwar Karley, Subba Rao. T. 2017. Antimicrobial Peptides Against Microbial Biofilms: Their Structures and Modes of Action. In: Frontiers in Anti- Infective Drug Discovery – Volume 6, Bentham Science Publishers, Sharjah, U.A.E. pp. 482-521.
19. Khan F, Niaz K, Abdollahi M. Toxicity of biologically active peptides and future safety aspects: an update. Current drug discovery technologies. 2018 Sep 1;15(3):236- 42.
20. Yildiz FH. Cyclic dimeric GMP signaling and regulation of surface-associated developmental programs. Journal of bacteriology. 2008 Feb 1;190(3):781-3.
21. Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. Journal of antimicrobial chemotherapy. 2010 Sep 1;65(9):1955-8.
22. Becker P, Hufnagle W, Peters G, Herrmann M. Detection of differential gene expression in biofilmforming versus planktonic populations of Staphylococcus aureus using micro-representational-difference analysis. Applied and Environmental Microbiology. 2001 Jul 1;67(7):2958-65.
23. Lewis K. Persister cells. Annual review of microbiology. 2010 Oct 13;64:357-72.
24. Aljabali AA, Obeid MA. Inorganic-Organic Nanomaterials for Therapeutics and Molecular Imaging Applications. Nanoscience&Nanotechnology-Asia, DOI : 10.2174/2210681209666190807145229.
25. Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, Qu D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007 Jul 1;153(7):2083-92.
26. Lebeaux D, Chauhan A, Rendueles O, Beloin C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens. 2013 Jun;2(2):288-356.
27. Gries CM, Rivas Z, Chen J, Lo DD. Intravital multiphoton examination of implant-associated Staphylococcus aureus biofilm infection. Frontiers in Cellular and Infection Microbiology. 2020;10.
28. Soares A, Alexandre K, Etienne M. Tolerance and Persistence of Pseudomonas aeruginosa in Biofilms Exposed to Antibiotics: Molecular Mechanisms, Antibiotic Strategies and Therapeutic Perspectives. Frontiers in microbiology. 2020 Aug 27;11:2057.
